Abstract
Objectives
Cardiovascular reactivity has been associated with future hypertension and cardiovascular mortality. Higher physical activity (PA) has been associated with lower cardiovascular reactivity in adults, but little data is available in children. The purpose of this study was to examine the relationship between PA and cardiovascular reactivity to mental stress in children.
Design
Cross-sectional study.
Methods
This study sample included children from the Oswego Lead Study (n=79, 46% female, 9-11 years old). Impedance cardiography was performed while children participated in a stress response protocol. Children were also asked to wear Actigraph accelerometers on their wrists for 3 days to measure intensity and duration of PA and sedentary time.
Results
In multivariable models, moderate to vigorous (MV) PA was associated with lower body mass index (BMI) percentile and lower total peripheral resistance (TPR) response to stress (beta = −0.025, p = 0.02; beta = −0.009, p = 0.05). After additional adjustment for BMI, MVPA was also associated with lower diastolic blood pressure response to stress (beta = −0.01, p = 0.03). Total PA and sedentary time were not associated with BMI or cardiovascular responses to stress.
Conclusions
A modest, inverse relation of PA to vascular reactivity to mental stress was observed in children. These data provide confirmatory evidence that the promotion of PA recommendations for children are important for cardiovascular health.
Keywords: blood pressure, heart rate, sedentary lifestyle, vascular resistance, pediatrics
Introduction
Exaggerated cardiovascular (CV) reactivity to stress is associated with cardiovascular disease (CVD) risk in adults.1 There is also consistent evidence showing that a high blood pressure (BP) response to stress is associated with a modest risk of future hypertension in both children and adults.2, 3 Physically inactive adults are generally observed to have higher CV reactivity to stress compared to more active peers,4 which may partially explain the elevated hypertension and CVD risk attributed to physical inactivity. However, there is considerably less evidence supporting a relationship between physical activity (PA) and CV reactivity to stress in children.5, 6
The underlying hemodynamics of the CV response may shed light on the relation of CV reactivity to CVD risk7 as well as the influence of PA. An exaggerated BP response may be driven by a combination of elevated cardiac output (CO), insufficient vasodilation and low vascular compliance.8 PA contributes to greater vascular compliance,9 influencing the balance of BP to CO, thus reducing total peripheral resistance (TPR).
The main purpose of this investigation was to examine the relation between PA and the interplay among hemodynamic changes (primarily BP, heart rate [HR], and TPR) in response to mental stress in children. PA is a multidimensional factor that includes different intensity levels and sedentary behavior that may have independent effects on CV health, and we hypothesized that each may have independent effects on the CV response to mental stress tasks. The use of accelerometer-determined measures of PA in the current investigation has the potential to improve the sensitivity and specificity for detecting the influences of the volume of PA at different intensities on CV reactivity.
Methods
Children between the ages of 9-11 years (n=100) were recruited for participation in the current investigation as part of the Oswego Lead Study, as previously described.10 Participant inclusion criteria involved willingness to undergo stress testing, agreeing to wear the PA monitor for 3 days and subjecting to a blood draw (for measures not included in the present investigation). Participants must have also reported no use of medication that could affect cardiovascular functioning (e.g., Ritalin) on the day of testing and have no significant developmental disorders that could affect task performance (e.g., autism, severe intellectual disability). Written informed assent and consent was obtained from all participants and their parents, and children were paid $100 for their participation. The Human Research Committee of the State University of New York (SUNY) at Oswego approved this study.
Participants arrived at the laboratory in the morning, at which time height, weight and questionnaires were administered. A 10-minute (min) rest period was given directly before beginning the stress-reactivity protocol. Experimental tasks were developed and run using E-Prime™ (Psychology Software Tools, Pittsburgh, PA). This software is programmed to present stimuli and record timing of choices made by clicking the mouse or keypad and records positioning of the cursor. The order of acute stress tasks were counter balanced, as previously described.10 Briefly, this protocol included: [1] a mirror tracing task (2 trials, 1.5-min each) in which participants use the mouse/cursor to trace a star (modified from 11), with the mouse control inverted so that the cursor moves to the right when the mouse moves to the left; [2] an inter-task rest (8-min); [3] a Go/No-Go task (2 trials, 2.5-min each) in which the goal is to respond as quickly as possible to the target tone but not to a non-target tone, sounding on 10-second (s) intervals (8 targets and 7 non-targets); [4] an inter-task rest (8-min); and [5] a continuous performance task (5 trials, 75-s each) in which numerical stimuli (0-9) were shown on the screen in rapid, random sequence (250-ms stimulus interval, 500-ms inter-stimulus interval), with “9” as the target number. CV reactivity was measured using impedance cardiography, electrocardiogram (ECG), and BP cuff (non-dominant arm) during experimental tasks as described below.
