Abstract
Background
Parents of children undergoing hematopoietic stem cell transplantation (HSCT) are at risk for psychological distress. This distress may result in aberrant immune, inflammatory or endocrine effects. These physiologic outcomes have not been reported previously.
Main Objective
To examine the feasibility of longitudinal testing of psychophysiological parameters of stress in parents of children undergoing HSCT.
Methods
This pilot study was conducted at a large children's hospital in the Midwest, and included parents of children who received autologous or allogeneic HSCT. Time points included: prior to start of HSCT conditioning, day +30, +60, and +100. Outcome variables included parent perceived stress, lymphocyte subsets, c-reactive protein (CRP), pro-inflammatory cytokines, salivary cortisol, and salivary amylase. Effect sizes were calculated for each outcome.
Results
Twelve parent-child dyads were enrolled (10 mothers, 2 fathers). Missing data was minimal. Parent perceived stress significantly increased from pre-HSCT through day +100, and parent CD3+ T lymphocyte counts decreased from pre-HSCT through day +100. No significant effects were observed for salivary studies, CRP, or pro-inflammatory cytokines. Effect sizes ranged from 1.23 (perceived stress) to 0.07 (CRP).
Conclusion
Results of this study suggest that it is feasible longitudinally measure parent psychophysiologic outcomes in the pediatric HSCT setting. Additionally, parent perceived stress increased linearly from start of conditioning through day +100, while parent T lymphocytes decreased concurrently.
Implications for practice
Routine psychological and physical health screening of parents of children undergoing HSCT are needed. Multidisciplinary psychosocial support services should be offered to parents at regular intervals during their child's HSCT.
Keywords: Parent, Distress, Hematopoietic Stem Cell Transplantation, Pediatrics, Quantitative Nursing Research
Introduction
Hematopoietic stem cell transplantation (HSCT) is an intense treatment modality offered with curative intent to children with severe oncologic, immunologic, metabolic or hematologic conditions. Although pediatric HSCT outcomes have improved over time, there remains a significant risk of disease relapse or complications related to the transplant procedure.1 Parents of children undergoing HSCT are at risk for increased psychological distress due to the high rate of transplant-related morbidity and mortality, caregiver burden, and potential adverse socioeconomic consequences.2-4 Substantial levels of depression, anxiety, and post-traumatic stress have also been reported in this parent population.5-8
Although psychological parameters of parent distress have been studied in the pediatric stem cell transplant setting, associated physiologic outcomes have not been measured despite reports of potentially harmful physiologic outcomes in parents caring for children with other medical or behavioral conditions. Altered neuroimmune and endocrine function have been observed in parents caring for children with cancer,9,10 while hypothalamic-pituitary-adrenal (HPA) axis dysfunction has been reported in mothers caring for children with cerebral palsy and parents of children with autism and attention deficit disorder.11,12
Little is known about alterations in physiological markers of stress in parents in the pediatric HSCT setting over time. Prior to conducting studies of candidate markers, such as parameters of immune, inflammatory and neuroendocrine functioning, data are needed regarding the feasibility of longitudinal physiological measurements in these parents. The purpose of this study was to describe alternations in parent perceived stress and physiological parameters of parent perceived stress over time in parents of children undergoing HSCT.
Objective #1: To explore the feasibility of collecting longitudinal questionnaire and physiologic stress response data in parents of children undergoing HSCT.
Objective #2: To describe changes in parent perceived stress and physiologic stress responses over the first 100 days of the child's HSCT.
Objective #3: To calculate effect sizes for relationships between parent perceived stress and physiologic stress response parameters.
Background
Hematopoietic stem cell transplantation is a stressful event for children and parents. Uncertainty about their child's outcome and risk of death are significant stressors for parents of children undergoing HSCT. Time constraints and increased financial demands are placed on these parents as well.13 Parents often provide intense, around-the-clock care during the acute and recovery phases of their child's HSCT.14 Parents are prone to lack of sleep and neglecting their own health care needs because their focus has shifted to the needs of their acutely ill child. Due to their child's lengthy hospital stays, parents often spend less time participating in restorative activities such as time with family and friends, rest, and exercise.15
This type of intense psychological stress is a stimulus that directly acts on the HPA axis and sympathetic nervous system.16 Activation of the HPA axis results in production and release of corticotropin releasing hormone from the hypothalamus, which in turn, causes the anterior pituitary to release adrenocorticotropic hormone. Glucocorticoids are then released into the systemic circulation by the adrenal cortex. Sympathetic nervous system (SNS) activation stimulates secretion of catecholamines (norepinephrine and epinephrine).
In certain doses, glucocorticoids and catecholamines can initiate an early inflammatory response causing increased production of cytokines and acute phase reactants.17 Glucocorticoid resistance, however, may result from chronic activation of the HPA and SNS axes. Potentially caused by the down-regulation of glucocorticoid receptors on the cell surface of immune cells, glucocorticoid resistance is a condition in which immune system cells become hypo-responsive to the effects of glucocorticoids.9 Glucocorticoid resistance can thereby create a pro-inflammatory state that may predispose individuals to inflammatory conditions such as coronary artery disease, type II diabetes and Alzheimer's disease.18,19
In support of these hypotheses, spousal caregivers of patients with Alzheimer's were 2.2 times more likely to demonstrate an ultrasound-confirmed carotid plaque than non-caregiver controls [95% confidence interval (CI), 1.01-4.73. p = .048] when controlling for other risk factors for atherosclerosis.20 In a prospective study of US nurses, caregiving was associated with an increased risk of coronary artery disease (RR 1.82, 95% confidence interval, 1.08-3.05) when controlling for age and relevant risk factors.21 It is reasonable to extrapolate these results to parents of children undergoing HSCT due to the intensely stressful nature of the transplant process and burden of care placed on parents. Taken together, these findings suggest that parent caregivers are also at risk for adverse health outcomes, and potentially those outcomes mediated by psychoneuroimmunology and inflammatory pathways.
