Abstract
Background
Chronic ethanol consumption causes alcoholic liver disease (ALD) and disruption of the circadian system facilitates the development of ALD. Small heterodimer partner (SHP) is a nuclear receptor and critical regulator of hepatic lipid metabolism. This study aims at depicting circadian metabolomes altered by chronic ethanol-plus-binge and Shp-deficiency using high throughput Metabolomics.
Methods
Wild-type (WT) C57Bl/6 and Shp-/- mice were fed the control diet (CD) or Lieber De-Carli ethanol liquid diet (ED) for 10 days followed by a single bout of maltose (CD+M) or ethanol (ED+E) binge on the 11th day. Serum and liver were collected over a 24 hr light-dark (LD) cycle at Zeitgeber time ZT12, ZT18, ZT0 and ZT6 and metabolomics was performed using GC-MS.
Results
A total of 110 metabolites were identified in liver and of those 80 were also present in serum from pathways of carbohydrates, lipids, pentose phosphate, amino acids, nucleotides and tricarboxylic acid (TCA) cycle. In the liver, 91% of metabolites displayed rhythmicity with ED+E whereas in the serum, only 87% were rhythmic. Bioinformatics analysis identified unique metabolome patterns altered in WT CD+M, WT ED+E, Shp-/- CD+M, and Shp-/- ED+E groups. Specifically, metabolites from the nucleotide and amino acid pathway (ribose, glucose-6-phosphate, glutamic acid, aspartic acid and seduheptulose-7-P) were elevated in Shp-/- CD+M mice during the dark cycle, whereas metabolites including N-methylalanine, 2-hydroxybutyric acid, 2-hydroxyglutarate were elevated in WT ED+E mice during the light cycle. The rhythmicity and abundance of other individual metabolites were also significantly altered by both control and ethanol diets.
Conclusions
Metabolomics provides a useful means to identify unique metabolites altered by chronic ethanol-plus-binge.
Keywords: metabolomics, circadian clock, nuclear receptor, alcohol
Introduction
The circadian system plays a pivotal role in coordinating the daily actions of physiology, behavior and metabolism (Adamovich et al., 2014, Huang et al., 2011). In mammals, circadian rhythms are under the control of a core circadian clock located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus (Panda et al., 2002). The SCN integrates a number of signals, such as feeding and light, and operates as a synchronizer for a multitude of peripheral clocks located in most tissues including liver, fat and muscle. Accumulative evidence demonstrates that a wide range of metabolic pathways are controlled by the circadian system and its disruption can evoke dysregulation of lipid metabolism, obesity and metabolic disease (Froy, 2010). Consequently, disruption of clock gene function in genetic mouse models results in impaired lipid metabolism (Turek et al., 2005, Oishi et al., 2006). The liver is an essential organ carrying out a number of biological processes underlying systemic energy regulation, metabolism, detoxification and hormonal productions, thus proper timing is key to maintenance of homeostasis in the system.
It has been well documented that chronic ethanol consumption causes steatosis and inflammation in rodents via disruption of key transcriptional regulators to facilitate the development of alcoholic fatty liver (AFL) (Fengler et al., 2016, You et al., 2008). In particular, exposure to ethanol has been reported to alter expression of lipid metabolism genes in livers of Clock mutant mice leading to the accumulation of lipids (Kudo et al., 2009) and disrupting the circadian rhythm of wildtype mice (Seggio et al., 2009).
The small heterodimer partner (SHP, NR0B2) is a unique nuclear receptor (Rudraiah et al., 2016) and a key transcription factor of genes involved in whole body and hepatic lipid metabolism (Huang et al., 2010, Huang et al., 2007, Wang et al., 2005, Tabbi-Anneni et al., 2010) and of circadian rhythms in the liver (Pan et al., 2010, Zhang et al., 2011b). Due to its lack of DNA binding domain, Shp exerts its transcriptional repression through protein-protein interactions with other nuclear receptors (Datta et al., 2006, Matsukuma et al., 2007, Zhou et al., 2010) and transcription factors (Zhang et al., 2014, Suh et al., 2006, Zhang et al., 2012). Shp-deficiency in mice has recently been shown to alter the rhythmicity of liver metabolic genes (Zhang et al., 2011a) by serving as an integral component of the liver circadian network to maintain hepatic lipid and homocysteine homeostasis (Lee et al., 2015, Tsuchiya et al., 2015). Furthermore, major genes regulating ethanol metabolism, Adh1 and Adh2, were upregulated in livers of ethanol-fed Shp-/- mice, pertinent to its role in ethanol catabolism (Park et al., 2016). Most recently, we used a modified chronic ethanol-binge model (Bertola et al., 2013) to examine cyclic lipid alterations in wild-type (WT) and Shp-/- mice over a 24 hr period and observed distinct responses in WT and Shp-/- mice upon ethanol diet plus ethanol-binge (ED+E) or control diet plus maltose-binge (CD+M) (Yang et al., 2016, The American Journal of Pathology, in press). In order to better understand the impact of ED+E vs CD+M in ALF, further study is needed to determine the rhythmicity of metabolic pathways controlled by alcohol and Shp.
