Abstract
Low-level laser therapy (LLLT) is known to enhance mitochondrial electron transfer and ATP production; thus, this study asked whether LLLT could stimulate the oxidative burst in human neutrophils (PMN) and improve their ability to kill microorganisms. Blood from healthy human subjects was collected and PMN were isolated from the samples. PMN were treated in vitro with 660 nm or 780 nm CW laser light at 40 mW power and increasing energies up to 19.2 J and were subsequently incubated with Candida albicans cells. Generation of hydroxyl radicals, hypochlorite anions and superoxide anions by PMN were checked using fluorescent probes and chemiluminescence assays; a microbicidal activity assay against C. albicans was also performed. LLLT excited PMN to a higher functional profile, which was translated as superior production of reactive oxygen species (ROS) and increased fungicidal capacity. The most efficacious energy was 19.2 J and, interestingly, the 660 nm light was even more efficacious than 780 nm at increasing the respiratory burst of PMN and the fungicidal capacity.
Human neutrophils (PMN) were stimulated in vitro with 660 nm or 780 nm CW laser light at 40 mW of power and a total energy of 19.2 J. Low-level laser therapy (LLLT) excited PMN to a higher functional profile, which was translated as a superior production of reactive oxygen species (ROS) such as hydroxyl radicals (HO•) and hypochlorite anions (ClO−) (Figure) and increased fungicidal capacity against Candida albicans cells.
Keywords: low-level laser therapy, photobiomodulation, neutrophils, reactive oxygen species, oxidative burst, Candida albicans
Graphical Abstract
1. Introduction
Low-level laser therapy (LLLT) or photobiomodulation uses light to stimulate cells and tissues via non-ionizing and non-thermal red or near infrared (NIR) wavelengths [1–3] and has been used to treat several medical conditions, including those typified by pain or inflammation [1, 4, 5]. However, there is uncertainty in the literature about whether LLLT has mainly an anti-inflammatory effect [6], or whether it can be regarded as a pro-inflammatory stimulus [7, 8].
Authors have used different models to study the pro-inflammatory and anti-inflammatory responses after LLLT. In order to recruit immune cells to a given anatomical site, some studies have employed chemical irritants such as formaldehyde to induce lung inflammation that mimics inflammation caused by pollutants [5], LPS to simulate mastitis in rats [9], or to cause acute respiratory distress syndrome (ARDS) in mice [10], or have even used live fungal cells to test the fungicidal response of neutrophils [11]. Many reports focus on polymorphonuclear (PMN) cells or neutrophils, as they possess specific receptors that allow them to recognize fungi and bacteria [12, 13] and are one of the principal mediators of the innate immune response against pathogens [14–16]. The degranulation process and the respiratory burst that are typical of PMN involve endogenous peroxidases and NADPH-oxidase, and these processes are essential for PMN to fight fungi [17].
We have previously studied the effects of LLLT upon the behavior of PMN acting against an important fungal infection (Paracoccidioides brasiliensis), and observed that LLLT induced lesser PMN migration to the infection site; however, the PMN that did reach the site of infection were significantly more activated towards the fungal cells and could efficiently kill the microorganism due to higher PMN viability, and greater production of ROS as well as pro-inflammatory proteins [11].
Therefore, in order to better investigate the activation of the respiratory burst of PMN after light irradiation we extracted and isolated these cells from the blood of healthy human subjects and subjected them to LLLT with different in vitro protocols, then assessed the production of intracellular and extracellular ROS and their fungicidal capacity against C. albicans cells.
2. Materials and methods
All experiments were performed in compliance with the relevant laws and institutional guidelines in accordance with the ethical standards of the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html). The present study was performed after approval of Human Research Ethics Committee of our institution (Protocol #292274), and experimentation with human subjects was only possible after obtaining an informed consent.