Psychoneuroimmunology studies have demonstrated that HPA axis dysfunction, increased production of inflammatory mediators, and immune dsyregulation may result from psychological stress.22 Psychological stress has been associated with cytokine dysregulation and increased in vivo levels of certain pro-inflammatory cytokines, namely IL-1 and IL-6.9,23-26 Furthermore, parents of children with cancer were found to have less dexamethasone suppression of IL-6 than parents of healthy children.9 In addition, studies of parent and other caregivers have shown elevations in c-reactive protein (a non-specific inflammatory marker) associated with psychological stress resulting from short- and long-term caregiving.25,27,28
Impaired cell-mediated immunity, and a shift of lymphocyte populations have been demonstrated in caregivers of adults with dementia.29-31 Benaroya-Milshtein et al. (2014) found aberrations in adaptive immunity in depressed parents of children with cancer. Cytotoxic T cells (CD8) were higher in depressed versus non-depressed parents, while helper-T cells (CD4) were decreased in depressed parents. In terms of innate immunity, parents of children newly diagnosed with cancer were found to have similar NK cell number and activity when compared to an immune reference group comprised of healthy adults.32 Benaroya-Milshtein et al. (2014) confirmed this finding; no differences in NK cell number were observed in depressed compared to non-depressed parents of children with cancer.
Psychological stress associated with parenting a sick child has also been associated with various neuroendocrine sequelae. Salivary cortisol, a proxy measure of HPA axis activity, was decreased in response to psychosocial stressors among parents of children with autism spectrum disorders, and those caregivers reported increased numbers of health complaints.33 Caregivers were found to have lower cortisol levels at all time-points. Miller, Cohen & Ritchey (2002) observed flatter diurnal cortisol slopes in parents of children with cancer. Benaroya-Milshtein et al. (2014) also studied parents of children with cancer and found no differences in serum cortisol between depressed and non-depressed parents. Salivary amylase is produced locally by the salivary glands, and has been associated with stress-induced activity of the SNS,34 however literature focused on the salivary amylase response to stress in parents caring for ill children is limited.
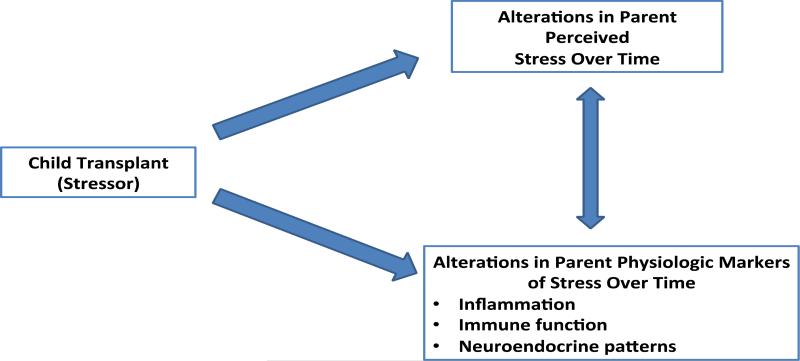
Figure 1 displays the conceptual model used to guide this study. This model depicts proposed links between the child transplant (the stressor) and the resulting psychological and physiologic consequences. It integrates interrelated biological and psychological constructs to describe health outcomes. The bidirectional arrow indicates an interdependent relationship between parent perceived stress and parent physiologic responses to stress. This conceptual model was adapted from a neurogenic inflammation model that explicates the complex, bidirectional interaction between the central nervous system and the immune system.17
Figure 1.
Conceptual Model
In summary, psychological stress can have adverse immune, inflammatory and endocrine effects, and may lead to the development of chronic inflammatory conditions. However, physiological consequences of stress have not been reported in parents of children undergoing HSCT.
Methods
Setting
The study was conducted between July 2013 and July 2014 at a large children's hospital in the Midwest. Approximately 40 to 50 stem cell transplants are performed annually at the center.
Sample
Parents were identified from a weekly referral list generated by the HSCT team and recruited prior to their child's initial HSCT admission. Eligible participants included parents of male or female children ages one month to 25 years with any disease requiring an autologous or allogeneic HSCT, and a pre-HSCT Karnofsky or Lansky score of ≥ 70%. Lansky and Karnofsky scores are performance scores that take into account impact of disease and disease treatment on daily function and activity status. Lansky scores are used for children less than 16 years,35 and Karnofsky scores are used for individuals aged 16 years and over.36 Performance status scoring was incorporated into the inclusion criteria to minimize a potential confounding factor of caring for a seriously ill child pre-HSCT.
Parents were excluded from the study if their child had a prior history of stem cell or solid organ transplantation, or if parents had a history of serious psychiatric or medical illness. A parent control group was not included in this feasibility study.
Measures
All parent measures including blood and salivary studies were completed at baseline, then monthly for three months from day 0 through day 100 after the child's HSCT. Parent blood was drawn by HSCT nurses. This venipuncture took place in the pediatric HSCT clinic or on the inpatient HSCT unit, and coincided with routine clinical care for the children so as not to add increased appointment burden for parents. Written and verbal instruction regarding saliva collection was provided to parents at baseline. Parents self-collected saliva within one to two days prior to their scheduled venipuncture, and delivered their saliva samples to the HSCT nurses at the time of venipuncture.
Pre-HSCT/Baseline Measures
Pre-HSCT Lansky or Karnofsky scores were extracted from the child's electronic medical record. Parents completed a demographic and health history questionnaire at study entry. This questionnaire was specifically created for this study, and addressed parent age, education, household income, employment and marital status, number of children, lifestyle factors such as exercise, alcohol, drug and tobacco use, medication use, pregnancy status, and health history.
Perceived Stress
The 14-item Perceived Stress Scale37 was used to measure psychological stress at each time point over the study duration. This 5-point Likert scale evaluates the extent to which one's life situations are perceived to be overwhelming, unpredictable, and uncontrollable over the past month. Participants rate responses from 0 (never) to 4 (very often). Higher mean scores indicate greater perceived stress. Positively worded items are reverse-scored. Composite scores are then obtained by summing across all 14 items. Total possible scores range from 0-56. Cronbach's alpha has ranged from 0.84 to 0.86 in previous studies.37-39 Concurrent validity has been demonstrated by high correlations with depressive and physical symptoms, as well as perceived impact of life events (all p values < .01).37,38
Inflammation and Immune Function
C-reactive protein is a non-specific marker of inflammation with normal levels typically below 1.0mg/ml.40 Protein concentration was measured using a latex immunoturbidometry assay.
Lymphocytes are normal components of the immune system. Total T cells (CD3+), T-helper cells (CD4+), cytotoxic T-cells (CD8+), B cells (CD19+), and natural killer cells (CD16+/56+) cells were measured using a flow cytometric assay. Normal absolute counts in adults are as follows: CD3+ (983-3572 cells/μl), CD4+ (491-2000 cells/μ), CD8+ (314-2087 cells/μ), CD19+ (64-800 cells/μ), CD16/56+ (27-693 cells/μ).41 The assay used Becton Dickinson Trucount™ beads and FACSCanto software to determine absolute counts of the lymphocyte subsets.