This series of computer tasks was designed to be challenging for all participants and thereby reliably produce mild stress and a cardiovascular response, rather than major physiological stressors that may be less typical of everyday stressors. Our protocol is very similar to other protocols used to study cardiovascular responses to stress in children2 and has been used extensively in the past.10
An Impedance Cardiograph (Model HIC-2000, Bio-Impedance Technology, Chapel Hill, NC) was used for the generation of the impedance wave forms using a tetra polar band electrode configuration. Electrodes for the ECG (to measure HR) were placed on each side of the abdomen below the impedance electrode bands, as well as a ground electrode beside the navel. This noninvasive technology measures changes in conductivity of the thorax. Calculations were performed as previously described12 to derive stroke volume (SV) and CO.
Systolic (S)BP and diastolic (D)BP were monitored using the Vasotrac device (APM205A; Medwave, Danvers, MA), which automatically computes TPR using the formula: TPR = ([(SBP-DBP)/3] + DBP)/CO X 80, where TPR is in dyne-s/cm−5, CO is in liters/min (calculated by the Impedence Cardiograph), and SBP and DBP are in mm Hg. During the last 3-min of the initial rest period and during the entire stress task protocol, BP measurements were recorded every 30-s. During the same time periods, HR and data for the impedance-derived variables were collected using a 15-s inter-sample interval and 14-s ensemble average duration (allowing 1-s for storing data). Per previous recommendations,12 measures based on volumetric calculations (i.e. SV, CO, and TPR) were only analyzed as change scores. The use of change scores has been reported to increase reliability of the measurements.13
SBP, DBP and HR change scores were computed by subtracting baseline levels of a variable from the task means. For impedance-derived variables involving volume measures (CO, SV, TPR), percent change from baseline to task was used because the accuracy of absolute levels of these variables is not reliable.12 For all analyses of responses to acute stress, cardiovascular change scores were standardized within the three acute stress tasks and then averaged across tasks (zMean Δ or zMean %Δ).2 This approach has been demonstrated to improve the reliability of cardiovascular reactivity assessment,12 and has been shown to predict future BP in children.2
PA was measured with the Actigraph Model GT1M accelerometers (Actigraph Inc., Pensacola, Florida), with a dynamic range of 0.05–2.5 g and frequency range of 0.25–2.5 Hz (Actigraph 2008). Participants were asked to wear accelerometers on their wrist from Friday morning when they arrived to the study visit (at 8 or 8:30 AM) until the next study visit (usually the following Monday) during all hours, except when bathing. Counts were collected in 1-min epochs.
Participants completed a sleep log, which was used as a general window to determine sleep and wake times. The actual sleep time was determined as starting with 10 consecutive minutes with no activity and wake time as 10 consecutive minutes of some activity, which was replicated successfully by independent coders (r = 0.96, p < 0.001). Sleep time was recoded as non-wear time for the current analysis. During waking hours, non-wear time was defined as 20 min or more 0 counts/min (allowing for 2 epoch interruptions). We received PA accelerometer data from 86 participants, of which 80 participants had 3 valid wear days with >10 h weartime per day, with sufficient wear time reported to provide high reliability in children.14 Of these 80 participants, one was excluded for incomplete stress-test data (n=79).
Sedentary time was defined as <420 counts per minute (cpm), moderate activity 4,332-13,559 cpm, and vigorous activity ≥13,560 cpm, disseminating from a regression equation validated by Crouter et al. for wrist-worn Actigraph monitoring in children.15 These cutpoints were chosen because they yielded better classification accuracy of activity intensity in previous studies.16, 17
The following covariates were used in multivariate models: age, sex, socioeconomic status (SES) score (using an average of parents’ education, occupation, and income; which were standardized prior to averaging), and season of accelerometer wear. Body mass index percentile (BMI%, age- and sex-adjusted by CDC standards18) was also added as a covariate in some models.