Majority of the circadian research have largely focused on dissecting out transcriptome expression profiles of core clock and output genes involved in lipid metabolism (Udoh et al., 2015, Smalling et al., 2013). However, this provides only a partial depiction of the changes in metabolic pathways involved as many processes are regulated beyond the transcriptional level. Limited studies however, have proceeded to screen other metabolic pathways and their products (including pathways of amino acids, carbohydrates and nucleotides) oscillating throughout the day (Abbondante et al., 2016, Eckel-Mahan et al., 2012). The present study took a novel and high-throughput approach to assess the oscillatory profile of metabolites in WT and Shp-/- mice fed with ED+E or CD+M using metabolomics analysis. The critical role of Shp in the metabolome associated with the deleterious effect of alcohol provides a therapeutic and preventative insight into the diagnosis and development of ALF.
Materials and Methods
Animals and diet
Male C57Bl/6 control and Shp-/- mice (Wang et al., 2002) of 8-10 weeks of age were housed individually in an environmentally controlled room and maintained on a 24h light/dark (LD) cycle (lights on at 6 am to 6 pm) with free access to water and food. Mice were fed a control liquid diet for 5 days to acclimatize to the liquid diet and tube feeding regime. After the acclimation period, mice were given either a control diet (Bio-Serv, product #F1259SD) or 5% Lieber-DeCarli ethanol liquid diet (Bio-Serv, product #F1258SP) for 10 days with diets changed daily at 5 pm as described previously (Tsuchiya et al., 2015). On the 11th day at 9 am, mice were gavaged with a single dose of maltose dextrin (Control, 9 g maltose dextrin per kg of body weight) or ethanol (5 g ethanol per kg of body weight), respectively. Nine hours after the binge, mice (n=3 per group per genotype) were euthanized and serum and liver collected every 6 hr over a 24h LD cycle at Zeitgeber time (ZT) ZT12, ZT18, ZT0 and ZT6. Both control and ethanol diets were provided throughout the sample collection period after the binge. A dim red light at intensity of 1 μmol/m2s was used to collect tissues in dark conditions. All experimental procedures were approved and performed according to the standards of the Institutional Animal Care and Use Committee (IACUC) and The University of Connecticut.
Metabolomics analyses
The metabolomics analysis was conducted by the Metabolomics Core at the University of Utah. A Waters GCT Premier mass spectrometer fitted with an Agilent 6890 gas chromatograph and a Gerstel MPS2 auto sampler was used for GC-MS analysis. A commercially available NIST library and pure purchased standards were used to determine metabolite identity. The data was normalized by means entering to the internal standard d4-succinate.
Data Analyses
Values are presented as means ± SEM (n=3/group). All data were analyzed by a students' t-test and P<0.05 was considered statistically significant. For heatmap clustering and boxplots, a one-way ANOVA was performed to determine the differential expression of selected metabolites.
Results
Comparative circadian metabolomics profile
The Gao-binge model closely resembles the drinking behavior and of acute chronic liver injury in patients with ALD (Bertola et al., 2013), thus we used this model to understand the circadian rhythmicity of metabolomes. In order to identify the relative abundance of cyclic metabolites throughout the circadian cycle, serum and liver samples from WT and Shp-/- mice that were fed ED+E or CD+M were collected at four time points over an entire 24 hr light/dark cycle.
Metabolomics analyses by GC-MS identified about 110 known metabolites in the liver belonging to major metabolic pathways such as carbohydrates, lipids, pentose phosphate, amino acids, nucleotides and tricarboxylic acid (TCA) cycle pathways (Table 1). Although a large fraction of metabolites was also identified in serum, only 80 of these metabolites (∼73%) were found to be present in serum compared to the liver. The total number of carbohydrate and lipid metabolites identified in serum was relatively less compared to its presence in the liver (Table 1). In the carbohydrate pathway, only 10 (50%) of these were detected in the serum. In the liver, a large proportion of metabolites (91%) displayed rhythmicity with chronic ethanol feeding whereas in the serum, only 87% were rhythmic. Analyses of amino acids revealed that a total of 29 amino acid metabolites were present in both serum and liver (Table 1). In addition, a similar number of metabolites from the pentose phosphate, urea and TCA pathway were identified in serum and liver (Table 1). Of the total number of nucleotide metabolites identified following metabolome analyses, only 54% of these were found to be in the serum compared with the liver.
Table 1. Comparative metabolomics profile of metabolic pathways in serum and liver.
Serum and liver were collected from wildtype (WT) control diet plus maltose-binge (CD+M), WT ethanol diet plus ethanol-binge (ED+E), Shp-/- CD+M and Shp-/- ED+E mice over a 24h light/dark (LD) cycle at Zeitgeber time ZT12, ZT18, ZT0 and ZT6 using the Gao-binge model. A GC/MS metabolomic analysis of serum and liver was performed to identify specific metabolites from the carbohydrate, amino acid, lipid, nucleotide, pentose phosphate and tricarboxylic acid (TCA) pathways.