Cytokines are important in cell signaling and produced by a wide variety of lymphocytes, although diagnostic reference ranges for these assays have yet to be established and validated in adults.42 Plasma concentrations of pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-8, and TNF-α, were measured using a sandwich immunoassay (V-Plex MSD Muli-spot system, Meso Scale Discovery, Rockville, MD) according to manufacturer's instructions. Pro-inflammatory cytokine samples were run in triplicate.
Neuroendocrine Patterns
Basal HPA axis and SNS activity were measured by assessment of salivary cortisol and alpha-amylase, respectively. Under normal circumstances, salivary cortisol peaks at waking then steadily declines throughout the day, while salivary amylase sharply drops at waking then steadily increases throughout the day.34,43
The saliva collection procedure was explained to parents in detail and supplemented with a handout that contained collection instructions and space to record saliva collection times. These handouts were returned with the collected saliva, and reviewed in order to assess parent adherence to the collection protocol. Participants were instructed to chew on a cotton, dental roll (Salivette, Sarstedt, Nümbrecht, Germany) for 1-2 minutes at each time point throughout a given day. The saturated cotton roll was returned to the tube and placed in the participant's refrigerator for up to 24 hours. The following time points were included: at waking, 30 minutes after waking, 1200 and 2200. These time points were selected to establish a diurnal pattern of salivary cortisol and amylase secretion.
Saliva samples that were returned were centrifuged to yield clear supernatants. These samples were subsequently frozen at −20°C until the assays were performed. Liquid chromatography/tandem mass spectrometry (LC-MS/MS) was used to quantify salivary cortisol. Cortisol samples were run in singlicate. Salivary alpha-amylase was quantified using an enzyme kinetic assay previously described.34 Salivary amylase samples were run in triplicate to ensure accuracy.
Area under the curve (AUC) was calculated for salivary cortisol and alpha-amylase using the trapezoidal method.44 In addition, cortisol-awakening response (CAR) was calculated by subtracting the cortisol at waking from the 30-minute post-waking value.
Procedures
The study was approved by the Institutional Review Board, and written informed consent was obtained from all participants prior to study enrollment. Eligible parents were approached for study enrollment in consecutive order as their children were admitted for conditioning. Parents were enrolled in the study for a three-month time period.
Study observations occurred at baseline (± 5 days of the start of the child's HSCT conditioning), at day +30, day +60, and day +100. Parents completed a baseline demographic and health history questionnaire designed specifically for this study. At each of the four time points, parents completed the perceived stress questionnaire, underwent venipuncture for a single blood sample, and provided four saliva samples throughout the day.
Statistical Analyses
Data analyses were performed using SPSS Statistical Analysis software, version 21. Descriptive statistics were calculated for parent demographics (age, gender, ethnicity, education, number of other children, marital status and baseline health complaints) and child characteristics (age, length of stay, diagnosis, transplant type, incidence of GVHD, and mortality), perceived stress, and physiologic outcome variables. Repeated measures analysis of variance (ANOVA) was performed to assess longitudinal trends of outcome variables. Probability statements were adjusted using Greenhouse-Geisser corrections. Effect sizes using Cohen's d were calculated for each variable.45
Results
Sample Description
Thirteen parents were approached for enrollment in consecutive order. One parent declined enrollment, citing extenuating personal circumstances. The final sample consisted of 12 parents (ten mothers and two fathers) of children undergoing HSCT. The attrition rate was 8%. One mother withdrew from the study after the baseline time point had been completed due to the death of her child.
Parent characteristics are shown in Table 1. The mean age of the parents was 33.1 years [standard deviation (SD) 7.3]. The majority of parents (58%) were Caucasian, and had other children in addition to the child undergoing HSCT (83%). All parents graduated from high school and the majority were college graduates or had some college education (83%). Fifty-eight percent of parents were married. Parents self-reported the following health complaints at the time of study enrollment (prior to their child's HSCT conditioning): migraines (58%), hypertension (42%), depression and/or anxiety (42%).
Table 1.
Parent Demographics (N=12)
| Mean Age (yrs) | 33.1 (7.3) | |
| N | % | |
| Gender | ||
| Male | 2 | 17 |
| Female | 10 | 83 |
| Ethnicity | ||
| Caucasian | 7 | 58 |
| African American | 3 | 25 |
| Other | 2 | 17 |
| Education | ||
| High School Graduate | 2 | 17 |
| ≥ Some College | 10 | 83 |
| No. Other Children | ||
| 0 | 2 | 17 |
| 1 | 7 | 58 |
| ≥2 | 3 | 25 |
| Marital Status | ||
| Single or Divorced | 5 | 42 |
| Married | 7 | 58 |
| Baseline Health Complaints | ||
| Hypertension | 5 | 42 |
| Migraines | 7 | 58 |
| Depression and/or Anxiety | 5 | 42 |
Child characteristics are shown in Table 2. Child mean age was 5.2 years (SD 4.6, range 6 months – 16 years). The majority of children were transplanted for a malignant condition (67%). Eight children received an allogeneic HSCT (67%), and four underwent an autologous HSCT (33%). Fifty-percent of children who underwent allogeneic HSCT met criteria for grade 2 to 4 acute graft-versus-host disease (GVHD). The mean length of stay for their initial transplant admission was 33.6 days (SD 14.1). One child died of causes related to disease progression on day −3 of her transplant conditioning. Pre-transplant observations were obtained from this parent, however, no follow-up parent data were obtained after the child's death.
Table 2.
Child Characteristics (N=12)
| Mean Age (yrs) | 5.2 (4.6) | |
| Mean LOS (days) | 33.6 (14.1) | |
| N | % | |
| Transplant Type | ||
| Allogeneic | 8 | 67 |
| Autologous | 4 | 33 |
| Diagnosis | ||
| Malignant | 8 | 67 |
| Non-Malignant | 4 | 33 |
|
Acute GVHD grade 2-4 Allogeneic patients only |
4 | 50 |
| Mortality | 1 | 8 |
Feasibility and Missing Data
Feasibility of obtaining longitudinal data from parents was supported by the low level of missing data and high level of parent adherence to the saliva collection protocol. All parents enrolled in the study (n=12) completed the baseline measurements including questionnaire completion, venipuncture and saliva collection. Prospectively, 92% (n=11) completed all of the study measures at each time point (day +30, +60, and +100). There were no missing parent blood samples during the study likely because venipuncture coincided with child clinic appointments or inpatient hospitalizations. Parents adhered to the salivary collection protocol based on review of the parent-completed, saliva collection handouts. Specifically, parent saliva was collected at the requested times without deviation from the collection protocol.
