Abstract
The single nucleotide polymorphism rs9804190 in the Ankyrin G (ANK3) gene has been reported in genome-wide association studies to be associated with bipolar disorder (BD). However, the neural system effects of rs9804190 in BD are not known. We investigated associations between rs9804190 with gray and white matter structure within a frontotemporal neural system implicated in BD. A total of 187 adolescent and adult European Americans were studied: a group homozygous for the C allele [52 individuals with BD and 56 controls] and a T-carrier group, carrying the high risk T allele (38 BD and 41 controls). Subjects participated in high-resolution structural magnetic resonance imaging and diffusion tensor imaging (DTI) scanning. Frontotemporal region of interest (ROI) and whole brain exploratory analyses were conducted. DTI ROI-based analysis revealed a significant diagnosis by genotype interaction within the uncinate fasciculus (p ≥ 0.05), with BD subjects carrying the T (risk) allele showing decreased fractional anisotropy compared to other subgroups, independent of age. Genotype effects were not observed in frontotemporal gray matter volume. These findings support effects of rs9804190 on frontotemporal white matter in adolescents and adults with BD and suggest a mechanism contributing to white matter pathology in BD.
Introduction
Converging evidence implicates abnormalities in a ventral frontotemporal system in the etiology of bipolar disorder (BD). Neuroimaging studies of BD have repeatedly shown structural abnormalities in gray matter (GM), particularly within the amygdala and orbitofrontal cortex (OFC)1, 2. Abnormalities within ventral frontotemporal GM structures, and in the connections between them, have also been reported in adolescents with BD3–8, suggesting these abnormalities may play a role in the neurodevelopment of BD. Recent research has increasingly implicated white matter (WM) connecting these regions in the etiology of BD. Diffusion tensor imaging (DTI) studies have shown decreased WM structural integrity within tracts carrying prefrontal cortex (PFC) connections, particularly within the uncinate fasciculus (UF)9–13, a tract that carries substantial amygdala-ventral PFC connections14, as well as the anterior limb of the internal capsule (ALIC)12, 15, 16, corpus callosum and cingulum17–23. There have been some conflicting findings in the UF24, 25. Genetic variation may be one mechanism leading to phenotypic heterogeneity within BD. Supporting this are studies of families multiply affected with BD21 and first degree relatives of individuals with BD13, 26, which implicates genetic liability in UF structural changes in BD.
Variation in the Ankyrin G (ANK3) gene, including an intronic single nucleotide polymorphism (SNP), rs9804190, has been reported in genome-wide association studies (GWAS) to be associated with BD27–32. The neural system effects associated with ANK3 variation in the context of BD are not yet understood. ANK3 may be associated with the development of structural brain abnormalities implicated in BD33 and may particularly influence WM. ANK3 is thought to participate in the stabilization and localization of ion channels and cell adhesion molecules to nodes of Ranvier and axonal initial segments34–36. ANK3 may also play a role in the developing cortex37, onset of myelination38, and neurogenesis in adults39. Additionally, lithium has been shown to alter ANK3 expression in the mouse brain40 and decrease hyperactivity observed in mice with altered ANK3 expression41, further supporting a role for ANK3 in BD pathology and as a potential therapeutic target.
Ventral frontotemporal dysfunction associated with rs9804190 in individuals with BD has been reported42, suggesting rs9804190 can influence frontotemporal systems in BD. In one study including 121 individuals with BD, no associations were detected with amygdala GM volume (GMV)43, suggesting rs9804190 may have its effects in other pathophysiological aspects of BD development. To our knowledge, there is only one neuroimaging report on effects of rs9804190 on structural integrity of WM. It was in 88 healthy individuals and an effect was not detected44.
In this study, we used DTI to investigate associations between rs9804190 and structural integrity of frontotemporal WM in BD and healthy control adolescents and adults. We hypothesized that rs9804190*T would be associated with decreased frontotemporal WM fractional anisotropy (FA), with the BD subgroup carrying the risk allele having the lowest UF FA, and with decreases independent of age, suggesting a role in the developmental pathophysiology of BD. Frontotemporal GMV was also assessed with structural magnetic resonance imaging (sMRI). WM FA and GMV in other brain regions were explored in whole brain analyses.
METHODS AND MATERIALS
Participants
Participants included 187 non-Hispanic European Americans: 90 with BD (76 BDI, 14 BDII), agemean ± standard deviation=27.1 ± 11.7 years, range=13–54 years, 68% female; 97 controls without personal history of an Axis I disorder or first-degree family member with a major mood or psychotic disorder, agemean=25.9 ± 11.0 years, range=12–55 years, 53% female. Psychiatric diagnoses and mood state at scanning were confirmed with the Structured Clinical Interview for DSM-IV Diagnosis45 for participants ≥18 years and the Kiddie-Schedule for Affective Disorders and Schizophrenia46 for participants <18 years. No subject had a history of a neurological illness, including head trauma with loss of consciousness for ≥5min, or major medical illness except treated hypothyroidism in BD subjects (N=7, 7.8%). No HC subject had substance (including alcohol) abuse/dependence history and no BD subject had a history within 6 months prior to scanning. On the scan day, urine toxicology screens were negative for substances of abuse for all subjects. After complete description of the study, written informed consent was obtained from subjects ≥18 years, and assent and parental permission from subjects <18 and their parent/guardian, in accordance with human investigation committees of the Yale School of Medicine and Department of Veterans Affairs.