Results are reported as mean ± standard deviation. Comparisons by HR response were examined using t tests. PA variables were ranked to reduce the impact of outliers before regression models were run. Multivariable linear regression models were used to examine associations between PA and cardiovascular reactivity measures, adjusting for possible confounders. Interactions with BMI% were explored. All statistical analysis were performed using Statistical Analysis System (SAS, version 9.4, SAS Institute Inc., Cary, NC).
Results
There were no significant differences in baseline physiological or PA variables between boys and girls, aged 9-11 years in this study (Table 1). Mean resting BP was 106/57 mm Hg, increasing to 112/61 mm Hg during the mental stress tasks; mean HR increased from 86 to 87 bpm during the stress tasks (Table 1). Girls had a higher mean percent change in SV during the mental stress tasks (p=0.04).
Table 1.
Participant demographics, cardiovascular stress response and physical activity variables (mean ± SD, unless otherwise described)
| Demographic Variables | Total (n=79) | Girls (n=36) | Boys (n=43) | p-value for difference by sex |
|---|---|---|---|---|
| Age, years | 10.0 ± 0.6 | 10.0 ± .6 | 10.1 ± .6 | 0.53 |
| Race, % white | 86 | 83 | 88 | 0.53 |
| BMI % a | 76 ± 22 | 74 ± 23 | 78 ± 22 | 0.38 |
| Obesity (>95th BMI %) a, n | 21 | 9 | 12 | 0.77 |
| Resting Heart Rate, bpm | 86 ± 11 | 87 ± 10 | 85 ± 11 | 0.32 |
| Resting Systolic BP, mm Hg | 106 ± 11 | 106 ± 11 | 105 ± 12 | 0.62 |
| Resting Diastolic BP, mm Hg | 57 ± 7 | 58 ± 7 | 56 ± 7 | 0.24 |
| Cardiovascular Stress Response Variables | ||||
| Mean Stress Task Heart Rate, bpm | 87 ± 10 | 88 ± 10 | 87 ± 10 | 0.60 |
| Mean Stress Task Systolic BP, mm Hg | 112 ± 11 | 111 ± 10 | 111 ± 12 | 0.99 |
| Mean Stress Task Diastolic BP, mm Hg | 61 ± 7 | 60 ± 7 | 60 ± 7 | 0.94 |
| Mean %Δ Stroke Volume | −0.36 ± 7.86 | 1.59 ± 8.70 | −2.00 ± 6.74 | 0.04 |
| Mean %ΔCardiac Output | 0.76 ± 9.31 | 2.00 ± 11.22 | −0.28 ± 0.07 | 0.28 |
| Mean %Δ Total Peripheral Resistance | 7.06 ± 12.51 | 5.71 ± 13.09 | 8.20 ± 12.04 | 0.38 |
| Physical Activity (PA) Variables | ||||
| Total Counts per min weartime | 2045 ± 468 | 2079 ± 434 | 2015 ± 498 | 0.56 |
| Sedentary, min/day | 227 ± 72 | 212 ± 70 | 239 ± 73 | 0.10 |
| % Sedentary time, min/min weartime | 28 ± 9 | 26 ± 8 | 30 ± 9 | 0.10 |
| Light, min/day | 468 ± 67 | 478 ± 57 | 459 ± 74 | 0.20 |
| MVPA, min/day | 114 ± 44 | 114 ± 40 | 113 ± 48 | 0.82 |
| Moderate, min/day | 111 ± 42 | 113 ± 39 | 110 ± 45 | 0.78 |
| Vigorous, min/dayb | 3.5 ± 5.2 | 3.3 ± 4.4 | 3.6 ± 5.7 | 0.67 |
| Participants meeting PA guidelines, n | 70 | 32 | 38 | 0.94 |
Abbreviations: Body mass index (BMI); beats per minute (bpm); blood pressure (BP); moderate-to-vigorous physical activity (MVPA).
Mean %Δ values indicate the percent change (from baseline to stress level) averaged across tasks.
CDC BMI percentiles
Mean vigorous min only calculated for children with any vigorous minutes (n=60)
Children included in this study wore the PA accelerometers for an average of 808 min/day. Only 11% of participants (n=9) averaged less than 60-min MVPA per day, consistent with not meeting the PA Guidelines for Americans.19 However, 85% of children spent less than 5 minutes per day in vigorous activities and 24% of participants spent less than 1 minute in vigorous activities during the 3-days when the monitor was worn.