Aside from the parent who was not evaluated following the child's death, there was minimal missing data. There were no missing perceived stress scale, cytokine, flow cytometry or CRP data. Out of 192 total data points for the saliva collection, 2.8% were missing, excluding the parent who was not evaluable after the pre-transplant time point.
Perceived Stress and Lymphocyte Subsets
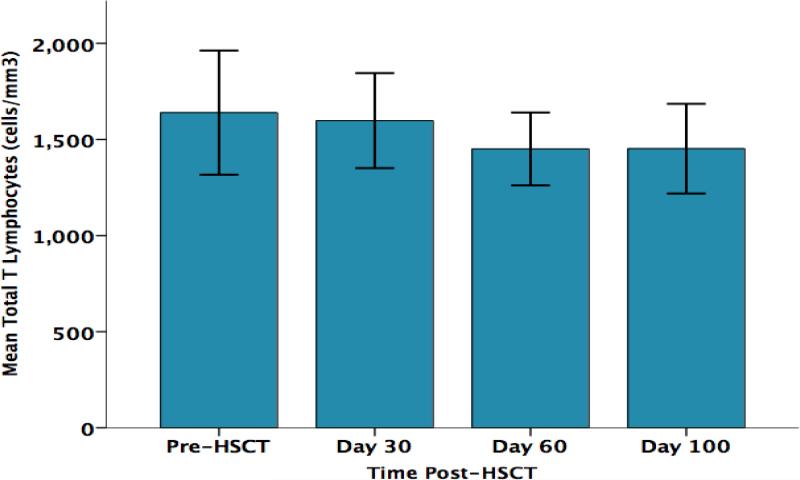
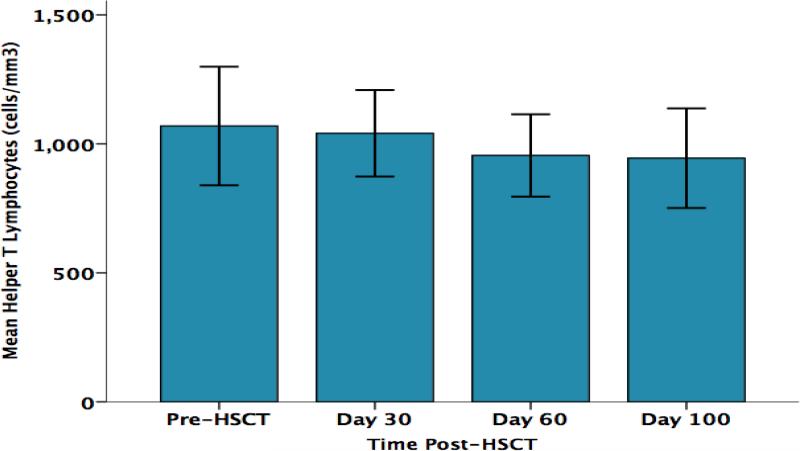
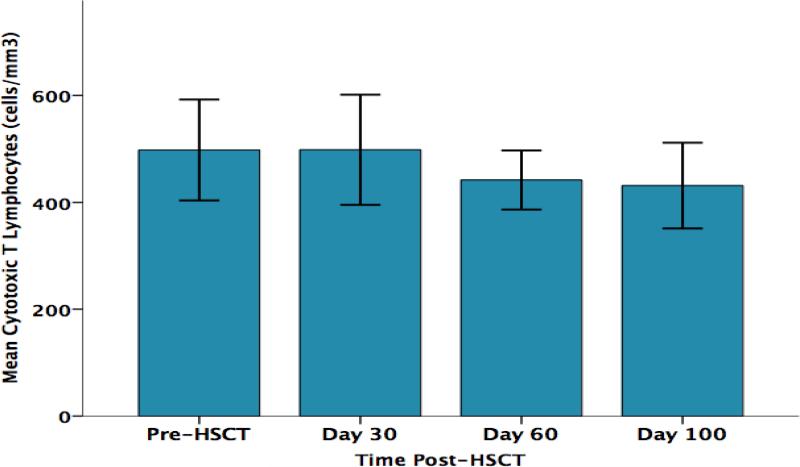
As shown in Figure 2, parent perceived stress significantly increased from pre-HSCT through day +100 F (3, 30) = 4.7, p = .015. As shown in Figure 3, parent CD3+ T lymphocyte counts (total T lymphocytes) progressively decreased from pre-HSCT through day +100 F (3,30) = 3.67, p= .031. Significant decreases over the same time points were also found for the CD3+/CD4+ subset (T helper cells) F (3,30) = 3.23, p= .047 (Figure 4) and for the CD3+/CD8+ subset (cytotoxic T cells) F (3,30) = 3.53, p= .049 (Figure 5). No significant changes over time were observed for B lymphocytes (CD19+), natural killer cells (CD16+/56+), or activated T cells (CD3+/DR+ cells) (data not shown). The results of parent lymphocyte subsets analyzed over time are presented in Table 3.
Figure 2.
Parents’ Perceived Stress Over Time
Figure 3.
Parents’ Total CD3+ T Lymphocyte Counts (cells/mm3) Over Time
Figure 4.
Parents’ Absolute CD3+/CD4+ T Lymphocyte Counts (cells/mm3) Over Time
Figure 5.
Parents’ Absolute CD3+/CD8+ T Lymphocyte Counts (cells/mm3) Over Time
Table 3.