DNA was extracted from whole blood. Genotypes at rs9804190 were determined by standard Taqman methods (Applied Biosystems; http://www.appliedbiosystems.com/). BD and control groups were each further subdivided into two groups: rs9804190 CC homozygotes (n=52 BD, 56 controls; agemean of whole CC group=25.6 ± 10.8 years; 58% female), and rs9804190 T-carriers (CT and TT genotypes) (n=38 BD, 41 controls; agemean of whole T-carrier group=27.6 ± 12.0 years, 62% female). Minor allele frequencies were 25% in the BD group and 26% in the control group. Genotype frequencies were consistent with Hardy-Weinberg equilibrium expectations. Table 1 provides demographic and clinical characteristics of the subgroups.
Table 1. Participant Characteristics.
Age was examined by a 2 [diagnostic group: control, bipolar disorder] x 2 [genotype: homozygous for the rs9804190 C allele (CC), carriers of the T (risk) allele (T-carriers)] ANOVA. All other factors were examined with Chi-square or Fisher’s exact tests.
| Healthy Control | Bipolar Disorder | p value | ||||
|---|---|---|---|---|---|---|
| CC group (n=56) | T-carrier Group (n=41) | CC Group (N=52) | T-carrier Group (n=38) | |||
|
|
||||||
| Demographics | Age (SD) | 23.9 (9.0) | 28.6 (12.9) | 27.5 (12.2) | 26.5(11.1) | 0.193 |
|
| ||||||
| Female (%) | 28 (50) | 23 (53) | 35 (67) | 26 (68) |
0.034 (HC vs. BD) 0.611 (CC vs. CT+TT) 0.911 (BD: CC vs. CT −TT) 0.552 (HC: CC vs. CT+TT) |
|
|
| ||||||
| Rapid Cycling (%)1 | -- | -- | 27 (52) | 12 (32) | 0.054 | |
|
| ||||||
| Lifetime Psychosis (%) | -- | -- | 18 (35) | 13 (34) | 0.998 | |
|
| ||||||
| Suicide Attempt (%) | -- | -- | 11 (22) | 12 (32) | 0.252 | |
|
| ||||||
| Mood State [Euthymic(%) / Depressed(%) / Elevated(%)] | -- | -- | 28 (54)/11 (21)/13 (25) | 20 (53)/10 (26)/8 (21) | 0.817 | |
|
|
||||||
| Medications | Medicated at scan (%) | -- | -- | 42 (81) | 30 (79) | 0.831 |
|
| ||||||
| Lithium (%) | -- | -- | 17 (33) | 6 (16) | 0.069 | |
|
| ||||||
| Anticonvulsant (%) | -- | -- | 22 (42) | 13 (34) | 0.436 | |
|
| ||||||
| Antipsychotic (%) | -- | -- | 26 (51) | 13 (35) | 0.140 | |
|
| ||||||
| Antidepressant (%) | -- | -- | 15 (29) | 14 (37) | 0.423 | |
|
| ||||||
| Stimulant (%) | -- | -- | 6 (12) | 8 (21) | 0.219 | |
|
| ||||||
| Benzodiazepine (%) | -- | -- | 8 (15) | 11 (29) | 0.119 | |
|
| ||||||
| Dopamine Agonist (%) | 2 (4) | 0 (0) | 0.507F | |||
|
| ||||||
| Opiate (%) | -- | -- | 1 (2) | 1 (3) | 1.000F | |
|
| ||||||
| Anticholinergic (%) | -- | -- | 1 (2) | 1 (3) | 1.000F | |
|
| ||||||
| Ketamine (%) | -- | -- | 1 (2) | 0 (0) | 1.000F | |
|
| ||||||
| Non-Benzodiazepine Hypnotic (%) | 1 (2) | 1 (3) | 1.000F | |||
|
| ||||||
| Adrenergic Agonist (%) | 0 (0) | 1 (3) | 0.422F | |||
|
| ||||||
| Levothyroxine (%)2 | -- | -- | 5 (10) | 2 (5) | 1.000F | |
|
|
||||||
| Lifetime Substance Use Disorders | Alcohol Abuse or Dependence (%) | -- | -- | 9 (17) | 9 (24) | 0.455 |
|
| ||||||
| Cannabis Abuse or Dependence (%) | -- | -- | 6 (12) | 8 (21) | 0.219 | |
|
| ||||||
| Polysubstance Abuse or Dependence (%) | -- | -- | 1 (2) | 1 (3) | 1.000F | |
|
| ||||||
| Stimulant Abuse or Dependence (%) | -- | -- | 2 (4) | 2 (5) | 1.000F | |
|
| ||||||
| Cocaine Abuse or Dependence (%) | -- | -- | 3 (6) | 2 (5) | 1.000F | |
|
| ||||||
| Sedative/Hypnotic Dependence (%) | -- | -- | 1 (2) | 1 (3) | 1.000F | |
|
| ||||||
| Opioid Dependence (%) | -- | -- | 1 (2) | 0 (0) | 1.000F | |
|
|
||||||
| Lifetime Other Psychiatric Disorders | Post Traumatic Stress (%) | -- | -- | 6 (12) | 2 (5) | 0.459F |
|
| ||||||
| Generalized Anxiety (%) | -- | -- | 4 (8) | 1 (3) | 0.392F | |
|
| ||||||
| Substance Induced Anxiety (%) | -- | -- | 1 (2) | 0 (0) | 1.000F | |
|
| ||||||
| Specific Phobia (%) | -- | -- | 4 (8) | 1 (3) | 0.397F | |
|
| ||||||
| Social Phobia (%) | -- | -- | 0 (0) | 3 (8) | 0.072F | |
|
| ||||||
| Panic Disorder (%) | -- | -- | 2 (4) | 3 (8) | 0.647F | |
|
| ||||||
| Panic Disorder with Agoraphobia (%) | -- | -- | 0 (0) | 3 (8) | 0.072F | |
|
| ||||||
| Obsessive Compulsive (%) | -- | -- | 3 (6) | 0 (0) | 0.260F | |
|
| ||||||
| Anorexia Nervosa (%) | -- | -- | 1 (2) | 2 (5) | 0.571F | |
|
| ||||||
| Bullmia (%) | -- | -- | 2 (4) | 1 (3) | 1.000F | |
|
| ||||||
| Binge Eating (%) | -- | -- | 1 (2) | 1 (3) | 1.000F | |
|
| ||||||
represents p-value calculated with Fisher’s exact test. Clinical factors assessed in both adolescents and adults are reported.