| Metabolic pathway | Number of metabolites in serum (%) | Number of metabolites in liver (%) |
|---|---|---|
| Total number of metabolites analyzed | 80 | 110 |
| Carbohydrates | 10 (12.5%) | 20 (18.1%) |
| Lipids | 20 (25%) | 28 (25.5%) |
| Amino acids | 29 (36.25%) | 29 (26.4%) |
| Nucleotides | 7 (8.75%) | 13 (12%) |
| Tricarboxylic acid | 9 (11.25%) | 9 (8%) |
| Urea | 2 (2.5%) | 3 (3%) |
| Others | 3 (3.75%) | 8 (7%) |
| % of metabolites rhythmic under ED+E | 91% | 87% |
Distinct cyclic pathways altered by maltose, ethanol and Shp-deficiency using bioinformatics analysis
The metabolomics data were subjected to non-supervised multivariate data analysis to identify unique metabolome patterns altered in WT CD+M, WT ED+E, Shp-/- CD+M, and Shp-/- ED+E groups. Correlation matrix clustering showed subsets of metabolites with strong correlation at each ZT time (Supp Figure S1-S4). PLS-DA scores plots revealed a maximal to minimal separation of metabolites among the four groups from the circadian dark phase between ZT12 (6 pm) and ZT18 (12 am) to light phase between ZT0 (6 am) and ZT6 (12 pm) (Figure 1A), indicating the effects of light (day) as well as food intake (night) on metabolic profiles.
Figure 1. Non-supervised multivariate data analysis of Metabolomics profiling.
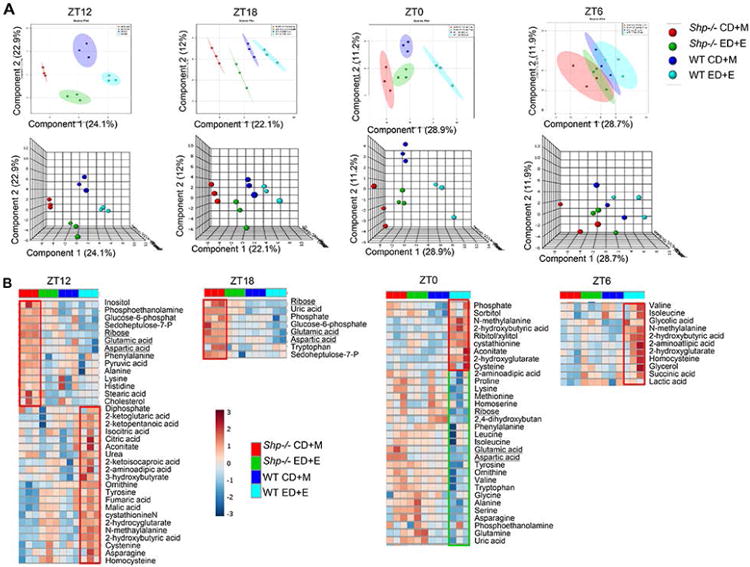
a) PLS-DA scores plot performed using two (top) or three (bottom) principal components corresponding to a model built using two or three latent variables aimed at discrimination among four groups. b) Heatmap showing differential abundance of metabolites in response to control maltose (CD+M) vs. ethanol diets (ED+E) in serum of wildtype (WT) and Shp-/- mice. Serum was collected from WT CD+M (dark blue), WT ED+E (light blue), Shp-/- CD+M (red) and Shp-/- ED+EH (green) mice over a 24h light/dark (LD) cycle at Zeitgeber time ZT12, ZT18, ZT0 and ZT6 using the Gao-binge model. A GC/MS metabolomics analysis was used to identify specific metabolite pathways.
Distinct metabolites were further visualized by Heatmap clustering. Interestingly, during the dark cycle between ZT12 and ZT18, Shp-/- CD+M mice had increased levels of metabolites from the nucleotide and amino acid pathway including ribose, glucose-6-phosphate, glutamic acid, aspartic acid and seduheptulose-7-P compared with other three groups (Figure 1B, left two panels, red box). In contrast, WT ED+E mice displayed several high levels of amino acid, TCA and lipid metabolites compared with other groups during the light cycle (ZT0 and ZT6) (Figure 1B, right two panels), which continued to onset of dark cycle at ZT12. It was also noted that some low levels of metabolites observed in WT ED+E at ZT0 (green box) (ribose, glutamic acid, aspartic acid) remained at constant low levels at other time points (Figure 1B). The differential expression of selected metabolites was elucidated by one-way ANOVA and boxplots (Supp Figure S5-S8).
Amino acid, nucleotide and lipid pathways differentially altered by maltose control and ethanol diets in Shp-/- serum
While only 73% (80 out of 110 metabolites) of overall circulating metabolomes were found in serum compared with liver, there are indeed striking differences between the two samples. Overall, more circulating serum metabolites from carbohydrate, amino acids, nucleotides, lipids and pentose phosphate pathway displayed loss of rhythmicity compared with the liver. Furthermore, differences were observed under ED+E or CD+M feeding conditions between WT and Shp-/- mice. Under CD+M conditions, Shp-/- mice had reduced amino acid (valine, threonine, methionine, homoserine, 4-aminobutyrate, β-alanine, aminomalonic acid and 4-hydroxyproline) metabolite levels during the dark phase. In particular, the levels were significantly reduced at ZT12, which was in contrast to their expected night time feeding behavior, whereas WT mice had increased levels (Figure 2A). In order to interpret the cyclic circadian pattern between WT and Shp-/- mice fed the control maltose or ethanol diet, a double plot was presented to represent these distinct changes. Aspartic acid and glutamic acid metabolite levels showed high to moderate rhythmicity in WT mice, where their amplitude was substantially increased in mice with Shp-deficiency during the dark phase from ZT12 to ZT18 (Figures 2B). Ribose, seduheptulose-7-phosphate, and phosphate metabolite levels from the nucleotide pathway were also significantly altered in Shp-/- mice that were fed CD+M during the onset of the dark phase (Figure 2C), as reflected by both increased amplitude as well as a shift in circadian phase.