Parents’ Lymphocyte Subsets Over Time, Absolute Counts (cells/mm3)
| Pre-HSCT | Day +30 | Day +60 | Day +100 | |
|---|---|---|---|---|
| Lymphocyte Subset | Mean/SD | Mean/SD | Mean/SD | Mean/SD |
| CD3+ (T cells) | 1640/508 | 1598/368 | 1451/282 | 1452/347 |
| CD3+CD4+ (Helper T cells) | 1069/362 | 1041/250 | 955/238 | 945/287 |
| CD3+CD8+ (Cytotoxic T cells) | 498/149 | 498/154 | 442/82 | 431/119 |
| CD19+ (B cells) | 338/180 | 313/106 | 296/95 | 316/119 |
| CD16+56+ (NK cells) | 154/75 | 174/82 | 160/62 | 177/72 |
| CD3+DR+ (Activated T cells) | 7/3 | 7/4 | 8/5 | 7/5 |
Pro-Inflammatory Cytokines
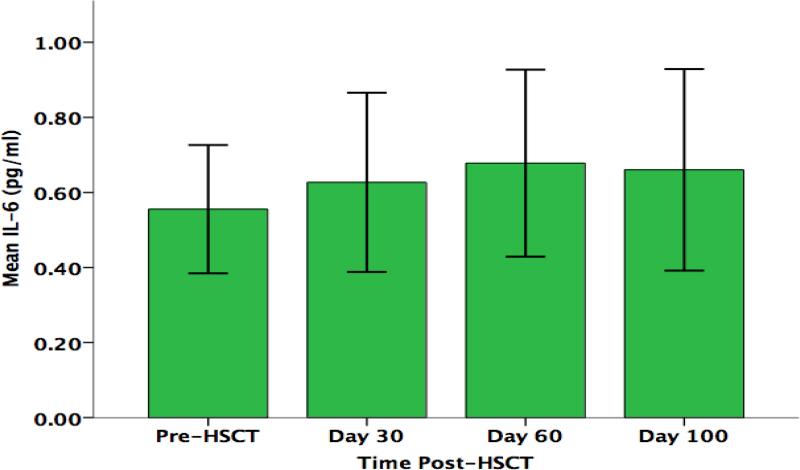
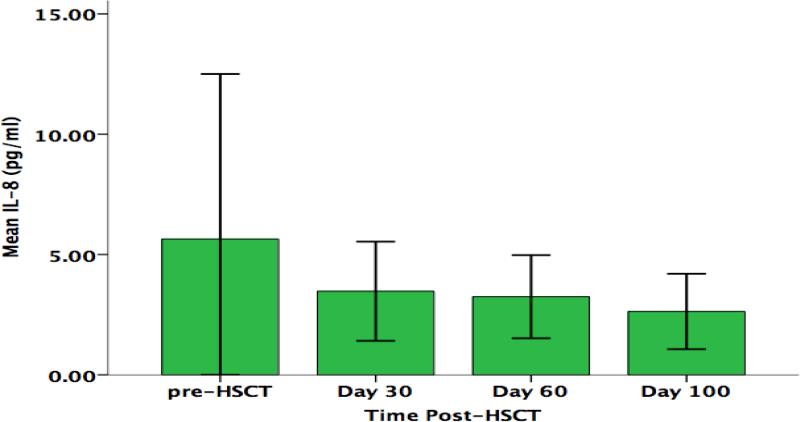
While no significant changes from pre-HSCT through day +100 were found in parents’ serum concentrations of IL-6 F (3,30) = 0.64, p= .53, or IL-8 F (3,30) = 0.66, p= .46, linear trends were found for both cytokines that approached statistical significance (Figures 6 & 7). Parent IL-6 concentration was found to increase over time, while IL-8 concentration was found to decrease over time. No trends were found with respect to longitudinal changes in parents’ concentrations of either TNF-α F (3,30) = 1.34, p= .0.28, or IL-1β F (3,30) = 0.31, p= .0.63 (data not shown).
Figure 6.
Parents’ Serum IL-6 Concentration (pg/ml) Over Time
Figure 7.
Parents’ Serum IL-8 Concentration (pg/ml) Over Time
C-Reactive Protein
C-reactive protein concentration was within the normal range for all enrolled parents, and remained normal for the duration of the study. No significant change was found for CRP over time F (3,30) = .39, p= .59 (data not shown).
Endocrine Markers
Salivary cortisol and amylase AUCs were calculated for each time point. No significant changes were found for either salivary cortisol AUC F (3,21) = .93, p= .37 or amylase AUC F (3,21) = .99, p= .40. Similarly, no significant changes were found for cortisol awakening response (cortisol at 30 minutes post-wakening minus cortisol at wakening), F (3,27) = 1.1, p= .33 (data not shown).
Effect Size and Power Analysis
Calculated effect sizes are presented in Table 4. Effect sizes ranged from 1.23 (perceived stress) to 0.07 (CRP). With the current sample size of 12, minor effects that were not statistically significant were observed for physiological variables with the exception of total, T-helper and cytotoxic T cells. Using the median effect size of 0.3, a sample of 140 would be necessary to have a power of 0.8.
Table 4.
Effect Sizes
| Variable | Effect Size |
|---|---|
| Perceived Stress | 1.23 |
| Salivary Cortisol | 0.75 |
| CD3+CD8+ (Cytotoxic T cells) | 0.63 |
| CD3+ (T cells) | 0.61 |
| CD3+CD4+ (Helper T cells) | 0.54 |
| IL-8 | 0.49 |
| TNF-α | 0.30 |
| IL-1 | 0.24 |
| CD19+ (B cells) | 0.24 |
| IL-6 | 0.21 |
| CD16+56+ (NK cells) | 0.18 |
| Salivary Amylase | 0.17 |
| CRP | 0.07 |
Discussion
Feasibility
Results of this pilot study suggest that it is feasible to measure longitudinal parent outcomes in the pediatric HSCT setting. Parents adhered to the saliva collection protocol, and there was no missing questionnaire or blood sample data. Furthermore, a low attrition rate (8%) was observed over the three-month observation period. This low attrition rate and minimal missing date suggest that the instruments and specimen collection were not overly burdensome for parents as their children undergo HSCT. The feasibility of this pilot study supports replicating this study with a larger sample to test hypotheses.
Psychologic Outcomes
Findings of this study suggest that parents’ perceived stress steadily increases from their child's transplant admission through the first 100 days post-transplant. Elevated stress levels have been previously reported in parents caring for children undergoing HSCT. Phipps, Dunavant, Lensing & Rai (2004) found that parents demonstrate significant increases in global distress (a combined measurement of mood disturbance, perceived stress and caregiver burden) from admission through day +42 post-HSCT, with resolution to less than admission levels noted by four to six months post-HSCT.4 The occurrence of transplant-related complications may explain increases in parental stress after their child's HSCT. In a prospective study of pediatric HSCT recipients, moderate to severe acute GVHD, organ toxicity, and systemic infection were associated with decreased parent emotional functioning between 45 days to three months post-HSCT.46
Although depression and anxiety were not included as outcome measures for this study, a significant number of parents self-reported symptoms of depression and/or anxiety at the time of study entry. Depression and anxiety have been previously observed in mothers of children undergoing HSCT.3,7,8,47,48 In one study, 15% of mothers (N=115) had moderate to severe depression based on the Beck Depression Inventory (BDI) and 18% of mothers had moderate to severe anxiety based on the Beck Anxiety Inventory.