Rapid-cycling reported is lifetime history of rapid cycling
Individuals on levothyroxine had hypothyroidism.
MRI Acquisition
High-resolution sMRI and DTI data were acquired in the same scanning session for each subject with a 3-Tesla Siemens Trio MR scanner (Siemens, Erlangen, Germany). The sMRI sagittal images were acquired with a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequence with parameters: repetition time (TR)=1500ms, echo time (TE)=2.83ms, matrix=256x256, field of view (FOV)=256mm x 256mm 2, and 160 one-mm slices without gap and two averages. DTI data were acquired in alignment with the anterior commissure-posterior commissure plane with diffusion sensitizing gradients applied along 32 non-collinear directions with b-value=1000s/mm2, together with an acquisition without diffusion weighting (b-value=0) (TR=7400ms; TE=115ms; matrix=128×128; FOV=256mm × 256mm2 and 40 three-mm slices without gap).
SMRI Processing
Images were processed with the Statistical and Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm). The SPM segmentation function was implemented for bias correction, spatial normalization and segmentation of the original structural images in the same model. SPM tissue probability maps (voxel size 2x2x2mm3) guided normalization and segmentation. To ensure the overall amount of tissue in a class was not altered, a “modulation” step was used during spatial normalization. After segmentation, normalization, and modulation steps, GM images were smoothed with an 8mm full-width-at-half-maximum (FWHM) isotropic kernel. Right and left a priori amygdala and OFC region of interests (ROIs) were defined with the WFU PickAtlas Tool (http://www.fmri.wfubmc.edu/download.htm). For OFC ROIs, the inferior, middle and superior orbital ROIs were combined with the rectus.
DTI Processing
Images were processed with SPM8. Diffusion-weighted data were interpolated to 1.5-mm isotropic voxels and denoised by a three-dimensional isotropic Gaussian kernel with Sigma 2mm FWHM Gaussian kernel. Diffusion eigenvectors and corresponding eigenvalues (λ1, λ 2, λ 3) were acquired after diagonalization of the DTI data. FA was then calculated according to previously published methodology9, 47. Whole brain FA maps were normalized to the standard Montreal Neurological Institute (MNI) space using a tissue probability map of WM as a template. FA maps were resampled to 2x2x2mm3 during normalization. Each FA map was spatially smoothed with a 10mm FWHM isotropic Gaussian kernel. The a priori UF ROI (right and left calculated separately) was obtained from the Johns Hopkins University DTI-based WM atlas (http://cmrm.med.jhmi.edu/)48.
ROI-Based and Voxel-Based sMRI and DTI Analyses
For ROI-based sMRI analysis, a linear mixed model was used, with diagnostic group (controls, BD) and genotype (CC, T-carriers) as between-subjects factors, hemisphere (left, right) as a within-subjects factor, and amygdala and OFC GMV as dependent variables. Main effects and the interaction between diagnostic group and genotype were modeled. Age and sex were considered as covariates with both main effects and interactions with genotype modeled. An equivalent model was used for ROI-based DTI analysis to assess UF FA.
Voxel-based exploratory analyses were completed with SPM8. Diagnostic group by genotype factorial models in SPM, covarying age and sex, with GMV or FA values as dependent variables, were run to assess for non-hypothesized regions showing a significant diagnostic group by genotype interaction. To further explore localization of gene effects within BD, two-sample (CC vs. T-carriers) t tests in SPM were conducted, covarying age and sex, with GMV or FA values as dependent variables. Findings were considered significant at p<0.001, ≥50voxels. Parallel t tests were conducted in control subjects.
Analyses of Demographic and Clinical Factors
A 2x2 (diagnostic group, genotype) analysis of variance (ANOVA) was used to examine differences in age. Chi-square tests were used to examine sex between diagnostic groups, between genotypes, and between genotypes within each diagnostic group. Within the BD group, Chi-square or Fisher’s exact tests were used to examine if clinical factors differed by genotype. Additionally, 2x2 (clinical factor, genotype) ANOVAs were performed for ROIs showing a significant gene effect. Clinical factors were assessed only if both CC and T-carrier groups had N>5 subjects with history of the factor. Mean GMV or FA from clusters showing significant gene effects in exploratory analyses were calculated and associations with clinical factors also explored.
RESULTS
Participant Characteristics
There were no significant differences in age between diagnostic or genotype groups. Overall, the BD group had more females (61%) (χ2=4.5, df=1, p<0.05). However, no sex differences were observed between the CC group and T-carriers within BD (χ2=0.01, df=1, p=0.91) or control (χ2=0.35, df=1, p=0.55) groups. There were no significant differences in other clinical factors by genotype (Table 1).
ROI-Based Analyses
SMRI
For amygdala and OFC GMV, no main effect of genotype or significant diagnosis by genotype interactions were detected (p ≥ 0.1). A main effect of diagnosis was observed for OFC GMV (F(1,180)=4.44, p<0.05); OFC GMV was lower in the BD than the control group. No main or interactive effects of diagnostic group or genotype were observed for amygdala GMV. No significant genotype interactions with age, sex, or hemisphere were observed.