Figure 2. Serum metabolites altered by maltose and ethanol diets in Shp-/- mice.
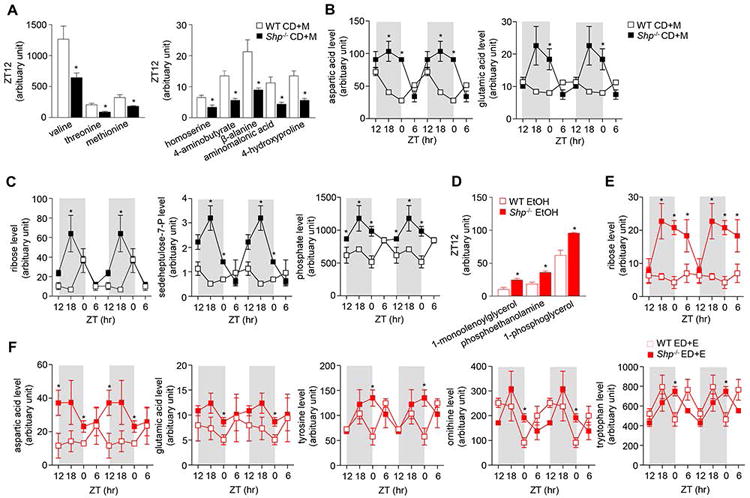
a-c: Comparison of serum metabolites between wildtype (WT) and Shp-/- mice fed with control diet + maltose binge (CD+M). a) amino acid metabolites at Zeitgeber time (ZT) 12, b) nucleotide metabolites and c) amino acid metabolites over a 24h light/dark (LD) cycle. d-f: Comparison between WT and Shp-/- mice fed with ethanol diet + ethanol binge (ED+E). d) lipid metabolites at ZT12, e) ribose metabolite and f) amino acid metabolites over a 24h LD cycle. Data are represented as means ± SEM (n=3/group). * P<0.05, Shp-/- versus WT. Serum was collected from WT CD+M (black open square), WT ED+E (red open square), Shp-/- CD+M (black closed square) and Shp-/- ED+E (red closed square) mice over a 24h LD cycle at ZT12, ZT18, ZT0 and ZT6 using the Gao-binge model. A GC/MS metabolomics analysis was used to identify specific metabolite pathways.
In response to ED+E feeding, Shp-/- mice had elevated levels of lipid metabolites (1-monoolenoylglycerol, phosphoethanolamine and 1-phosphoglycerol) at the onset of the dark phase at ZT12 compared with WT mice (Figure 2D). Additionally, ribose metabolite levels had no obvious rhythmicity in WT mice, while their levels showed a clear circadian oscillation due to increased amplification during the dark phase in Shp-/- mice (Figure 2E). Interestingly, Shp-/- mice had slightly higher magnitude of oscillation of circulating aspartic acid, glutamic acid, and tyrosine during the onset of light conditions compared with WT mice (Figure 2F), which caused enhanced amplification. In addition, plasma ornithine and tryptophan levels showed strong rhythmicity shift of the circadian cycle to the right in Shp-deficient mice (Figure 2F).
Oscillatory liver metabolic pathways altered by maltose diet in Shp-/- mice
WT mice that were fed CD+M had more pronounced effects on the carbohydrate pathway compared with the amino acids, lipid and nucleotide pathways in the liver (Figure 3). Indeed, carbohydrate metabolite levels of fructose, galactose and GalNac showed similar circadian rhythmicity which peaked during the dark phase in WT mice (Figure 3A), consistent with the composition of the control diet as well as increased activity and eating habits during the night cycle. In contrast, these metabolites in Shp-/- had reduced amplitude and blunted circadian rhythmicity (Figure 3A). Similarly, circadian rhythmicity of ribose and sedoheptulose-7-phosphate from the pentose pathway and uracil, adenosine, hypoxanthine, adenine and uric acid from the nucleotides pathway were similar despite reduced amplitude and circulating metabolite levels in Shp-/- mice compared with WT mice (Figures 3B-3C). Additionally, there was a decrease in oscillatory amplitude of palmitiladic acid, linoleic acid, 1-monolinoleoylglycerol and 1-monopalmitolylglycerol in Shp-/- mice (Figure 3D). Majority of metabolites from the carbohydrate, lipid and nucleotide pathways from Shp-/- mice lack strong rhythmicity under CD+M conditions in contrast to WT mice who displayed strong rhythmicity (Figures 3B-3D). Similar to serum levels, glutamic acid in the liver showed a significant shift in circadian phase where metabolite levels were elevated in Shp-/- mice during the dark phase and reduced at the onset of the light phase compared with WT mice (Figure 3E).