2.1 Human neutrophil isolation
Human PMN were obtained from eight (8) healthy male donors (aged 20–40 years) who had not received any medication for at least 7 days prior to blood collection and who signed an informed consent. The cells were purified from heparin blood samples diluted in phosphate-buffered saline solution (PBS) by sequential Ficoll–Hypaque differential density centrifugation (5810R Centrifuge, Eppendorf, NY, USA) according to Boyum [18]. The degree of purity was greater than 95% and was verified by light microscopy, after Leishman staining, in order to confirm the neutrophil morphology [19]. PMN in the pellet were resuspended in 2 mL of PBS. The cell viability was evaluated with 0.2% Trypan blue (Sigma-Aldrich, St. Louis, MO, USA) and found to be >92%. PMN were quantified using a hemocytometer, and the final concentration was adjusted to 106 PMN/mL [19, 20]. The extracted and isolated PMN from individuals were gathered to compose pools of cells that were further distributed into distinct experimental groups.
2.2 Low-level laser irradiation of PMN
Cells were irradiated using a CW semiconductor diode laser (Twin Flex; MMO, São Carlos, SP, Brazil) with a spot size of 0.04 cm2, at 660 nm or 780 nm wavelengths with 40 mW total energy, irradiance of 1 W/cm2 per irradiation, fluence of 6 J/cm2 per irradiation, and giving 0.24 J of energy per irradiation period (6 seconds per irradiation). Different numbers of irradiations were used up to a maximum of 80 pulses (480 sec total) equivalent to 19.2 J total energy. The laser tip always touched the bottom of the well-plate and the laser beam remained perpendicular to the surface of the plate at all times. PMN viability was assessed with an MTT assay (Sigma-Aldrich) and the minimum viability was 98 ± 2% for non-irradiated and 97 ± 3% for irradiated groups. There were no statistically significant differences among the groups. Irradiated and non-irradiated PMN (1 × 106 cells/mL) were added into a 96-well plate. After 3 hours of incubation (5% CO2 and 37 °C) 10 μL of MTT (5 mg/mL) was added to the wells and the plate was further incubated for 4 hours. Subsequently, 10 μL of DMSO (Sigma-Aldrich) was added and the plate was read in a microplate reader at 570 nm (Anthos Zenyth 200, Biochrom, Cambridge, UK). Triton-X-100 (10%) was used as positive control.
2.3 Measurement of hydroxyl radical and hypochlorite anion intra- and extracelullar
Intracellular and extracellular hydroxyl radical (HO•) and hypochlorite anion (ClO−) were measured with the fluorescent probes, hydroxyphenyl fluorescein (HPF; H36004) and aminophenyl fluorescein (APF; A36003) (Molecular Probes, Life Technologies, New York, NY, USA). PMN suspension (1 × 106 cells/mL) was incubated with 10 mM glucose, 1 mM CaCl2, 1.5 mM MgCl2 in a 96-well plate. Laser groups were irradiated with 660 nm or 780 nm wavelengths until fluorescence emission reached a plateau (maximum of 80 irradiation periods – total energy of 19.2 J delivered for 480 seconds). APF or HPF were dissolved in 25 μM DMSO and were added to the wells. The same concentration of DMSO was also added to the remaining experimental groups. The fluorescence emission was measured (Varian Cary Eclipse Fluorescence Spectrophotometer, Varian, Mulgrave, Australia) using excitation/emission wavelengths of 500/520 nm [21].