DTI
UF FA levels were lower in the T-carriers than CC homozygotes (F(1,181)=14.6, p<0.0005) and BD than control group (F(1,181)=14.7, p<0.0005). A significant diagnosis by genotype interaction was observed (F(1,181)=3.88, p=0.05); FA levels were lowest in BD T-carriers compared to BD CC homozygotes and both control genotype groups (p<0.0005, Figure 1). No other groups differed (p ≥ 0.15). No genotype interactions with age, sex, or hemisphere were observed.
Figure 1. Fractional Anisotropy Genotype by Diagnosis Effects in the Uncinate Fasciculus.
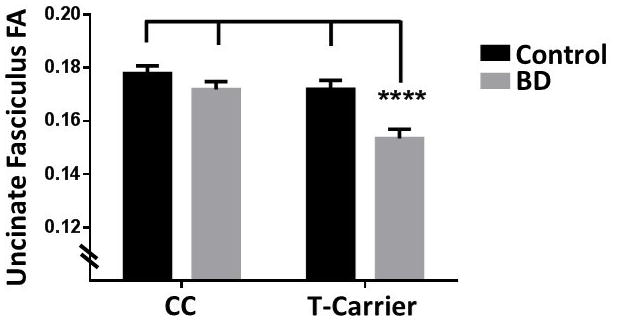
A significant diagnostic group [control, bipolar disorder (BD)] by genotype group [homozygous for the rs9804190 C allele (CC), heterozygous and homozygous carriers of the T (risk) allele (T-carriers)] interaction was observed for fractional anisotropy (FA) in the a priori uncinate fasciculus (UF) region of interest. T-carriers with BD showed lower UF FA compared to each of the other groups (****p<0.0005, between group comparisons). Control CC homozygotes N=56, BD CC homozygotes N=52, Control T-carriers N=41, BD T-carriers N=38.
Effects of Demographic and Clinical Factors
Clinical factors including whether subjects were medicated at the time of scan (yes/no, overall and medication subclasses) and history (yes/no) of rapid-cycling, lifetime psychosis, suicide attempt, mood states, alcohol abuse/dependence, or cannabis abuse/dependence, did not show significant effects on UF FA measures within BD. These clinical variables did not show significant genotype interactions.
Voxel-Based Analyses
SMRI
No significant diagnostic group by genotype interaction was observed. Compared to BD CC homozygotes, BD T-carriers showed significantly smaller GMV bilaterally in the thalamus (MNI coordinates: x=4mm, y=−10mm, z=−2mm, cluster=286voxels). Compared to control CC homozygotes, control T-carriers showed significantly smaller GMV in the right thalamus (x=16mm, y=−14mm, z=10mm, cluster=82voxels). No regions showing greater GMV in BD or control T-carrier groups were observed.
DTI
Clusters in non-hypothesized regions that showed significant diagnostic group by genotype interactions, with lower FA in BD T-carriers than all other subgroups, were in areas of right temporoparietal WM (z=32mm, y=−32mm, z=20mm, cluster=130voxels), left posterior dorsal cingulum (z=−10mm, y=−28mm, z=30mm, cluster=54voxels) and posterior corpus callosum/fornix (x=−6mm, y=−34mm, z=12mm, cluster=90voxels). Compared to BD CC homozygotes, BD T-carriers had lower FA in the left UF cluster extending into ventral frontal WM, including anterior cingulum and corpus callosum, and dorsal frontal WM, including forceps minor (x=−16mm, y=−2mm, z=−12mm, cluster=53voxels; x=−16mm, y=48mm, z=0mm, cluster=453voxels), and in the right UF cluster into ventral frontal WM, including anterior cingulum and corpus callosum, and ALIC (x=20mm, y=28mm, z=−4mm, cluster=747voxels). There was also a decrease within right superior temporal WM (x=44mm, y=−8mm, z=−12mm, cluster=103voxels).
Lower FA in BD T-carriers, compared to BD CC homozygotes, was observed in dorsomedial frontal WM, including areas of right dorsal anterior cingulum (x=20mm, y=4mm, z=42mm, cluster=522voxels) and left dorsal anterior cingulum extending into the corona radiata and external capsule (x=−16mm, y=12mm, z=34mm, cluster=862voxels), of left temporoparietal WM (x=−34mm, y=−42mm, z=24mm, cluster=247voxels), and of posterior dorsomedial WM, including areas of left dorsal cingulum (x=−12mm, y=−30mm, z=32mm, cluster=65voxels), left parietal/occipital WM (x=−18mm, y=−60mm, z=32mm, cluster=777voxels), and right parietal WM (x=24mm, y=−56mm, z=34mm, cluster=1117voxels) extending into temporoparietal WM. There were no areas showing greater FA in BD T-carriers and no areas of significant differences by genotype in controls.
Effects of Demographic and Clinical Factors
Individuals with lifetime history of rapid-cycling, compared to those without, showed higher FA in the right dorsal anterior cingulum (F(1,84)=3.97, p<0.05) and left parietal/occipital WM (F(1,84)=5.90, p<0.05). Individuals with a history of suicide attempt, compared to those without, showed lower FA in the left UF (F(1,80)=4.44, p<0.05) and higher FA in left parietal/occipital WM (F(1,80)=5.98, p<0.05). Individuals with a history of alcohol abuse/dependence showed higher FA in left temporoparietal WM (F(1,84)=5.55, p<0.05). No other clinical factors showed significant effects on clusters showing gene effects in DTI exploratory analysis. Clinical factors did not show significant effects on thalamic GMV. There were no significant clinical variable by genotype interactions.