In univariate analysis, BMI categories were not related to resting HR, SBP, DBP, or PA variables(Supplemental Table 1). But in regression models adjusted for covariates (age, sex, SES, and season the accelerometer was worn), MVPA was associated with lower BMI%. After adjusting for BMI%, PA was not associated with resting HR, SBP or DBP (Table 2).
Table 2.
Multivariable-adjusted regression models to assess the relations between physical activity measures and baseline variables.
| Sedentary Time (%/day) | Total counts/min | MVPA (min/day) | |||||
|---|---|---|---|---|---|---|---|
| Baseline Variables | Models | estimate | p | estimate | p | estimate | p |
| BMI% | Multivar. Model | −0.02 | 0.86 | −0.18 | 0.11 | −0.25 | 0.02 |
| Resting Heart Rate | Multivar. Model | 0.04 | 0.51 | −0.11 | 0.04 | −0.12 | 0.03 |
| + BMI% | 0.04 | 0.48 | −0.09 | 0.08 | −0.09 | 0.08 | |
| Resting Systolic BP | Multivar. Model | −0.03 | 0.60 | 0.05 | 0.42 | 0.06 | 0.28 |
| + BMI% | −0.03 | 0.61 | 0.06 | 0.35 | 0.08 | 0.21 | |
| Resting Diastolic BP | Multivar. Model | −0.04 | 0.30 | 0.05 | 0.17 | 0.06 | 0.11 |
| + BMI% | −0.04 | 0.31 | 0.06 | 0.13 | 0.07 | 0.06 | |
Abbreviations: Body mass index CDC percentile (BMI%); blood pressure (BP); moderate-to-vigorous physical activity (MVPA); socioeconomic status (SES)
All models were analyzed using ranked PA variables.
Multivariable model: adjusted for age, sex, SES, season
+ BMI% : Multivar. Model additionally adjusted for BMI%
Significant associations were bolded for emphasis (p≤0.05)
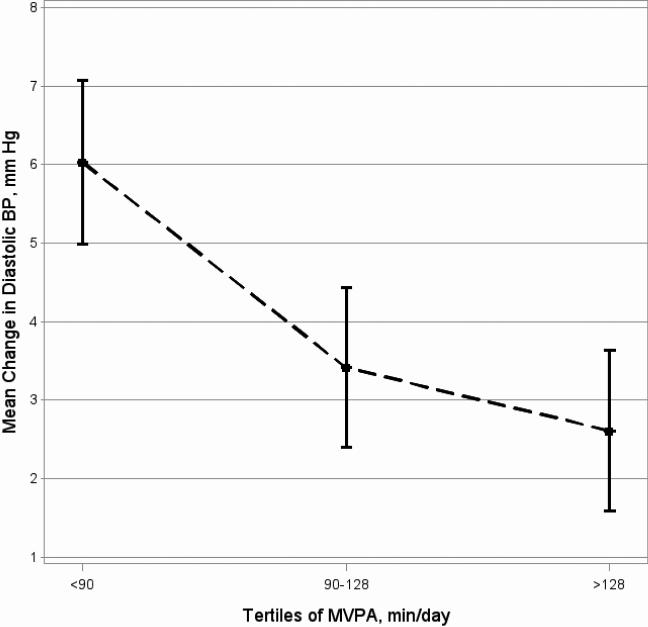
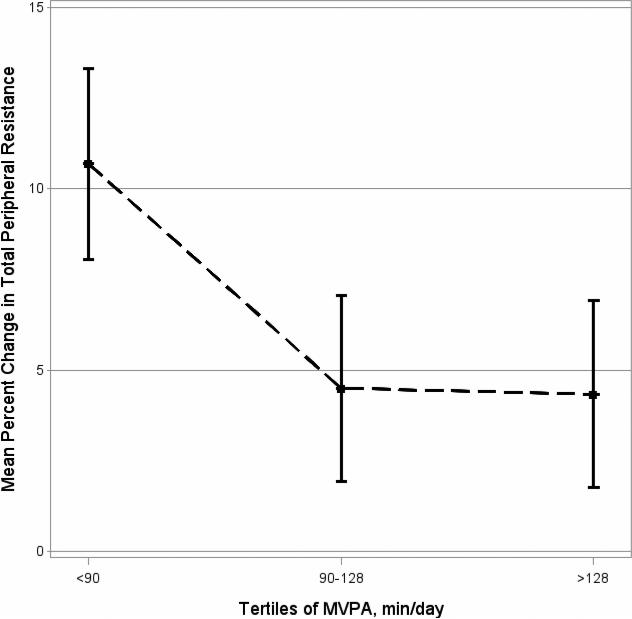
In regression models adjusted for covariates (age, sex, SES, and season), MVPA was associated with lower TPR response to mental stress (Table 3, beta=−0.009, p=0.05). After additional adjustment for BMI%, higher MVPA was also associated with lower DBP response to stress (beta=−0.009, p=0.05, Table 3 and Figure 1), but the relation of MVPA to TPR response was no longer statistically significant (beta=−0.008, p=0.08, Table 3 and Figure 2). Total PA and time spent in sedentary activities did not appear to have significant relation to stress-reactivity in any of the analysis presented. There were no significant interactions with BMI%.