Figure 3. Liver oscillatory metabolic pathways disrupted by maltose-diet in Shp-/- mice.
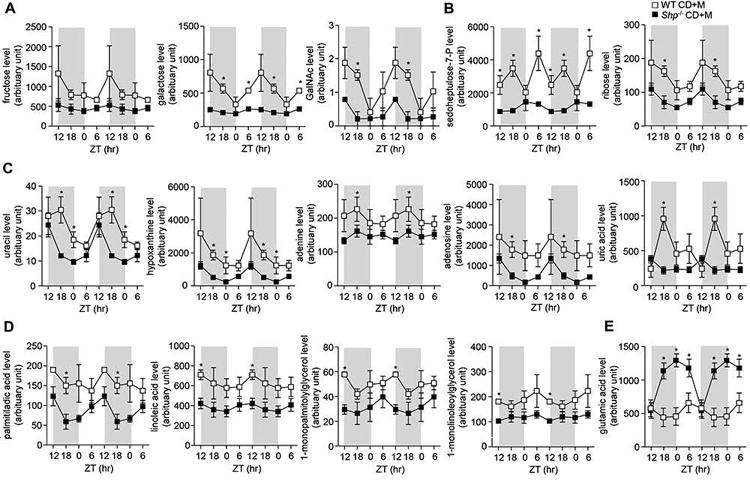
a-d: Comparison of liver metabolites between wildtype (WT) and Shp-/- mice fed with control diet + maltose binge (CD+M). a) carbohydrate metabolites, b) pentose phosphate metabolites, c) nucleotide metabolites, and d) lipid metabolites in WT and Shp-/- mice. Data are represented as means ± SEM (n=3/group). * P<0.05, Shp-/- versus WT. Livers were collected from WT CD+M (black open square) and Shp-/- CD+M (black closed square) mice over a 24h LD cycle at Zeitgeber time ZT12, ZT18, ZT0 and ZT6. A GC/MS metabolomics analysis was used to identify specific metabolite pathways.
Oscillatory liver metabolic pathways altered by ethanol diet in Shp-/- mice
Mice fed ED+E displayed rhythmicity in their lipid pathway but the amplitude and expression levels were lower (linoleic acid, oleic acid, elaidic acid, 1-monolinoleoylglycerol and 2-hydroxybutyric acid) in Shp-/- mice, which was significant at the beginning of the dark phase (ZT12) (Figure 4A). Furthermore, Shp-/- mice had low rhythmicity throughout the 24 hr light-dark cycle in contrast to the strong rhythmicity of WT mice (Figure 4A). The circadian profile from the nucleotide pathway showed a shift of circadian phase of adenine, ribose and hypoxanthine in Shp-/- mice where levels were higher at the onset of the light phase compared with WT mice (Figure 4B). Consistent with this, there was also a shift of circadian phase of carbohydrate levels of GalNac in Shp-/- mice with lower expression levels compared with ethanol-fed WT mice (Figure 4C). In the amino acid pathway, lower levels of β-alanine, 4-hydroxyproline, N-methylalanine and 4-aminobutyrate amino acids were found in livers of Shp-/- mice (Figure 4D). In particular, the oscillatory shift and amplitude of β-alanine, 4-hydroxyproline and 4-aminobutyrate amino acids was noticeably altered by Shp-deficiency in comparison to WT mice (Figure 4D). Increased levels of BCAAs valine, leucine and isoleucine, as well as AAAs glutamic acid, asparagine and phenylalanine were evident in Shp-/- mice at the onset of the light phase at ZT0 compared with WT mice (Figure 4D-4E). In particular glutamic acid, valine, leucine, isoleucine, phenylalanine and asparagine showed a shift in circadian phase where it peaked at the onset of the light cycle and lower expression during the dark cycle compared with WT mice (Figure 4E).
Figure 4. Liver oscillatory metabolic pathways disrupted by ethanol-diet in Shp-/- mice.
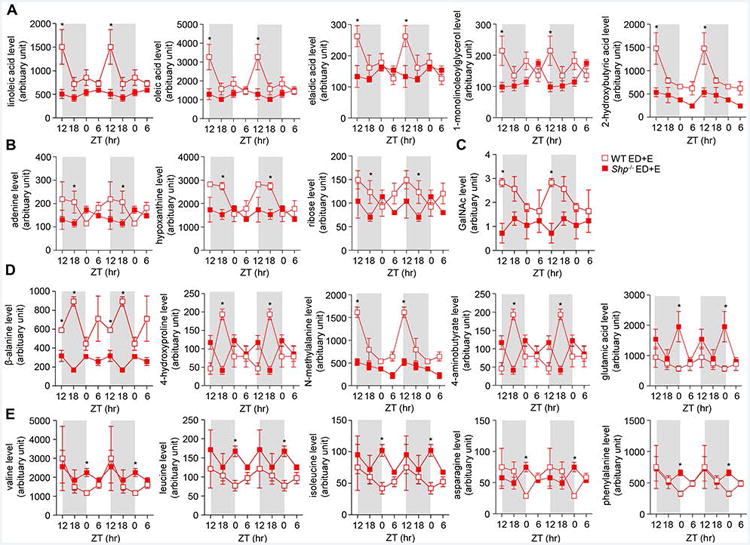
a-d: Comparison of liver metabolites between wildtype (WT) and Shp-/- mice fed with ethanol diet + ethanol binge (ED+E). a) lipid metabolites, b) nucleotide metabolites, c) carbohydrate metabolites, and d) amino acid metabolites in WT and Shp-/- mice. Data are represented as means ± SEM (n=3/group). * P<0.05, Shp-/- versus WT. Livers were collected from WT ED+E (red open square) and Shp-/- ED+E (red closed square) mice over a 24h light/dark (LD) cycle at Zeitgeber time ZT12, ZT18, ZT0 and ZT6 using the Gao-binge model. A GC/MS metabolomics analysis was used to identify specific metabolite pathways.