DISCUSSION
Decreases in UF FA were observed among BD subjects carrying the ANK3 rs9804190 risk allele (T), compared to CC and T-carrier control subjects and BD CC homozygotes. Additionally, exploratory analyses showed decreases in BD T-carriers extending further into ventral and dorsal WM regions, including the ALIC, anterior and posterior cingulum, and corpus callosum. Findings were independent of age.
UF FA decreases in the BD T-carrier group suggest the risk allele is associated with decreased frontotemporal WM integrity in BD. Negative DTI findings in healthy individuals is consistent with a previous report44, suggesting rs9804190 may not be associated with WM deficits in typical development. Decreased UF FA in BD was observed in this and previous BD studies9–13. The UF has been suggested to play a role in emotional regulation, reward processing, and impulsive decision-making49, processes disrupted in BD. More research is needed to identify mechanisms by which WM in individuals with BD may be more vulnerable to effects of rs9804190. Effects of rs9804190 specifically among BD subjects may be due to an effect of rs9804190 on BD progression or an effect of higher genetic liability for BD among the BD subjects compared to control subjects. Changes in FA are suggested to reflect altered myelination, fiber density, axonal damage and/or axonal diameter50–53. A role for ANK3 has been implicated during the onset of myelination38. Postmortem studies in BD have reported reduced glia cells, including oligodendrocytes, and downregulation of genes related to myelination54–57, particularly in frontal brain regions. We suggest a possible multi-hit hypothesis in BD. Increased vulnerability to ANK3 variation in the neural circuitry implicated in BD in individuals with the disorder could result from interactions with effects of other myelination genes, oligodendrocyte function or other mechanisms contributing to neural circuitry pathology in the disorder. ANK3 variation may cross diagnostic boundaries58–60, although results are mixed28. Negative findings in HCs reported here suggest some gene specificity; future work including other psychiatric diagnoses is needed to investigate specificity of ANK3 pathophysiological mechanisms to BD.
DTI exploratory analyses suggest that, in BD, regions of the ALIC, anterior and posterior cingulum, and corpus callosum are also affected by rs9804190. Decreased FA within these regions has been observed in BD12, 15–23, with studies suggesting genetic variation may contribute to heterogeneity21, 61, 62. Within BD, diffusivity within cingulum and corpus callosum has been suggested to relate to differences in attention and executive functions63, diffusivity in ALIC to working memory63, altered set shifting and greater risk-taking13. Rs9804190 has been shown previously to be associated with externalizing behavior, including substance abuse64. Studies also support a role of the ankyrin gene family in nicotine dependence and alcohol use-related phenotypes65–69. We did not observe significant differences between BD CC and T-carrier groups with respect to histories of alcohol or cannabis abuse/dependence, and history of alcohol abuse/dependence did not contribute to the FA decreases observed. More research is needed to understand behavioral consequences of gene-associated brain changes in BD.
We did observe an effect of genotype on GMV in exploratory analyses: both control and BD T-carrier groups showed smaller thalamic GMV than control and BD CC homozygotes. Decreased thalamic volume has been observed in BD subjects and their unaffected family members70. The thalamus plays a key role in sensory processing and dysfunction can affect cognition and emotional behavior71. It is possible that ANK3 variation may contribute to thalamic effects in individuals with, and those at risk for, BD.
The developmental timing of genetic effects on brain structure cannot be determined from this study. No significant effects of age, and no interactions with age, were observed for UF FA. This suggests gene effects observed are present by adolescence in BD and could be associated with the development of BD pathology. Exploratory analyses controlled for age and therefore gene effects observed in dorsal WM and ALIC appear to also be independent of age. However, care must be taken with making developmental inferences from this study’s cross-sectional design and future longitudinal studies are needed.
Diagnostic group differences, independent of genotype, in frontotemporal GMV were observed. Supporting previous research1, 2, we found individuals with BD had smaller OFC GMV compared to controls. While not shown, adolescents/young adults (<22 years) with BD showed significantly smaller amygdala GMV, compared to adolescent/young adult controls. Reports of GMV differences in adolescents with BD have been relatively consistent in showing amygdala decreases, whereas reports in adults have varied1. The source of this heterogeneity is not clear, but could in part be related to age of onset, course of illness, and medication history72. No effects of genotype were observed on amygdala GMV in this study suggesting ANK3 may not contribute to this heterogeneity.
Other ANK3 SNPs have been associated with altered GM and WM structure. Rs1938526 has been reported to be associated with cortical thinning in inferior frontal, orbital frontal, and temporal gyrus in individuals with first episode psychosis73; however, in that study only one BD subject had the risk allele. Another ANK3 SNP, rs10994336, also supported by GWAS studies as conferring risk for BD32, has been reported to be associated with variation in ALIC FA and greater risk-taking in a study of healthy individuals without BD44. Studies in BD implicate rs10994336 in altered ventral anterior cingulate cortex responses during a working memory task42 and ventral PFC activation and connectivity during a facial affect-processing task74. These studies suggest other ANK3 SNPs may contribute to altered deficits within corticostriatal-limbic systems implicated in BD, including frontotemporal deficits. Previous studies suggest high linkage disequilibrium (LD) between rs10994336 and rs193852673, but low LD between rs10994336 and rs984190, indicating rs984190 may contribute independently to BD27, 75. More work is needed examining differences between, and effects of, multiple SNPs in BD and HCs.