2.4 Measurement of superoxide anion production by cytochrome c reduction
Superoxide dismutase (SOD)-inhibitable cytochrome c reduction assay was used to measure the superoxide anion ( ) production by PMN. The experiment was performed in triplicate. PMN suspension (5 × 105 cells/mL) in PBS was added into a 96-well plate with 50 U/ml SOD for 10 minutes, followed by addition of 10 mM glucose, 1 mM CaCl2, 1.5 mM MgCl2, and 25 μM cytochrome c. In the control group, PMN were stimulated with non-opsonized C. albicans at a m.o.i of 10 : 1 [22], or 40 μM N-formyl-methionyl-leucyl-phenylalanine (fMLP). In the laser groups, PMN were previously irradiated with laser 660 nm or 780 nm wavelengths for 80 irradiations (total energy of 19.2 J delivered over 480 seconds), following stimulation with non-opsonized C. albicans at a m.o.i of 10 : 1, or 40 μM fMLP. The 96-well plate was incubated at 37 °C for 3 hours. The reaction was monitored by spectrophotometry at a wavelength of 550 nm. The results were expressed in nmol of per 5 × 105 cells per minute, as follows [23, 24]:
2.5 Chemiluminescence
The quantification of total and extracellular ROS produced by the human PMN oxidative “burst” was carried out using the luminol or isoluminol chemiluminescence assays, respectively. While luminol is thought to cross cell membranes, hence measuring the general chemiluminescence [25, 26], isoluminol possess the amino group located distant from the oxygen group [27], thus making this molecule not trespass membranes [28, 29]; in that way, isoluminol only detects the extracellular chemiluminescence [26]. PMN suspension (1 × 106 cells/mL) was incubated with 10 mM glucose, 1 mM CaCl2, 1.5 mM MgCl2, 50 μM luminol or 50 μM isoluminol, and 8 U/mL horseradish peroxidase (HRP); then, in the control group, PMN were stimulated with non-opsonized C. albicans at a m.o.i of 10 : 1, or 40 μM fMLP. In the laser groups, PMN were previously irradiated with 660 nm or 780 nm wavelengths at 80 irradiation periods (total energy of 19.2 J delivered for 480 seconds), following stimulation with non-opsonized C. albicans at an m.o.i of 10 : 1. A luminometer (Glomax 20/20 Luminometer, Promega, USA) was used to measure the chemiluminescence signal over 30 minutes. The results were expressed as relative light units (RLU) [23]. Negative control (32 μM DPI, Sigma-Aldrich) was performed. The experiment was performed in triplicate.
2.6 Yeast preparation
C. albicans yeast (ATCC 10231 – American Type Culture Collection, VA, USA) was cultived in brain heart infusion (BHI) medium overnight at 37 °C. Fungal cells (10 mL) were centrifuged (5810R Centrifuge, Eppendorf, NY, USA) at 2200 g for 8 minutes, and the pellet was re-suspended in PBS. The fungal suspension was adjusted to 3 × 107 colony-forming units (CFU)/mL using a spectrophotometer (1 OD600 = 3 × 107 CFU/mL). C. albicans was inactivated by heat for 30 minutes at 100 °C for the oxidative burst experiments, whereas for the microbicidal activity assay we used fully viable microorganisms. C. albicans was opsonized by 20% human serum incubated at 37 °C for 1 hour followed by centrifugation at 1000 g for 10 minutes. The pellet was re-suspended in PBS.
2.7 Microbicidal activity assay
The microbicidal activity assay was performed according to Green et al. [22]. Opsonized C. albicans suspension (1 × 107 CFU/mL) was incubated with PMN (106 cells/mL), 10 mM glucose, 1 mM CaCl2, and 1.5 mM MgCl2 at 37 °C over 90 minutes. In the laser groups, PMN were previously irradiated with laser 660 nm or 780 nm wavelengths for 80 irradiation periods (total energy of 19.2 J delivered for 480 seconds). After different incubation times (5, 30 and 90 minutes) all experimental groups were treated with cold PBS and were diluted in H2O (pH = 11) at 3 different concentrations: 103, 106 and 109 cells/mL. The suspensions were dispensed on Tryptone Soy Agar (TSA) into Petri dishes, and they were incubated at 37 °C for 24 hours. Viable C. albicans were measured from the number of colonies grown on Petri dishes and was expressed in CFU/mL. Negative control (32 μM DPI, Sigma-Aldrich) was performed.
2.8 Statistical analysis
Experimental groups were compared using one-way analysis of variance (ANOVA) followed by Tukey test for multiple comparisons. The analyses adopted a significance level of 5% (p < 0.05).