Table 3.
Multivariable-adjusted regression models to assess the relations between physical activity measures and cardiovascular responses to stress
| Cardiovascular Stress Response Variables | Models | Sedentary Time (%/day) | Total Counts/min | MVPA (min/day) | |||
|---|---|---|---|---|---|---|---|
| estimate | p | estimate | p | estimate | p | ||
| zMean Δ Heart Rate | Multivar. Model | −0.0002 | 0.96 | 0.003 | 0.46 | 0.003 | 0.47 |
| + BMI% | −0.0003 | 0.94 | 0.003 | 0.55 | 0.003 | 0.59 | |
| zMean Δ Systolic BP | Multivar. Model | −0.005 | 0.31 | −0.003 | 0.49 | −0.004 | 0.35 |
| + BMI% | −0.005 | 0.31 | −0.004 | 0.44 | −0.005 | 0.29 | |
| zMean Δ Diastolic BP | Multivar. Model | −0.0006 | 0.90 | −0.007 | 0.11 | −0.008 | 0.08 |
| + BMI% | −0.0006 | 0.89 | −0.008 | 0.09 | −0.009 | 0.05 | |
| zMean %Δ Stroke Volume | Multivar. Model | 0.004 | 0.43 | 0.0004 | 0.93 | 0.004 | 0.34 |
| + BMI% | 0.003 | 0.45 | −0.001 | 0.83 | 0.002 | 0.58 | |
| zMean %Δ Cardiac Output | Multivar. Model | 0.005 | 0.29 | 0.00004 | 0.99 | 0.004 | 0.39 |
| + BMI% | 0.004 | 0.31 | −0.0012 | 0.78 | 0.002 | 0.64 | |
| zMean %Δ Total Peripheral Resistance | Multivar. Model | −0.001 | 0.83 | −0.007 | 0.15 | −0.009 | 0.05 |
| + BMI% | −0.0009 | 0.85 | −0.006 | 0.21 | −0.008 | 0.08 | |
Abbreviations: Body mass index CDC percentile (BMI%); blood pressure (BP); moderate-to-vigorous physical activity (MVPA); socioeconomic status (SES);
zMean Δ values indicate the change (from baseline to stress level), standardized within each task, and then averaged across tasks.
zMean %Δ values indicate the percent change (from baseline to stress level), standardized within each task, and then averaged across tasks.
All models were analyzed using ranked PA variables.
Multivariable (Multivar.) Model : adjusted for age, sex, SES, season
+ BMI %: Multivar. Model additionally adjusted for BMI (CDC percentile)
Significant associations were bolded for emphasis (p≤0.05)
Figure 1. Adjusted means of change in diastolic BP (during mental stress) by tertiles of MVPA (min/day)
Figure 1. Adjusted means of change in diastolic BP (during mental stress) by tertiles of MVPA (min/day).
Least Square Means (±SE) of mean change in diastolic BP to tertiles of moderate-to-vigorous physical activity (MVPA), adjusted for the following potential confounders: age, sex, socioeconomic status, season and body mass index percentile.
Figure 2. Adjusted means of percent change in total peripheral resistance (during mental stress) by tertiles of MVPA (min/day).
Least Square Means (±SE) of mean percent change in total peripheral resistance to tertiles of moderate-to-vigorous physical activity (MVPA), adjusted for the following potential confounders: age, sex, socioeconomic status, season and body mass index percentile.