Physiologic Outcomes
Parents’ total, helper, and cytotoxic T lymphocyte counts significantly decreased from baseline to day +100 (Figures 3, 4 and 5). However, all three T lymphocyte populations remained within the clinically normal ranges at each time point.
Decreased T-helper cells with concomitant increases in cytotoxic T cells have been reported in other caregiving populations and individuals under intense stress.10,49,50 Those results differ from the results reported in this study, in that cytotoxic T cells also decreased over time in these parents of children undergoing HSCT. Benaroya-Milshtein et al. (2014) sampled parents caring for children with cancer, and based on results of the BDI divided parents into depressed and non-depressed groups. Compared to non-depressed parents, depressed parents had significantly lower CD4 counts, higher CD8 counts, and lower CD4/CD8 ratios; additionally, BDI scores were negatively correlated with CD4/CD8 ratios.10
In spousal caregivers of dementia patients, depression was associated with lower T cell proliferation to mitogens and a shift to increased CD8 cells.49 Older female caregivers (>45 years) of handicapped patients were also found to have a more pronounced shift from T-helper cells to cytotoxic T cells,50 suggesting that age and/or years of caregiving may play an important role in the impact of caregiving stress on the immune system. However, in the current study, cytotoxic T cells did not increase in this parent sample, potentially due to their relatively young age (median age of 33 years). Furthermore, the duration of caregiving is relatively short in parents caring for children in the acute phase of HSCT, compared to spousal caregivers of dementia patients. The chronicity of caregiving may be positively associated with a shift in T cell populations from T helper cells to cytotoxic T cells, and also may explain the absence of such a shift in these parents of children undergoing HSCT.
The mechanism responsible for the observed alterations in T cell subsets may relate to changes in regulatory T cell populations (Tregs), neurotransmitters, and neurotransmitter receptor expression as a function of psychological state. The brain neurotransmitter, 5-hydroxytryptamine (5-HT), is low in individuals with depression, and has been shown to modulate the immune system.51,52 Furthermore, the 5-HT1a receptor (5-HT1aR) mediates the function of 5-HT in depression and peripheral immune function.53 Decreased expression of 5-HT and 5-HT1a receptor has been found in peripheral blood of patients with major depression (MD) compared with healthy controls, while regulatory T cells and 5-HT1aR expression within Tregs were significantly decreased in patients with MD.53 These observations suggest a potential interaction between Tregs and neurotransmitters that may result in immune dysfunction in patients with MD.
Longitudinal analysis of inflammatory markers such as CRP and pro-inflammatory cytokines did not reveal any significant changes from baseline, although trends were observed for an increase in IL-6 and a decrease in IL-8 over time. A systematic review and meta-analysis of the impact of psychological stress on inflammatory markers found robust effects for increases in IL-6 following episodes of acute stress.54 The post-stress effect for CRP was less robust, and there were too few studies examining IL-8 to include in the review. However, some studies of individual caregiving populations have reported alternations in pro-inflammatory cytokines that coincide with periods of increased caregiver stress,9,26,55 while other studies have not observed such trends.25,28 This discrepancy may be due to differences in measurement technique and timing of measurements, in addition to variability in cytokine release by multiple tissues, random fluctuations, and transient cytokine responses to changes in mood and dietary intake.54
In this study, there were no significant time effects observed for parameters related to salivary cortisol or amylase. Previous cross-sectional studies have reported significant cortisol changes related to parenting stress.9 Parents caring for children with cancer displayed flatter diurnal cortisol slopes and reduced cortisol-awakening response in comparison to age and gender matched controls.9 With respect to the current study, the use of age and gender matched controls would have enabled direct normative comparison of these endocrine markers at each time point, and should be considered for future research of parents of children undergoing HSCT.
Clinical Implications
The results of this study suggest that parents of children undergoing HSCT experience high levels of perceived stress. Significant effects were also observed with respect to longitudinal decreases in T cell subsets, suggesting that parents of children undergoing HSCT may be at risk for alterations in lymphocyte-mediated immune function. Pediatric transplant clinicians should perform routine screenings of parent physical and psychological health during the acute phase of HSCT. Factors that have been shown to relate to parent psychological distress in the pediatric HSCT setting, and should be assessed by clinicians as part of a pre-transplant psychological evaluation, include parent psychiatric history,7,56 methods of coping and cognitive processing,8,57 prior history of traumatic life events,58 family environment,47,56 and availability of social support.5
Psychosocial support services should be readily available for parents before and after their child's transplant. A combination of individualized counseling services and easily accessible parent support groups should be offered to these parents periodically throughout their child's HSCT. A multidisciplinary team consisting of nurses, physicians, social workers, psychologists, music, art and child-life therapists should work together to provide seamless psychosocial support.
Limitations
The primary limitation of this pilot study is the small number of subjects. As a result, longitudinal variations of some physiologic parameters such as cytokines, CRP and salivary studies may not have been detectable. Furthermore, the impact of confounding factors such as child demographic and transplantation characteristics could not be assessed reliably. However, this study did establish the feasibility of evaluating parent psychophysiological outcomes during their child's HSCT as evidenced by an overall lack of missing data.
A second limitation is the lack of a parent control group. The use of an age and gender-matched control group consisting of parents of medically healthy children would enable direct comparisons across psychophysiological parameters of stress, while controlling for potentially confounding factors.
A third limitation is the lack of a comprehensive psychosocial assessment of parents. Depression and anxiety are prevalent in this parent population and have been shown to impact physiological parameters of stress.25,53,55,59 The use of standardized depression and anxiety inventories may have revealed correlations with physiologic parameters. Social support and family environment are additional variables that have been shown to influence psychophysiological parameters of stress,6,8,47,56,60 and are worth investigating.