3. Results
3.1 HO• and ClO− generation by PMN
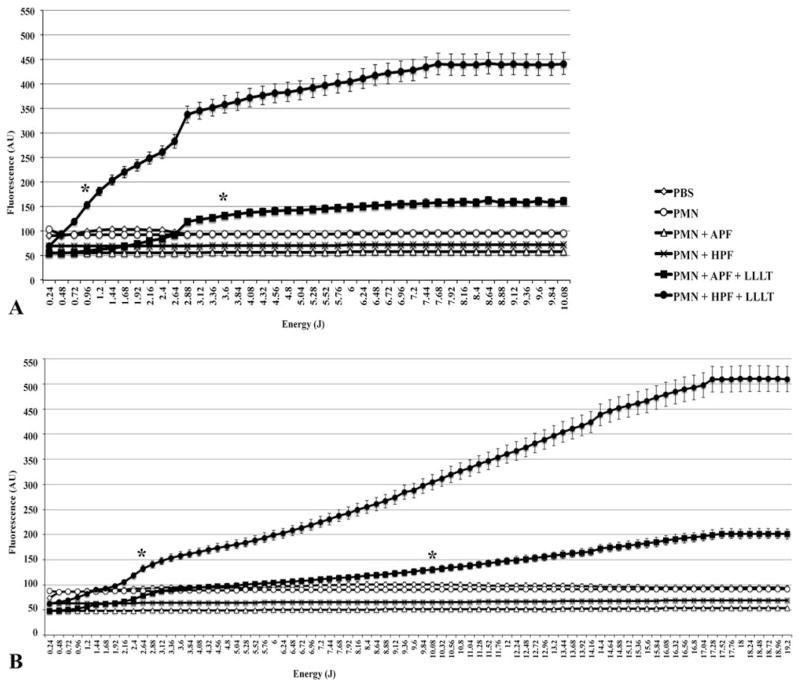
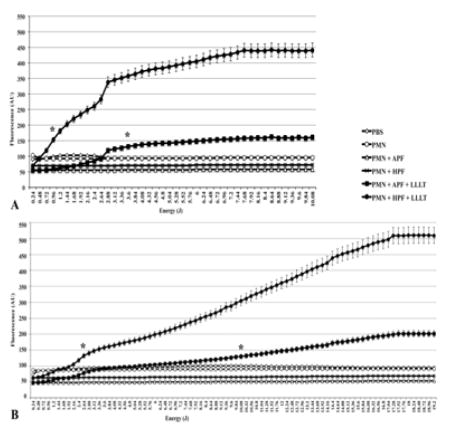
HO• and ClO− were produced in significantly higher quantities by irradiated PMN than they were by control PMN (p < 0.01). Both red and infrared wavelengths stimulated ROS production and the most efficacious energy dose for 660 nm light was 10.08 J (p < 0.01), while for 780 nm it was 19.2 J (p < 0.01) (Figure 1A depicts red LLLT and 1B depicts infrared LLLT).
Figure 1.
ROS generation is significantly increased with LLLT. Fluorescent probes APF and HPF helped detecting the ROS production after increasing doses of (A) red and (B) infrared LLLT. Results represented by means ± standard deviations. Asterisks indicate a p < 0.01 from that point on and in comparison to any other group. All groups contained the same amount of diluted DMSO.
3.2 generation by PMN stimulated with LLLT, fMLP or C. albicans
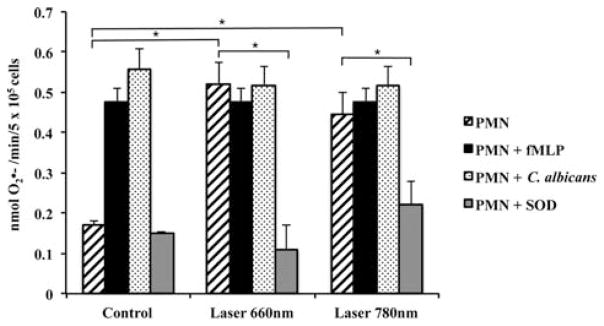
was highly generated by both 660 nm or 780 nm irradiated PMN (p < 0.01) compared to control PMN; no significant differences were found between the irradiated cells and the cells stimulated with fMLP (Figure 2). In addition, the increased levels of by irradiated PMN were similar to the levels of cells that were stimulated with C. albicans (Figure 2). Finally, SOD was able to significantly quench the levels generated by irradiated PMN. This was done as a control to confirm extracellular generated superoxide (Figure 2).