Discussion
Analysis of serum metabolomes for markers of liver dysfunction have been extensively used to identify liver disease states, however majority of the studies have yet to provide thorough screening beyond this level. Here we provide a novel and high throughput comparative oscillatory analysis of the serum metabolome compared with the liver metabolome over a 24hr circadian cycle to elucidate the role of Shp and the deleterious effects of alcohol on the development of ALD.
The current study demonstrates that the majority of metabolites identified by GC-MS was found in the liver compared with the serum. The cycling of carbohydrates and lipids in particular, were predominately found in the liver compared with its presence in the serum. Indeed, the liver is the primary site of glucose uptake and glucose production, where the majority of the cycling carbohydrates (including glucose and fructose) are broken down and stored as glycogen and excess lipids undergo fatty acid oxidation and its energy is stored as fatty acids. The abundance of these particular metabolites from the carbohydrate and lipid pathways were either not detected or were below the detection limit in the serum compared with their abundance in the liver. Interestingly, there was a phase shift of serum aspartic acid and glutamic acid cycle where metabolite levels peaked during the dark cycle in Shp-/- mice by the maltose-binge diet. Aspartic acid and glutamic acid are two main excitatory neurotransmitters playing important roles in protecting the liver by aiding the removal of ammonia. High levels of ammonia in the system can result in severe liver dysfunction (Sawhney et al., 2016). This was also evident in liver where glutamic acid levels were higher in Shp-/- mice than in WT mice irrespective of diet consumed. The elevated levels may be associated with changes in the transsulfuration pathway previously reported where hepatic levels of betaine-homocysteine methyltransferase (Bhmt) and cystathionine gamma lyase (Cth) protein were significantly upregulated in Shp-deficient mice (Tsuchiya et al., 2015). These observations suggests that maltose-binge may have trigged a defensive response in Shp-/- mice in protecting the liver against harmful levels of toxic metabolites.
Although the majority of metabolites identified by the metabolomics analysis were present in both CD+M and ED+E feeding conditions, there were in fact differences between the two diets in both serum and liver despite a similar volume intake of the CD and ED that both WT and Shp-/- mice consumed. Furthermore, differences were also observed between the two genotypes under control conditions. The CD+M diet is rich in monosaccharides and disaccharides that are not present in normal chow diet; this is required for the purpose of balancing caloric intake from the ethanol diet. The lower levels of metabolites observed in the carbohydrate, lipid, pentose phosphate and nucleotide pathway in Shp-/- mice compared with WT mice under CD+M conditions suggest that lack of Shp may result in a fast turnover in the circulating levels of these metabolites. However the composition of the control maltose diet may affect to some extent the expression profile of particular metabolites compared with a regular chow diet. It is also worth noting that Shp-deficient mice treated with ED+E showed a similar low expression pattern in the carbohydrate, lipid and pentose phosphate pathways irrespective of the diet consumed. These distinct changes in individual metabolites altered by ethanol feeding and Shp-deficiency may correlate with changes in hepatic gene expression recently reported (Yang et al.; 2016, in press). Specifically, the lower expression levels may correlate with activating transcription factor-4 (ATF4) protein which was down regulated in Shp-/- compared with WT mice. The higher levels of liver lipid and carbohydrate metabolites in WT mice fed the ethanol diet was consistent with the increased lipid accumulation, which was diminished in mice deficient for Shp (Yang et al., 2016, in press).
The essential amino acids leucine, isoleucine and valine are classified as branched chain amino acids (BCAAs) which are poorly metabolized in the liver compared with other essential amino acids. Studies have previously reported that an imbalance between BCAA and AAA can lead to liver failure (Tajiri and Shimizu, 2013) as well as other diseases such as obesity, cardio-metabolic dysfunction and insulin resistance (Tajiri and Shimizu, 2013, Newgard et al., 2009, Wang et al., 2011). Majority of these studies have largely focused on AAAs phenylalanine and tyrosine because of its precursor with brain catecholamine and to a smaller extent, tryptophan, which is converted into the inhibitory neurotransmitter serotonin. In patients with advanced liver disease and liver cirrhosis, BCAA levels were found to be reduced while methionine and AAAs tyrosine and phenylalanine were increased (Morgan et al., 1978), possibly due to impaired hepatic metabolism and portal systemic shunting of blood. Patients with fulminant hepatic failure also had elevated levels of AAA but concentration levels of BCAA were largely normal (Chase et al., 1978).