Lower UF FA was associated with both risk allele and history of suicide attempt. Future work is needed to directly investigate this relationship to determine whether ANK3 variation increases vulnerability to suicide-related behavior. This study included individuals with BDI and BDII. Genetic effects remained the same within BD when covarying for these BD subtypes and there were no significant BD subtype by genotype interactions (data not shown) in ROIs, suggesting genetic effects may cross subtype classifications. No relationships between other clinical factors and gene effects on GMV or WM FA were observed: however, sample size may have limited power to detect effects. We did not observe significant effects of medication, which was further limited by lack of systematic study of medications. Regions identified in whole-brain analyses may include false positives as we did not account for multiple comparisons since analyses were exploratory. Future studies are needed to confirm these findings, including of larger samples, with more homogeneous clinical backgrounds, and systematic study of medication and other clinical features.
In summary, ANK3 rs9804190 variation was associated with decreased WM structural integrity within the UF, as well as in ventral and dorsal frontal, ALIC, cingulum and corpus callosum regions. Effects of rs9804190 on WM were observed in participants with BD, not controls, with results independent of age, suggesting there may be vulnerability to alterations in WM development in these regions in BD. Effects of the genotype were also detected in thalamus GMV within BD and control groups. More work, including preclinical, is needed to understand mechanisms underlying the emergence of genetic effects and associated behavioral consequences. Dissecting the roles of genetic variation on neural and behavioral pathophysiology of BD could identify new targets for detecting and treating BD that are more specific to an individual based on their genetics.
Figure 2. Fractional Anisotropy Decreases in Bipolar Disorder by ANK3 Genotype.
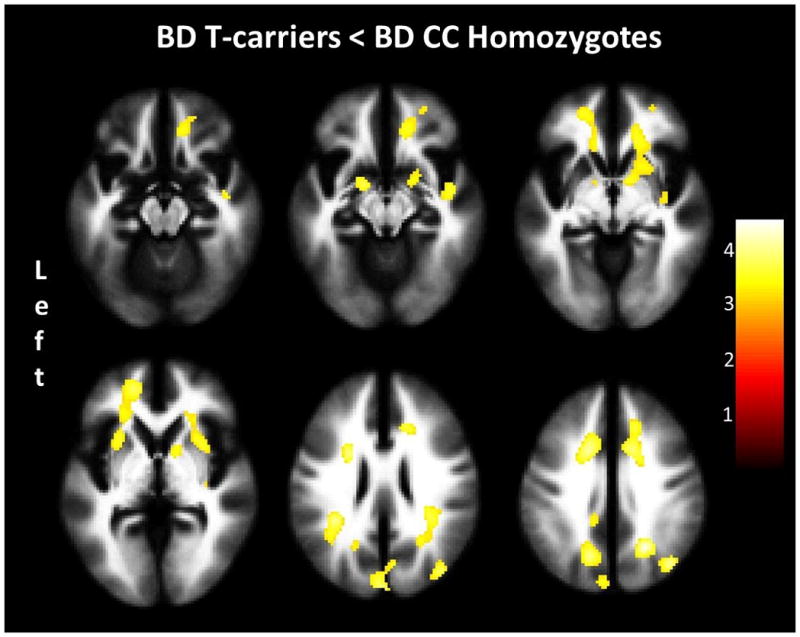
The images show the regions of fractional anisotropy decreases in the bipolar disorder (BD) rs9804190 T-carrier group [heterozygous and homozygous carriers of the T (risk) allele], compared to bipolar disorder CC homozygotes. No regions of fractional anisotropy increases were observed in the BD T-carrier group compared to BD CC homozygotes. Significance threshold is p<0.001, cluster >50 voxels. ‘Left’ on left of figure denotes left side of brain. The color bar represents the range of T values. BD CC homozygotes N=52, BD T-carriers N=38.
Acknowledgments
The authors were supported by research grants from the National Institute of Health (NIH) R01MH69747(HPB), R01MH070902(HPB), R01DA12690(JG), R01DA12849(JG), RC1MH088366(HPB), T32MH014276(ETCL), T32DA022975(ETCL), and K01MH086621(FW); CTSA UL1TR000142 from the NIH National Center for Advancing Translational Science, US Department of Veterans Affairs Research Enhancement Award Program(FW, HPB, and JG) and Career Development Award(KPJ); International Bipolar Foundation(HPB); Brain and Behavior Research Foundation(FW, and HPB); Attias Family Foundation(HPB); Klingenstein Third Generation Foundation(FW); Women’s Health Research at Yale(HPB); and The John and Hope Furth Endowment(HPB). We thank Susan Quatrano and Philip Markovich for their assistance with the research, Cheryl Lacadie, Karen Martin, Terry Hickey, and Hedy Sarofin for their technical expertise, Ann Marie Lacobelle for her genotyping assistance, Dr. Andrew McIntosh for his comments on an earlier version of this manuscript, and the research subjects for their participation.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Blond BN, Fredericks CA, Blumberg HP. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar disorders. 2012;14(4):340–355. doi: 10.1111/j.1399-5618.2012.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar disorders. 2012;14(4):313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(6):636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, et al. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain : a journal of neurology. 2011;134(Pt 7):2005–2012. doi: 10.1093/brain/awr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh MK, Chang KD, Chen MC, Kelley RG, Garrett A, Mitsunaga MM, et al. Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar disorders. 2012;14(6):585–596. doi: 10.1111/j.1399-5618.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mwangi B, Spiker D, Zunta-Soares GB, Soares JC. Prediction of pediatric bipolar disorder using neuroanatomical signatures of the amygdala. Bipolar disorders. 2014;16(7):713–721. doi: 10.1111/bdi.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Bobrow L, Liu J, Spencer L, Blumberg HP. Corticolimbic functional connectivity in adolescents with bipolar disorder. PloS one. 2012;7(11):e50177. doi: 10.1371/journal.pone.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biological psychiatry. 2009;66(5):516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. Journal of affective disorders. 2011;131(1–3):299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh AM, Munoz Maniega S, Lymer GK, McKirdy J, Hall J, Sussmann JE, et al. White matter tractography in bipolar disorder and schizophrenia. Biological psychiatry. 2008;64(12):1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Munoz Maniega S, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar disorders. 2009;11(1):11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 13.Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biological psychiatry. 2013;74(12):908–916. doi: 10.1016/j.biopsych.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex; a journal devoted to the study of the nervous system and behavior. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh AM, Job DE, Moorhead TW, Harrison LK, Lawrie SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biological psychiatry. 2005;58(3):254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Lu LH, Zhou XJ, Fitzgerald J, Keedy SK, Reilly JL, Passarotti AM, et al. Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar disorders. 2012;14(6):597–606. doi: 10.1111/j.1399-5618.2012.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biological psychiatry. 2008;64(8):730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Kale Edmiston E, Chen K, Tang Y, Ouyang X, Jiang Y, et al. A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry research. 2014;221(1):58–62. doi: 10.1016/j.pscychresns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Barysheva M, Jahanshad N, Foland-Ross L, Altshuler LL, Thompson PM. White matter microstructural abnormalities in bipolar disorder: A whole brain diffusion tensor imaging study. NeuroImage Clinical. 2013;2:558–568. doi: 10.1016/j.nicl.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Jackowski M, Kalmar JH, Chepenik LG, Tie K, Qiu M, et al. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. The British journal of psychiatry : the journal of mental science. 2008;193(2):126–129. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emsell L, Chaddock C, Forde N, Van Hecke W, Barker GJ, Leemans A, et al. White matter microstructural abnormalities in families multiply affected with bipolar I disorder: a diffusion tensor tractography study. Psychological medicine. 2014;44(10):2139–2150. doi: 10.1017/S0033291713002845. [DOI] [PubMed] [Google Scholar]
- 22.Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-Based Meta-Analytical Evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Sarrazin S, Poupon C, Linke J, Wessa M, Phillips M, Delavest M, et al. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA psychiatry. 2014;71(4):388–396. doi: 10.1001/jamapsychiatry.2013.4513. [DOI] [PubMed] [Google Scholar]
- 24.Versace A, Almeida JR, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Archives of general psychiatry. 2008;65(9):1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Molecular psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 26.Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TW, et al. White matter integrity in individuals at high genetic risk of bipolar disorder. Biological psychiatry. 2011;70(4):350–356. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J, et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Molecular psychiatry. 2009;14(5):487–491. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesli M, Koefoed P, Athanasiu L, Mattingsdal M, Gustafsson O, Agartz I, et al. Association analysis of ANK3 gene variants in nordic bipolar disorder and schizophrenia case-control samples. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156b(8):969–974. doi: 10.1002/ajmg.b.31244. [DOI] [PubMed] [Google Scholar]
- 29.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Molecular psychiatry. 2008;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nature communications. 2014;5:3339. doi: 10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- 31.Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nature genetics. 2008;40(9):1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leussis MP, Madison JM, Petryshen TL. Ankyrin 3: genetic association with bipolar disorder and relevance to disease pathophysiology. Biology of mood & anxiety disorders. 2012;2(1):18. doi: 10.1186/2045-5380-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kordeli E, Lambert S, Bennett V, Ankyrin G. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. The Journal of biological chemistry. 1995;270(5):2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 35.Komada M, Soriano P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. The Journal of cell biology. 2002;156(2):337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. The Journal of cell biology. 2008;183(4):635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durak O, de Anda FC, Singh KK, Leussis MP, Petryshen TL, Sklar P, et al. Ankyrin-G regulates neurogenesis and Wnt signaling by altering the subcellular localization of beta-catenin. Molecular psychiatry. 2015;20(3):388–397. doi: 10.1038/mp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ching W, Zanazzi G, Levinson SR, Salzer JL. Clustering of neuronal sodium channels requires contact with myelinating Schwann cells. Journal of neurocytology. 1999;28(4–5):295–301. doi: 10.1023/a:1007053411667. [DOI] [PubMed] [Google Scholar]
- 39.Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, et al. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71(1):61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenetics and genomics. 2007;17(8):605–617. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- 41.Leussis MP, Berry-Scott EM, Saito M, Jhuang H, de Haan G, Alkan O, et al. The ANK3 bipolar disorder gene regulates psychiatric-related behaviors that are modulated by lithium and stress. Biological psychiatry. 2013;73(7):683–690. doi: 10.1016/j.biopsych.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Delvecchio G, Dima D, Frangou S. The effect of ANK3 bipolar-risk polymorphisms on the working memory circuitry differs between loci and according to risk-status for bipolar disorder. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2015;168b(3):188–196. doi: 10.1002/ajmg.b.32294. [DOI] [PubMed] [Google Scholar]
- 43.Tesli M, Egeland R, Sonderby IE, Haukvik UK, Bettella F, Hibar DP, et al. No evidence for association between bipolar disorder risk gene variants and brain structural phenotypes. Journal of affective disorders. 2013;151(1):291–297. doi: 10.1016/j.jad.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Linke J, Witt SH, King AV, Nieratschker V, Poupon C, Gass A, et al. Genome-wide supported risk variant for bipolar disorder alters anatomical connectivity in the human brain. NeuroImage. 2012;59(4):3288–3296. doi: 10.1016/j.neuroimage.2011.10.083. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 46.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 48.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson IR, Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Developmental cognitive neuroscience. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. Journal of neuroscience research. 2006;83(3):392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- 51.Larvaron P, Boespflug-Tanguy O, Renou JP, Bonny JM. In vivo analysis of the post-natal development of normal mouse brain by DTI. NMR in biomedicine. 2007;20(4):413–421. doi: 10.1002/nbm.1082. [DOI] [PubMed] [Google Scholar]