In addition, potential selection biases were created by a gender imbalance towards mothers, and enrollment from a single institution that offers a wide variety of support services. Previous research has demonstrated that the transplant center may be a significant factor in parent stress associated with pediatric HSCT.46
Directions for Future Research
The effect sizes generated by testing the constructs presented in Figure 1 can be used to power future hypothesis-testing studies of parent perceived stress and physiologic responses to stress in the pediatric HSCT setting. Although this study is the first to report psychophysiologic outcomes of parents of children undergoing HSCT, its limitations preclude statements regarding causality. A larger, prospective, multi-institutional, comparison group-controlled study of outcomes for this parent population would permit correlation analyses between psychological and physiologic outcomes, and reduce potential biases. Simultaneous short and long-term monitoring of parent physical health outcomes could aid in interpretation of physiologic outcome results. Furthermore, parent psychiatric outcomes such as anxiety, depression, and post-traumatic stress disorder should be incorporated into future parent research in the pediatric HSCT setting.
Future studies of parent psychophysiologic outcomes should include a comprehensive set of parent and child parameters. Co-factors that may alleviate or exacerbate parent distress should be studied simultaneously and might include social support, coping, and demographic factors. Furthermore, parent neuroendocrine, immunologic and inflammatory markers should be prospectively studied at regular intervals from pre-admission. In vitro proliferative responses to mitogens and antigens, antibody responses to vaccination, and NK cell cytotoxicity would be informative regarding functional aspects of immune function.
Finally, multi-institutional studies are necessary to develop and evaluate the efficacy of interventions for parents of children undergoing HSCT on parent psychophysiologic outcomes. Psychophysiologic parameters of parent distress may be utilized as outcome measures for intervention studies. Randomized, longitudinal, repeated measure, cohort studies would be necessary to detect differences in parent distress outcomes between control and intervention groups.
Acknowledgments
This research was supported by a Nursing Research Traineeship funded through the Children's Oncology Group Chair's grant (U10CA98543-Adamson) and the National Clinical Trials Network Operations grant (U10 CA180886-Adamson).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors-the ALL-SCT-BFM-2003 trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.9747. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl Norberg A, Mellgren K, Winiarski J, Forinder U. Relationship between problems related to child late effects and parent burnout after pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2014;18(3):302–309. doi: 10.1111/petr.12228. [DOI] [PubMed] [Google Scholar]
- 3.Riva R, Forinder U, Arvidson J, et al. Patterns of psychological responses in parents of children that underwent stem cell transplantation. Psychooncology. 2014 doi: 10.1002/pon.3567. [DOI] [PubMed] [Google Scholar]
- 4.Phipps S, Dunavant M, Lensing S, Rai SN. Patterns of distress in parents of children undergoing stem cell transplantation. Pediatr Blood Cancer. 2004;43(3):267–274. doi: 10.1002/pbc.20101. [DOI] [PubMed] [Google Scholar]
- 5.Nelson AE, Gleaves L, Nuss S. Mothers' responses during the child's stem cell transplantation: Pilot study. Pediatr Nurs. 2003;29(3):219–223. [PubMed] [Google Scholar]
- 6.Manne S, DuHamel K, Nereo N, et al. Predictors of PTSD in mothers of children undergoing bone marrow transplantation: The role of cognitive and social processes. J Pediatr Psychol. 2002;27(7):607–617. doi: 10.1093/jpepsy/27.7.607. [DOI] [PubMed] [Google Scholar]
- 7.Manne S, Nereo N, DuHamel K, et al. Anxiety and depression in mothers of children undergoing bone marrow transplant: Symptom prevalence and use of the beck depression and beck anxiety inventories as screening instruments. J Consult Clin Psychol. 2001;69(6):1037–1047. doi: 10.1037//0022-006x.69.6.1037. [DOI] [PubMed] [Google Scholar]
- 8.Manne S, Duhamel K, Ostroff J, et al. Coping and the course of mother's depressive symptoms during and after pediatric bone marrow transplantation. J Am Acad Child Adolesc Psychiatry. 2003;42(9):1055–1068. doi: 10.1097/01.CHI.0000070248.24125.C0. [DOI] [PubMed] [Google Scholar]
- 9.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 10.Benaroya-Milshtein N, Apter A, Yaniv I, et al. Neuroimmunological function in parents of children suffering from cancer. J Neural Transm. 2014;121(3):299–306. doi: 10.1007/s00702-013-1098-6. [DOI] [PubMed] [Google Scholar]
- 11.Bella GP, Garcia MC, Spadari-Bratfisch RC. Salivary cortisol, stress, and health in primary caregivers (mothers) of children with cerebral palsy. Psychoneuroendocrinology. 2011;36(6):834–842. doi: 10.1016/j.psyneuen.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Lovell B, Moss M, Wetherell M,A. The psychophysiological and health corollaries of child problem behaviours in caregivers of children with autism and ADHD. J Intellect Disabil Res. 2013;59(2):150–157. doi: 10.1111/jir.12081. [DOI] [PubMed] [Google Scholar]
- 13.Meehan KR, Fitzmaurice T, Root L, Kimtis E, Patchett L, Hill J. The financial requirements and time commitments of caregivers for autologous stem cell transplant recipients. J Support Oncol. 2006;4(4):187–190. [PubMed] [Google Scholar]
- 14.Von Ah D, Spath M, Nielsen A, Fife B. The caregiver's role across the bone marrow transplantation trajectory. Cancer Nurs. 2016;39(1):E12–9. doi: 10.1097/NCC.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 15.Polomeni A, Lapusan S, Bompoint C, Rubio MT, Mohty M. The impact of allogeneichematopoietic stem cell transplantation on patients' and close relatives' quality of life and relationships. Eur J Oncol Nurs. 2015 doi: 10.1016/j.ejon.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav Immun. 2002;16(6):622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 18.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: A systematic review. Can J Cardiol. 2011;27(2):174–182. doi: 10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Roepke SK, Chattillion EA, von Kanel R, et al. Carotid plaque in alzheimer caregivers and the role of sympathoadrenal arousal. Psychosom Med. 2011;73(2):206–213. doi: 10.1097/PSY.0b013e3182081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: A prospective study. Am J Prev Med. 2003;24(2):113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 22.Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: A review. Neuroimmunomodulation. 2008;15(4-6):251–259. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol. 2009;27(18):2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- 26.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71(1):57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovell B, Moss M, Wetherell M. The psychosocial, endocrine and immune consequences of caring for a child with autism or ADHD. Psychoneuroendocrinology. 2012;37(4):534–542. doi: 10.1016/j.psyneuen.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Lovell B, Wetherell MA. The cost of caregiving: Endocrine and immune implications in elderly and non elderly caregivers. Neurosci Biobehav Rev. 2011;35(6):1342–1352. doi: 10.1016/j.neubiorev.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Bauer ME, Vedhara K, Perks P, Wilcock GK, Lightman SL, Shanks N. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol. 2000;103(1):84–92. doi: 10.1016/s0165-5728(99)00228-3. [DOI] [PubMed] [Google Scholar]