In the current study, the modest increase in serum AAA levels (aspartic acid, glutamic acid, tyrosine, ornithine and tryptophan) in Shp-/- mice during the dark cycle were not associated with overt changes in serum BCAA (valine, leucine and isoleucine) levels. Although there was a shift in the oscillating profile of BCAAs and phenylalanine in livers of Shp -/- mice fed the alcohol diet, levels were moderately higher compared with WT mice, consistent with levels reported in serum. On the contrary, the lower expression levels of a number of amino acids including BCAAs (valine, leucine, isoleucine), glucogenic amino acids (glutamic acid, aspartic acid, glycine, alanine, proline) and AAAs (ornithine, tyrosine, tryptophan, asparagine) as well as other amino acids (serine, methionine, lysine, homoserine, glutamine) in WT mice fed the ethanol diet is intriguing and warrants further investigation. It is worth noting however, that serum ALT levels were transiently increased after a binge in WT mice fed the ED+E but returned to basal levels after 24 hours (Tsuchiya et al., 2015). While elevated levels of serum AST and ALT may point towards acute liver injury and reduced plasma BCAA is a hallmark of liver cirrhosis, the required levels need to reach a certain threshold for prominent signs of liver dysfunction. Our findings of reduced BCAA levels and elevated serum ALT previously reported in WT mice fed ED+E may be an indicator of early liver dysfunction that has yet to manifest. Nevertheless, it appears that Shp-deficiency may afford protection or at least delay the onset of liver dysfunction due to the elevated BCAA metabolite levels observed.
Several lines of evidence suggest that disruption of the circadian system may be a risk factor for alcohol-induced intestinal hyperpermeability, endotoxemia, dysbiosis and hepatic steatosis (Forsyth et al., 2015, Mutlu et al., 2009, Summa et al., 2013, Yan et al., 2011). Gut-derived endotoxemia is a key cofactor of ALD and accumulative evidences have reported a strong correlation between plasma endotoxin levels and the severity of liver injury (Summa et al., 2013, Mutlu et al., 2009, Yan et al., 2011). Similarly, impairments in the circadian cycle leading to dysregulated lipid metabolism have been reported (Froy, 2010). Indeed, the increased steatosis reported in WT mice following exposure to chronic ethanol consumption may be associated with increased intestinal permeability and endotoxemia but further investigation is warranted which is beyond the scope of the current study. Lower liver triglycerides levels were reported in Shp-/- mice in response to ED+E (Yang et al.; 2016, in press) which may be associated with the reduced circulating lipid metabolites observed in the current study.
This study provides a novel insight into the role of Shp and the effects of control maltose and alcohol on serum and liver metabolomes, and demonstrate that observations reported in the serum do not necessarily translate to the findings reported in the liver. On a whole, metabolomics analysis revealed distinct cyclic changes in metabolites by ethanol binge and Shp-deficiency and that prolonged ED+E feeding may further unmask a distinct phenotype compared with the mild changes observed in the current study. However, further studies including the use of RNA sequencing is warranted to link the metabolomics changes observed to a specific set of genes that may be altered by Shp-deficiency. Nevertheless, alterations in amino acid metabolite levels due to ethanol feeding highlight important characteristics that may provide therapeutic approaches into the diagnosis and prevention of ALD.
Supplementary Material
Acknowledgments
We thank Dr. James Cox for the metabolomics analysis.
Grant Support: L.W. is supported by NIH R01DK104656, R01DK080440, R01ES025909, R21AA022482, R21AA024935, VA Merit Award 1I01BX002634, P30 DK34989 (Yale Liver Center) and the National Natural Scientific Foundation of China (Grant No. 81572443). S.L. is supported by VA Merit Award 1I01CX000361, NIH U01AA021840, US DOD W81XWH-12-1-0497 and R21AA024935.
Footnotes
No conflict of interest to disclose for all authors.
Author's contributions: M.T., Z.Y., S.L., and L.W. analyzed the data. M.T. and L.W. prepared the manuscript.