Discussion
In this study, we demonstrated that children with higher levels of MVPA had lower vascular resistance (TPR) response and a lower DBP response to mental stress. Results displayed in our figures suggest that the effect of lower vascular reactivity may occur at PA levels higher than the current PA Guidelines for Americans (for children to achieve 60 minutes or more MVPA each day). To our knowledge, this is the first study that has reported an association between accelerometer-determined habitual PA and vascular reactivity to mental stress in 9-11 year old children.
During stress, elevated CO causes a transient increase in arterial blood volume, but simultaneous vasodilation counter-balances these effects to maintain basal peripheral resistance (i.e., TPR).8 In individuals who have an imbalance of these underlying hemodynamic responses, the resulting changes in TPR can define individuals as either vascular reactors (increased TPR) or cardiac reactors (resulting in decreased or maintained TPR). These responses appear to be reproducible in individuals.20 Cardiac reactivity to stress (increase in HR, SV, and CO) does not appear to be atherogenic and has not been associated with subclinical CVD or future CVD risk.21 Instead, higher cardiac reactivity (and maintenance or decrease in TPR) may be viewed as an appropriate fight-or-flight response to stress.22
An acute exercise bout may dampen perceived stress, resulting in lower cardiac reactivity to stress;23 however, there is no strong evidence linking HR (cardiac) reactivity directly to CVD risk. HR reactivity may play an indirect role within the compensatory interactions among the hemodynamic variables. The stress tasks performed in the current investigation traditionally elicit cardiac responses acutely,2 but with the prolonged stress protocol in this study the response has been shown to become more vascular, increasing TPR.7 We did not observe an association between PA and cardiac reactivity to stress in the current study, possibly because there was not a strong, sustained cardiac response to our stress protocol.
Although there was only a limited cardiac response to stress in our study, we did observe a sustained vascular response. Vascular reactivity occurs when an insufficient compensatory vascular compliance or vasodilation leads to increased TPR and increased BP (as CO increases in response to stress). This vascular reactivity is consistent with a maladaptive pattern of CV response to stress and is generally considered responsible for higher CVD risk in adults with higher stress reactivity.1 Vascular damage may begin in childhood.24, 25 Growing evidence also supports the conclusion that exaggerated vascular (pressor) response in children may lead to future pathology. For example, increased BP response in children has been associated with increased artery wall thickness26 and future BP levels.3, 27 Our investigation provides evidence for a relation of higher PA to lower vascular reactivity in children, which supports literature in adults suggesting that higher habitual PA promotes greater vascular compliance,9 contributing to lower vascular reactivity. These relationships have not been well-studied in children, but some supporting evidence exists,28, 29 suggesting that PA is important for vascular health in children. The acute effects of an exercise bout on subsequent vascular reactivity to stress has been more consistently documented in both adults30 and children.5, 6 Furthermore, differing research methodologies, demographics, and small sample sizes may have contributed to null findings between habitual PA and CV reactivity in other studies.5
Habitual PA was measured in children using accelerometers for three days in this study, which has been validated previously to be a sufficient length of wear time to provide reliable data.14 One major limitation to the use of accelerometers is the lack of standardization of accelerometer results collected using different placement sites (e.g. wrist versus waist), limiting our ability to make meaningful comparisons to other studies. Participants included in our study appeared to have accumulated more PA compared to other (more nationally-representative) samples. In this study, 89% of children achieved the Guidelines of 60 MVPA min/day, whereas only 42% of 6-11 year olds met the PA Guidelines in the National Health and Nutrition Examination Survey (NHANES, 2003-2004).32 Unfortunately, due to different placement of the accelerometer in this study (wrist) compared to previous NHANES and Canadian study examinations (waist), we are unable to make meaningful comparisons. The most recent NHANES exam (2013-2014) collected wrist-worn accelerometry data in children; therefore, data from our present investigation will be comparable to future NHANES publications. At this time, there have been limited validation studies using wrist-placed Actigraph accelerometers in children, and the intensity cutpoint used in the current investigation to define MVPA has only been validated by a single study.15 Eventual standardization of accelerometer measurements and comparisons across placement sites would be a significant advancement for this field of research.