Figure 2.
LLLT shifts up the generation in PMN. generation determined by SOD-inhibitable reduction of cytochrome c for Control and Laser groups. Human PMN were irradiated with 660 nm or 780 nm laser light; p < 0.01 (*) between PMN irradiated with laser 660 nm and non-irradiated PMN; p < 0.01 (*) between PMN irradiated with laser 780 nm and non-irradiated PMN; p < 0.01 (*) between PMN irradiated with 660 nm and PMN irradiated with 660 nm along with SOD; p < 0.01 (*) between PMN irradiated with 780 nm and PMN irradiated with 780 nm and SOD. Irradiated and non-irradiated PMN stimulated by fMLP showed no statistically differences between them (p > 0.05). Irradiated and non-irradiated PMN stimulated by C. albicans showed no statistically differences between them (p > 0.05). Results represent means ± standard deviations. p value obtained with ANOVA followed by Tukey test (level of significance of 5%). fMLP, N-Formyl-methionyl-leucyl-phenylalanine; SOD, superoxide dismutase.
3.3 Intracellular and extracellular production of total oxidants by PMN
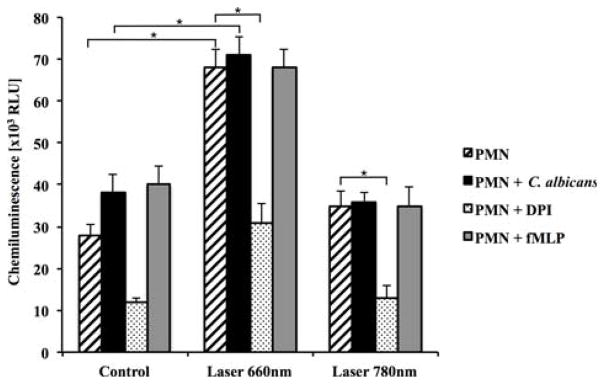
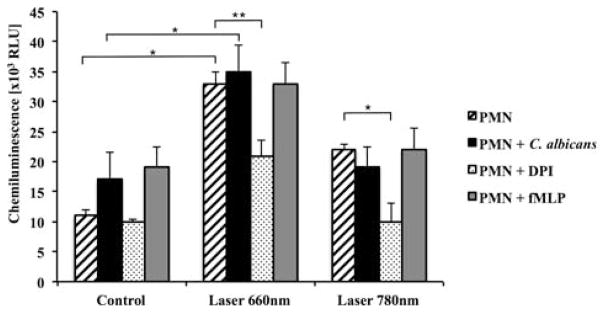
The total amount of ROS produced by the 660 nm-irradiated PMN (detected with luminol) was clearly higher than that of control or 780 nm-irradiated PMN, and the oxidants produced after LLLT were efficaciously inhibited by the use of a specific inhibitor of NADPH oxidase called diphenyleneiodonium (DPI) (Figure 3). Similarly, we only found significantly higher levels of extracellular ROS (detected by isoluminol) with red LLLT, and DPI neutralized the greater part of these oxidants (Figure 4). It is worth mentioning that the 660 nm irradiation was more effective than fMLP or C. albicans stimulation for both intra and extracellular production of oxidants (Figures 3 and 4).
Figure 3.
LLLT enhances PMN luminol chemiluminescence. p < 0.01 (*) between non-irradiated and irradiated (660 nm) PMN; p < 0.01 (*) between non-irradiated and irradiated (660 nm) PMN stimulated with C. albicans; p < 0.01 (*) between irradiated (660 nm) PMN and irradiated (660 nm) PMN with DPI; p < 0.01 (*) between irradiated (780 nm) PMN and irradiated (780 nm) PMN with DPI. Results represented by means ± standard deviations. RLU, relative light units; fMLP, N-Formyl-methionyl-leucyl-phenylalanine; DPI, diphenyleneiodonium.