References
- Abbondante S, Eckel-mahan KL, Ceglia NJ, Baldi P, Sassone-corsi P. Comparative Circadian Metabolomics Reveal Differential Effects of Nutritional Challenge in the Serum and Liver. J Biol Chem. 2016;291:2812–28. doi: 10.1074/jbc.M115.681130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamovich Y, Rousso-noori L, Zwighaft Z, Neufeld-cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–30. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase RA, Davis M, Trewby PN, Silk DBA, Williams R. Plasma amino acid profiles in patients with fulminant hepatic failure treated by polyacrylonitrate membrane hemodialysis. Gastroenterology. 1978;78:1033–40. [PubMed] [Google Scholar]
- Datta S, Wang L, Moore DD, Osborne TF. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter by nuclear receptors liver receptor homologue-1 and small heterodimer partner: a mechanism for differential regulation of cholesterol synthesis and uptake. J Biol Chem. 2006;281:807–12. doi: 10.1074/jbc.M511050200. [DOI] [PubMed] [Google Scholar]
- Eckel-mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci U S A. 2012;109:5541–6. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fengler VH, Macheiner T, Kessler SM, Czepukojc B, Gemperlein K, Muller R, Kiemer AK, Magnes C, Haybaeck J, Lackner C, Sargsyan K. Susceptibility of Different Mouse Wild Type Strains to Develop Diet-Induced NAFLD/AFLD-Associated Liver Disease. PLoS One. 2016;11:e0155163. doi: 10.1371/journal.pone.0155163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A. Circadian rhythms, alcohol and gut interactions. Alcohol. 2015;49:389–98. doi: 10.1016/j.alcohol.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, Wang L. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–57. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- Huang J, Tabbi-anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1211–21. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–41. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Tamagawa T, Shibata S. Effect of chronic ethanol exposure on the liver of Clock-mutant mice. J Circadian Rhythms. 2009;7:4. doi: 10.1186/1740-3391-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Zhang Y, Tsuchiya H, Smalling R, Jetten AM, Wang L. Small heterodimer partner/neuronal PAS domain protein 2 axis regulates the oscillation of liver lipid metabolism. Hepatology. 2015;61:497–505. doi: 10.1002/hep.27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukuma KE, Wang L, Bennett MK, Osborne TF. A key role for orphan nuclear receptor liver receptor homologue-1 in activation of fatty acid synthase promoter by liver X receptor. J Biol Chem. 2007;282:20164–71. doi: 10.1074/jbc.M702895200. [DOI] [PubMed] [Google Scholar]
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut. 1978;19:1068–73. doi: 10.1136/gut.19.11.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–46. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006;580:127–30. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174–86. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Park JE, Lee M, Mifflin R, Lee YK. Enhanced ethanol catabolism in orphan nuclear receptor SHP-null mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G799–807. doi: 10.1152/ajpgi.00343.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudraiah S, Zhang X, Wang L. Nuclear Receptors as Therapeutic Targets in Liver Disease: Are We There Yet? Annu Rev Pharmacol Toxicol. 2016;56:605–26. doi: 10.1146/annurev-pharmtox-010715-103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney R, Holland-fischer P, Rosselli M, Mookerjee RP, Agarwal B, Jalan R. Role of ammonia, inflammation, and cerebral oxygenation in brain dysfunction of acute-on-chronic liver failure patients. Liver Transpl. 2016;22:732–42. doi: 10.1002/lt.24443. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–12. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalling RL, Delker DA, Zhang Y, Nieto N, Mcguiness MS, Liu S, Friedman SL, Hagedorn CH, Wang L. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G364–74. doi: 10.1152/ajpgi.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Huang J, Park YY, Seong HA, Kim D, Shong M, Ha H, Lee IK, Lee K, Wang L, Choi HS. Orphan nuclear receptor small heterodimer partner inhibits transforming growth factor-beta signaling by repressing SMAD3 transactivation. J Biol Chem. 2006;281:39169–78. doi: 10.1074/jbc.M605947200. [DOI] [PubMed] [Google Scholar]
- Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS One. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbi-anneni I, Cooksey R, Gunda V, Liu S, Mueller A, Song G, Mcclain DA, WANG L. Overexpression of nuclear receptor SHP in adipose tissues affects diet-induced obesity and adaptive thermogenesis. Am J Physiol Endocrinol Metab. 2010;298:E961–70. doi: 10.1152/ajpendo.00655.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol. 2013;19:7620–9. doi: 10.3748/wjg.v19.i43.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H, Da Costa KA, Lee S, Renga B, Jaeschke H, Yang Z, Orena SJ, Goedken MJ, Zhang Y, Kong B, Lebofsky M, Rudraiah S, Smalling R, Guo G, Fiorucci S, Zeisel SH, Wang L. Interactions Between Nuclear Receptor SHP and FOXA1 Maintain Oscillatory Homocysteine Homeostasis in Mice. Gastroenterology. 2015;148:1012–1023 e14. doi: 10.1053/j.gastro.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, Mcdearmon E, Laposky A, Losee-olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, BASS J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udoh US, Valcin JA, Gamble KL, Bailey SM. The Molecular Circadian Clock and Alcohol-Induced Liver Injury. Biomolecules. 2015;5:2504–37. doi: 10.3390/biom5042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, Moore DD. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–31. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, Moore DD. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab. 2005;2:227–38. doi: 10.1016/j.cmet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, Mccabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–8. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andrews GK, Wang L. Zinc-induced Dnmt1 expression involves antagonism between MTF-1 and nuclear receptor SHP. Nucleic Acids Res. 2012;40:4850–60. doi: 10.1093/nar/gks159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonzo JA, Gonzalez FJ, Wang L. Diurnal regulation of the early growth response 1 (Egr-1) protein expression by hepatocyte nuclear factor 4alpha (HNF4alpha) and small heterodimer partner (SHP) cross-talk in liver fibrosis. J Biol Chem. 2011a;286:29635–43. doi: 10.1074/jbc.M111.253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011b;1812:893–908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu N, Xu J, Kong B, Copple B, Guo GL, Wang L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology. 2014;60:919–30. doi: 10.1002/hep.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Zhang Y, Macchiarulo A, Yang Z, Cellanetti M, Coto E, Xu P, Pellicciari R, Wang L. Novel polymorphisms of nuclear receptor SHP associated with functional and structural changes. J Biol Chem. 2010;285:24871–81. doi: 10.1074/jbc.M110.133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