- 52.Takagi T, Nakamura M, Yamada M, Hikishima K, Momoshima S, Fujiyoshi K, et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. NeuroImage. 2009;44(3):884–892. doi: 10.1016/j.neuroimage.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 53.Underwood CK, Kurniawan ND, Butler TJ, Cowin GJ, Wallace RH. Non-invasive diffusion tensor imaging detects white matter degeneration in the spinal cord of a mouse model of amyotrophic lateral sclerosis. NeuroImage. 2011;55(2):455–461. doi: 10.1016/j.neuroimage.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 54.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet (London, England) 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 55.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophrenia research. 2004;67(2–3):269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 57.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophrenia research. 2007;94(1–3):273–280. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Wirgenes KV, Tesli M, Inderhaug E, Athanasiu L, Agartz I, Melle I, et al. ANK3 gene expression in bipolar disorder and schizophrenia. The British journal of psychiatry : the journal of mental science. 2014;205(3):244–245. doi: 10.1192/bjp.bp.114.145433. [DOI] [PubMed] [Google Scholar]
- 59.Yuan A, Yi Z, Wang Q, Sun J, Li Z, Du Y, et al. ANK3 as a risk gene for schizophrenia: new data in Han Chinese and meta analysis. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2012;159b(8):997–1005. doi: 10.1002/ajmg.b.32112. [DOI] [PubMed] [Google Scholar]
- 60.Nie F, Wang X, Zhao P, Yang H, Zhu W, Zhao Y, et al. Genetic analysis of SNPs in CACNA1C and ANK3 gene with schizophrenia: A comprehensive meta-analysis. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2015;168(8):637–648. doi: 10.1002/ajmg.b.32348. [DOI] [PubMed] [Google Scholar]
- 61.Benedetti F, Bollettini I, Barberi I, Radaelli D, Poletti S, Locatelli C, et al. Lithium and GSK3-beta promoter gene variants influence white matter microstructure in bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(2):313–327. doi: 10.1038/npp.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benedetti F, Bollettini I, Poletti S, Locatelli C, Lorenzi C, Pirovano A, et al. White matter microstructure in bipolar disorder is influenced by the serotonin transporter gene polymorphism 5-HTTLPR. Genes, brain, and behavior. 2015;14(3):238–250. doi: 10.1111/gbb.12206. [DOI] [PubMed] [Google Scholar]
- 63.Poletti S, Bollettini I, Mazza E, Locatelli C, Radaelli D, Vai B, et al. Cognitive performances associate with measures of white matter integrity in bipolar disorder. Journal of affective disorders. 2015;174:342–352. doi: 10.1016/j.jad.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 64.Logue MW, Solovieff N, Leussis MP, Wolf EJ, Melista E, Baldwin C, et al. The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology. 2013;38(10):2249–2257. doi: 10.1016/j.psyneuen.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen XD, Xiong DH, Yang TL, Pei YF, Guo YF, Li J, et al. ANKRD7 and CYTL1 are novel risk genes for alcohol drinking behavior. Chinese medical journal. 2012;125(6):1127–1134. [PMC free article] [PubMed] [Google Scholar]
- 66.Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, et al. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcoholism, clinical and experimental research. 2007;31(10):1645–1653. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 67.Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Human molecular genetics. 2006;15(24):3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- 68.Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Human molecular genetics. 2007;16(23):2844–2853. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
- 69.Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Haplotypic variants in DRD2, ANKK1, TTC12, and NCAM1 are associated with comorbid alcohol and drug dependence. Alcoholism, clinical and experimental research. 2008;32(12):2117–2127. doi: 10.1111/j.1530-0277.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hajek T, Carrey N, Alda M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar disorders. 2005;7(5):393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell AS, Sherman SM. Advances in understanding mechanisms of thalamic relays in cognition and behavior. 2014;34(46):15340–15346. doi: 10.1523/JNEUROSCI.3289-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foland-Ross LC, Thompson PM, Sugar CA, Narr KL, Penfold C, Vasquez RE, et al. Three-dimensional mapping of hippocampal and amygdalar structure in euthymic adults with bipolar disorder not treated with lithium. Psychiatry research. 2013;211(3):195–201. doi: 10.1016/j.pscychresns.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cassidy C, Buchy L, Bodnar M, Dell’elce J, Choudhry Z, Fathalli F, et al. Association of a risk allele of ANK3 with cognitive performance and cortical thickness in patients with first-episode psychosis. Journal of psychiatry & neuroscience : JPN. 2014;39(1):31–39. doi: 10.1503/jpn.120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dima D, Jogia J, Collier D, Vassos E, Burdick KE, Frangou S. Independent modulation of engagement and connectivity of the facial network during affect processing by CACNA1C and ANK3 risk genes for bipolar disorder. JAMA psychiatry. 2013;70(12):1303–1311. doi: 10.1001/jamapsychiatry.2013.2099. [DOI] [PubMed] [Google Scholar]
- 75.Fiorentino A, O’Brien NL, Locke DP, McQuillin A, Jarram A, Anjorin A, et al. Analysis of ANK3 and CACNA1C variants identified in bipolar disorder whole genome sequence data. Bipolar disorders. 2014;16(6):583–591. doi: 10.1111/bdi.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]