Figure 4.
LLLT enhances PMN isoluminol chemiluminescence. p < 0.01 (*) between non-irradiated and irradiated (660 nm) PMN; p < 0.01 (*) between non-irradiated and irradiated (660 nm) PMN stimulated with C. albicans; p < 0.05 (**) between irradiated (660 nm) PMN and irradiated (660 nm) PMN with DPI; p < 0.01 (*) between irradiated (780 nm) PMN and irradiated (780 nm) PMN with DPI. Results represented by means ± standard deviations. RLU, relative light units; fMLP, N-Formyl-methionyl-leucyl-phenylalanine; DPI, diphenyleneiodonium.
3.4 Fungicidal capacity of irradiated PMN
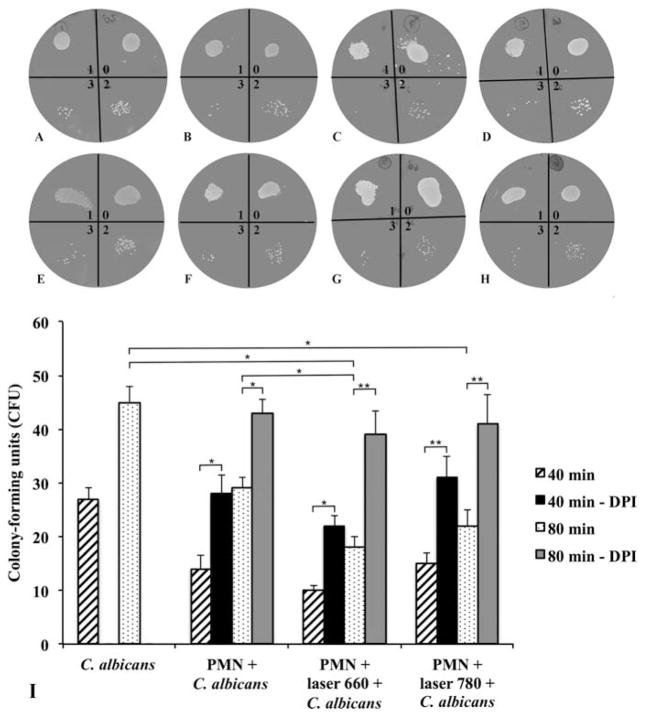
We observed that the ability to kill C. albicans was enhanced after red light stimulation. This was demonstrated by a diminished number of fungal colonies after 80 minutes of co-culture; however, 40 minutes of fungal co-culture did not lead to any difference in the results between the control and irradiated groups (Figure 5).
Figure 5.
Fungicidal capacity of PMN is improved with LLLT. Colony-forming units (CFU) of C. albicans at 40 (A) Control; (B) PMN + C. albicans; (C) PMN + C. albicans + laser 660 nm; (D) PMN + C. albicans + laser 780 nm) and 80 minutes (E) Control; F – PMN + C. albicans; (G) PMN + C. albicans + laser 660 nm; (H) PMN + C. albicans + laser 780 nm) evaluation for non-irradiated and irradiated PMN, being 0: no dilution; 1: 103 cells/mL; 2: 106 cells/mL and 3: 109 cells/mL; and (I) Quantification of CFU. p < 0.05 (*) between non-irradiated and irradiated (laser 660 nm) PMN after 80 minutes of fungal growth; p < 0.05 (*) between C. albicans and irradiated (laser 660 nm) PMN after 80 minutes of fungal growth; p < 0.05 (*) between C. albicans and irradiated (laser 780 nm) PMN after 80 minutes of fungal growth; p < 0.05 (*) between non-irradiated PMN and non-irradiated PMN with DPI after 40 minutes and 80 minutes of fungal growth; p < 0.05 (*) between irradiated (laser 660 nm) PMN and irradiated (laser 660 nm) PMN with DPI after 40 minutes; p < 0.01 (**) between irradiated (laser 660 nm) PMN and irradiated (laser 660 nm) PMN with DPI after 80 minutes; p < 0.01 (**) between irradiated (laser 780 nm) PMN and irradiated (laser 780 nm) PMN with DPI after 40 minutes and 80 minutes of fungal growth. Results represented by means ± standard deviations. DPI, diphenyleneiodonium.