- 30.Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8(1):69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- 31.Glaser R, MacCallum RC, Laskowski BF, Malarkey WB, Sheridan JF, Kiecolt-Glaser JK. Evidence for a shift in the th-1 to th-2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci. 2001;56(8):M477–82. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 32.Stehl MJ, Kazak AE, Hwang WT, Pai AL, Reilly AF, Douglas SD. Innate immune markers in mothers and fathers of children newly diagnosed with cancer. Neuroimmunomodulation. 2008;15(2):102–107. doi: 10.1159/000148192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Andres-Garcia S, Moya-Albiol L, Gonzalez-Bono E. Salivary cortisol and immunoglobulin A: Responses to stress as predictors of health complaints reported by caregivers of offspring with autistic spectrum disorder. Horm Behav. 2012;62(4):464–474. doi: 10.1016/j.yhbeh.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60(7):1651–1656. doi: 10.1002/1097-0142(19871001)60:7<1651::aid-cncr2820600738>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CC, editor. Evaluation of chemotherapeutic agents in cancer. Columbia University Press; New York: 1949. pp. 191–205. [Google Scholar]
- 37.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 38.Levenstein S, Prantera C, Varvo V, et al. Development of the perceived stress questionnaire: A new tool for psychosomatic research. J Psychosom Res. 1993;37(1):19–32. doi: 10.1016/0022-3999(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 39.Stawski RS, Sliwinski MJ, Almeida DM, Smyth JM. Reported exposure and emotional reactivity to daily stressors: The roles of adult age and global perceived stress. Psychol Aging. 2008;23(1):52–61. doi: 10.1037/0882-7974.23.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: Clinical importance. Curr Probl Cardiol. 2004;29(8):439–493. [PubMed] [Google Scholar]
- 41.Valiathan R, Deeb K, Diamante M, Ashman M, Sachdeva N, Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of south florida. Immunobiology. 2014;219(7):487–496. doi: 10.1016/j.imbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: Comparison of the meso scale discovery electrochemiluminescence assay and the cytometric bead array. J Immunol Methods. 2011;372(1-2):71–77. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxbe DE, Repetti RL, Nishina A. Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychol. 2008;27(1):15–25. doi: 10.1037/0278-6133.27.1.15. [DOI] [PubMed] [Google Scholar]
- 44.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Statisical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- 46.Terrin N, Rodday AM, Tighiouart H, Chang G, Parsons SK, Journeys to Recovery Study Parental emotional functioning declines with occurrence of clinical complications in pediatric hematopoietic stem cell transplant. Support Care Cancer. 2013;21(3):687–695. doi: 10.1007/s00520-012-1566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jobe-Shields L, Alderfer MA, Barrera M, Vannatta K, Currier JM, Phipps S. Parental depression and family environment predict distress in children before stem cell transplantation. J Dev Behav Pediatr. 2009;30(2):140–146. doi: 10.1097/DBP.0b013e3181976a59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindwall JJ, Russell K, Huang Q, et al. Adjustment in parents of children undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(4):543–548. doi: 10.1016/j.bbmt.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castle S, Wilkins S, Heck E, Tanzy K, Fahey J. Depression in caregivers of demented patients is associated with altered immunity: Impaired proliferative capacity, increased CD8+, and a decline in lymphocytes with surface signal transduction molecules (CD38+) and a cytotoxicity marker (CD56+ CD8+). Clin Exp Immunol. 1995;101(3):487–493. doi: 10.1111/j.1365-2249.1995.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pariante CM, Carpiniello B, Orru MG, et al. Chronic caregiving stress alters peripheral blood immune parameters: The role of age and severity of stress. Psychother Psychosom. 1997;66(4):199–207. doi: 10.1159/000289135. [DOI] [PubMed] [Google Scholar]
- 51.Abdouh M, Albert PR, Drobetsky E, Filep JG, Kouassi E. 5-HT1A-mediated promotion of mitogen-activated T and B cell survival and proliferation is associated with increased translocation of NF-kappaB to the nucleus. Brain Behav Immun. 2004;18(1):24–34. doi: 10.1016/s0889-1591(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 52.Suguro T, Watanabe T, Kanome T, et al. Serotonin acts as an up-regulator of acyl-coenzyme A:Cholesterol acyltransferase-1 in human monocyte-macrophages. Atherosclerosis. 2006;186(2):275–281. doi: 10.1016/j.atherosclerosis.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Xiao B, Qiu W, et al. Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord. 2010;124(1-2):68–75. doi: 10.1016/j.jad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Rohleder N, Miller GE. Acute deviations from long-term trait depressive symptoms predict systemic inflammatory activity. Brain Behav Immun. 2008;22(5):709–716. doi: 10.1016/j.bbi.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Phipps S, Dunavant M, Lensing S, Rai SN. Psychosocial predictors of distress in parents of children undergoing stem cell or bone marrow transplantation. J Pediatr Psychol. 2005;30(2):139–153. doi: 10.1093/jpepsy/jsi002. [DOI] [PubMed] [Google Scholar]
- 57.DuHamel KN, Manne S, Nereo N, et al. Cognitive processing among mothers of children undergoing bone marrow/stem cell transplantation. Psychosom Med. 2004;66(1):92–103. doi: 10.1097/01.psy.0000108104.23738.04. [DOI] [PubMed] [Google Scholar]
- 58.DuHamel KN, Rini C, Austin J, et al. Optimism and life events as predictors of fear appraisals in mothers of children undergoing hematopoietic stem cell transplantation. Psychooncology. 2007;16(9):821–833. doi: 10.1002/pon.1132. [DOI] [PubMed] [Google Scholar]
- 59.Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems --2011 curt richter award winner. Psychoneuroendocrinology. 2012;37(3):307–316. doi: 10.1016/j.psyneuen.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Lovell B, Moss M, Wetherell MA. With a little help from my friends: Psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil. 2012;33(2):682–687. doi: 10.1016/j.ridd.2011.11.014. [DOI] [PubMed] [Google Scholar]