Sedentary behavior is notoriously difficult to measure with wrist-worn monitors, especially in children. Many sedentary behaviors involve large amounts of hand/arm movement, such as when playing video/computer games, often recorded at levels over 1200 counts/min activity.16 Therefore, in the current investigation, some sedentary behavior may have been misclassified as light activity. The sedentary behavior as defined in the present investigation may represent the extreme sedentary behavior. However, the proportion of time spent sedentary in this study, 30%, was only somewhat lower than the 40% sedentary time reported in 6-11 year olds in the NHANES 2003-2004 exam (using different accelerometer placement and cutpoints).33 It is possible that our use of different cutpoints to define activity intensity would have altered results, however, sensitivity analysis we performed using a higher validated sedentary cutpoint (<1261 counts/min15) did not alter significance of any of the presented analysis (data not shown).
An additional measurement error was possibly introduced by the use of 1 minute epoch lengths in this study, which may have underestimated the amount of time spent in vigorous activities due to spontaneous nature of vigorous activity in children. However, this study sample appears to be a group of active children (at moderate-intensity levels), of whom most were meeting the PA Guidelines and performing little sedentary behavior. Therefore, results cannot be generalized to populations of very inactive children. We may actually expect an even stronger effect if this study was replicated in a group of children with a greater range of PA levels. We would typically expect diminishing returns with higher levels of PA in the relation of PA to vascular health in adults; but our results support a linear relationship between PA and vascular reactivity to stress in children. Replication will be necessary to confirm these results.
PA may interact with demographic or CV risk factors to differentially affect health outcomes in subgroups of the population. Previous studies have suggested that the relation between PA and vascular compliance in children may be driven by BMI.31 Our results do not support this hypothesis, however, as we reported a stronger relationship between MVPA and DBP reactivity after adjusting for BMI percentile in the maximally adjusted model, suggesting that the observed relationship between lower PA and higher vascular reactivity was not due to differences in BMI. Our results suggest that longer durations of moderate activity than currently recommended may be necessary for a vascular benefit. With larger studies we may be able to tease apart the influence of covariates and identify specific subgroups of children, possibly those at higher risk for CV disease later in life, that may benefit most from PA interventions.
Conclusion
his study supports the conclusion that higher levels of MVPA are associated with a lower vascular response to mental stress in children. Importantly, we also reported that total physical activity (total accelerometer counts) and sedentary time were not associated with the vascular response to mental stress. Vascular damage begins early in life and prevention of this damage, potentially through increased PA in childhood, may have implications for future development of CVD. Continued study of this physiological relationship will be important, including identification of the volume of PA at different intensity levels necessary to sustain health benefits.
Supplementary Material
Practical Implications.
Higher moderate to vigorous physical activity was associated with lower blood pressure response to mental stress in children.
Total physical activity (total accelerometer counts) and sedentary time were not associated with the cardiovascular response to mental stress.
These data provide confirmatory evidence that the promotion of moderate to vigorous physical activity recommendations for children are important for cardiovascular health.
Acknowledgements
This work was supported by National Institutes of Health grants R15-ES15619 (BBG), R01-ES023252 (BBG) and T32-HL07224 (NLS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- 1.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 2.Matthews KA, Salomon K, Brady SS, et al. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosom Med. 2003;65(3):410–5. doi: 10.1097/01.psy.0000057612.94797.5f. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22(4):479–85. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- 4.Huang CJ, Webb HE, Zourdos MC, et al. Cardiovascular reactivity, stress, and physical activity. Frontiers in physiology. 2013;4:314. doi: 10.3389/fphys.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roemmich JN, Lambiase M, Salvy SJ, et al. Protective effect of interval exercise on psychophysiological stress reactivity in children. Psychophysiology. 2009;46(4):852–61. doi: 10.1111/j.1469-8986.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 6.Lambiase MJ, Barry HM, Roemmich JN. Effect of a simulated active commute to school on cardiovascular stress reactivity. Medicine and science in sports and exercise. 2010;42(8):1609–16. doi: 10.1249/MSS.0b013e3181d0c77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ring C, Burns VE, Carroll D. Shifting hemodynamics of blood pressure control during prolonged mental stress. Psychophysiology. 2002;39(5):585–90. doi: 10.1017.S0048577202011320. [DOI] [PubMed] [Google Scholar]