4. Discussion
In this study we detected elevated HO• and ClO− formation upon the illumination of human PMN; such ROS generation is considered to be an important mechanism for PMN to kill microorganisms [30–32]. ROS generation by PMN has even been implicated in the killing of neoplastic cells [32–34] and could be involved in the immune response against tumors. Immune cells play a crucial role in virtually all types of infections, and need to be effective against a wide range of bacteria, viruses and fungi. Cellular and humoral immune responses essentially work jointly to eradicate microbes such as C. albicans, which is a common opportunistic pathogen that can lead to a form of invasive fungal disease in immunocompromised individuals [35]. Candida ranks as one of the most important nosocomial fungal infections, with the potential to be lethal if it reaches the bloodstream [36, 37].
PMN are well known to be the principal participants in the innate immune response that develops after Candida infections [14–16]. These leukocytes possess receptors that allow them to recognize fungi and other microbes, namely the beta-2 subfamily of integrins also known as CD18 [12, 13]. Thus, we asked whether LLLT could cause PMN to fight C. albicans more effectively by stimulating their inner production and discharge of ROS such as hydroxyl radicals. In fact, hydroxyl radicals are effective against Gram-negative bacteria in third-degree burn infections [31] and can also kill neoplastic cells [33]. We found that not only were HO• and ClO− produced in higher quantities after LLLT, but superoxide ( ) was also increased to a level equivalent to the fMLP treated positive-control.
The chemotactic peptide fMLP induces the respiratory burst in PMN by increasing the phosphorylation of various tyrosine domains of proteins [38] and increasing NADPH oxidase activity [39]. Thus, increasing the production of extracellular superoxide with LLLT to the same extent as fMLP is remarkable, and indicates that 19.2 J (80 irradiations of 6 seconds each) represented an effective stimulatory energy dose. Moreover, stimulating PMN with C. albicans led to the same superoxide generation as LLLT. This again means that PMN were effectively stimulated to produce ROS to very high levels.
The assay of the amount of oxidants using luminol and isoluminol showed that light stimulation increased generation of both intracellular and extracellular ROS; the concentrations of oxidants were raised by light by as much or even more than they were with other stimuli (fMLP and Candida). In that way, LLLT probably elevated the oxidative burst to the highest extent possible, since fMLP did not increase ROS production higher than light irradiation. Additionally, we have reasons to believe that the mechanism of stimulation of ROS generation in PMN by light is NADPH oxidase dependant, since the NADPH oxidase inhibitor DPI efficaciously reduced the amount of oxidants to their basal levels. DPI prevents flavin adenine dinucleotide from binding to NADPH oxidase [40], and therefore impairs the ROS production of PMN even when treated with fMLP [39].
There is a dichotomy that underlies the role of LLLT on inflammation: sometimes, LLLT has pro-inflammatory effects [7, 8], while at other times LLLT has mainly anti-inflammatory effects [6]. The anti-inflammatory effects are usually manifested as a lesser influx of PMN into a given site of inflammation; for instance, 650 nm LLLT diminished the number of LPS-induced PMN entering the breast alveoli in a rat model of mastitis [9], while a 830 nm LLLT protocol was able to significantly reduce the total number of cells and the number of PMN in a model in BALB/c mice in which LPS was used to induce a disease that mimicked acute respiratory distress syndrome (ARDS) [10]. In addition, three out of four doses of 660 nm LLLT significantly reduced myeloperoxidase (MPO) activity in PMN in an ARDS model in mice [41].
Nevertheless, we believe that although LLLT can reduce the influx of PMN into a given site of inflammation, somewhat paradoxically, LLLT may also be able to increase the individual activity levels of those PMN. In a recent study we were able to demonstrate that even though irradiated PMN were present in lower numbers, they were far more active against virulent forms of Paracoccidioides brasiliensis (Pb), which is the fungus responsible for a neglected tropical disease known as paracoccidioidomycosis (PCM) [11].