- 8.James JE, Gregg ME, Matyas TA, et al. Stress reactivity and the Hemodynamic Profile- Compensation Deficit (HP-CD) Model of blood pressure regulation. Biological psychology. 2012;90(2):161–70. doi: 10.1016/j.biopsycho.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270–5. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 10.Gump BB, Mackenzie JA, Bendinskas K, et al. Low-level Pb and cardiovascular responses to acute stress in children: the role of cardiac autonomic regulation. Neurotoxicology and teratology. 2011;33(2):212–9. doi: 10.1016/j.ntt.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balslev D, Christensen LO, Lee JH, et al. Enhanced accuracy in novel mirror drawing after repetitive transcranial magnetic stimulation-induced proprioceptive deafferentation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(43):9698–702. doi: 10.1523/JNEUROSCI.1738-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherwood A, Allen MT, Fahrenberg J, et al. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 13.Llabre MM, Spitzer SB, Saab PG, et al. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28(6):701–11. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 14.Rich C, Geraci M, Griffiths L, et al. Quality control methods in accelerometer data processing: defining minimum wear time. PLoS One. 2013;8(6):e67206. doi: 10.1371/journal.pone.0067206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crouter SE, Flynn JI, Bassett DR., Jr. Estimating Physical Activity in Youth Using a Wrist Accelerometer. Medicine and science in sports and exercise. 2014 doi: 10.1249/MSS.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Lee JM, Peters BP, et al. Examination of different accelerometer cut-points for assessing sedentary behaviors in children. PLoS One. 2014;9(4):e90630. doi: 10.1371/journal.pone.0090630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Routen AC, Upton D, Edwards MG, et al. Discrepancies in accelerometer-measured physical activity in children due to cut-point non-equivalence and placement site. Journal of sports sciences. 2012;30(12):1303–10. doi: 10.1080/02640414.2012.709266. [DOI] [PubMed] [Google Scholar]
- 18.CDC. A SAS Program for the CDC Growth Charts. 2008 < http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm>.
- 19.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee report, 2008. US Department of Health and Human Services; Washington DC: 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kasprowicz AL, Manuck SB, Malkoff SB, et al. Individual differences in behaviorally evoked cardiovascular response: temporal stability and hemodynamic patterning. Psychophysiology. 1990;27(6):605–19. doi: 10.1111/j.1469-8986.1990.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 21.Low CA, Salomon K, Matthews KA. Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosom Med. 2009;71(9):927–31. doi: 10.1097/PSY.0b013e3181ba18ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EM, Hughes BM. Trait dominance is associated with vascular cardiovascular responses, and attenuated habituation, to social stress. Int J Psychophysiol. 2014;92(2):79–84. doi: 10.1016/j.ijpsycho.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Roemmich JN, Lambiase MJ, Balantekin KN, et al. Stress, behavior, and biology: risk factors for cardiovascular diseases in youth. Exercise and sport sciences reviews. 2014;42(4):145–52. doi: 10.1249/JES.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvisalo MJ, Putto-Laurila A, Jartti L, et al. Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes. 2002;51(2):493–8. doi: 10.2337/diabetes.51.2.493. [DOI] [PubMed] [Google Scholar]
- 25.Urbina EM, Kimball TR, Khoury PR, et al. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28(8):1692–8. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambiase MJ, Dorn J, Roemmich JN. Metabolic and cardiovascular adjustments during psychological stress and carotid artery intima-media thickness in youth. Physiology & behavior. 2012;105(5):1140–7. doi: 10.1016/j.physbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JK, Alpert BS, Walker SS. Ethnicity, pressor reactivity, and children's blood pressure. Five years of observations. Hypertension. 1992;20(3):327–32. doi: 10.1161/01.hyp.20.3.327. [DOI] [PubMed] [Google Scholar]
- 28.Palve KS, Pahkala K, Magnussen CG, et al. Association of physical activity in childhood and early adulthood with carotid artery elasticity 21 years later: the cardiovascular risk in Young Finns Study. J Am Heart Assoc. 2014;3(2):e000594. doi: 10.1161/JAHA.113.000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesa CC, Sbruzzi G, Ribeiro RA, et al. Physical activity and cardiovascular risk factors in children: meta-analysis of randomized clinical trials. Preventive medicine. 2014;69c:54–62. doi: 10.1016/j.ypmed.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Hamer M, Taylor A, Steptoe A. The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biological psychology. 2006;71(2):183–90. doi: 10.1016/j.biopsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Nettlefold L, McKay HA, Naylor PJ, et al. The relationship between objectively measured physical activity, sedentary time, and vascular health in children. Am J Hypertens. 2012;25(8):914–9. doi: 10.1038/ajh.2012.68. [DOI] [PubMed] [Google Scholar]
- 32.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 33.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.