The highest fungicidal capacity was activated in the PMN by red light compared to NIR light. We do not understand exactly why the shorter wavelength was more effective, but the literature suggests that red light is indeed efficacious at accelerating the electron transport in the mitochondria leading to higher ATP production since red light is absorbed by the recognized photoacceptor, cytochrome C oxidase [42–45]. It is known that the respiratory burst in PMN is driven by ATP as an energy source. Repeated 632.8 nm laser treatment to the thymus projection areas and to the hind limbs of mice led to a rise in production of cytokines, NO and Hsp70 by immune cells [46].
Moreover, even blue light from a light emitting diode may increase ROS generation in a dose-dependent manner and without altering the viability of fibroblasts [47]; blue laser light may also result in greater production of ROS in fibroblasts, human cervix adenocarcinoma cells, macrophages, and several other types of cells [48]. Inducing the production of ROS by PMN makes them better at killing C. albicans, and considering that some Candida strains may present resistance to commonly used antimycotics [49, 50], while the ROS burst is not target-specific and so far no fungal resistance has been found against PMN killing.
5. Conclusion
In summary, the paradox of LLLT being a potent anti-inflammatory or pro-inflammatory tool, as well as the pros and cons of shining light on an infected tissue have to be considered before recommending laser-therapy to any clinical application; here we show how specific and safe low-level light parameters can boost the innate immune response towards an opportunistic fungus, what may possibly help treating clinical fungal infections in the near future.
Acknowledgments
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES AUX PE PNPD 2386/2011) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq –INCT of Redox Processes in Biomedicine – Redoxoma) for financially supporting this study. MR Hamblin was supported by US NIH grant R01AI050875.
Footnotes
Conflict of interest All authors of the present manuscript declare no conflict of interest.
Author biographies Please see Supporting Information online.
References
- 1.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperandio FF, Simoes A, Correa L, Aranha AC, Giudice FS, Hamblin MR, Sousa SC. J Biophotonics. 2015;8:795–803. doi: 10.1002/jbio.201400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperandio FF, Giudice FS, Correa L, Pinto DS, Jr, Hamblin MR, de Sousa SC. J Biophotonics. 2013;6:839–847. doi: 10.1002/jbio.201300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperandio FF, Simoes A, Aranha AC, Correa L, Orsini Machado de Sousa SC. Photomed Laser Surg. 2010;28:581–587. doi: 10.1089/pho.2009.2601. [DOI] [PubMed] [Google Scholar]
- 5.Miranda da Silva C, Peres Leal M, Brochetti RA, Braga T, Vitoretti LB, Saraiva Camara NO, Damazo AS, Ligeiro-de-Oliveira AP, Chavantes MC, Lino-Dos-Santos-Franco A. PLoS One. 2015;10:e0142816. doi: 10.1371/journal.pone.0142816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim W, Choi H, Kim J, Kim S, Jeon S, Zheng H, Kim D, Ko Y, Kim D, Sohn H, Kim O. J Oral Pathol Med. 2015;44:94–102. doi: 10.1111/jop.12204. [DOI] [PubMed] [Google Scholar]
- 7.Viegas VN, Abreu ME, Viezzer C, Machado DC, Filho MS, Silva DN, Pagnoncelli RM. Photomed Laser Surg. 2007;25:467–473. doi: 10.1089/pho.2007.1098. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS. Photomed Laser Surg. 2004;22:241–247. doi: 10.1089/1549541041438623. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, He X, Hao D, Yu D, Liang J, Qu Y, Sun D, Yang B, Yang K, Wu R, Wang J. J Vet Med Sci. 2014;76:1443–1450. doi: 10.1292/jvms.14-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira MC, Jr, Greiffo FR, Rigonato-Oliveira NC, Custodio RW, Silva VR, Damaceno-Rodrigues NR, Almeida FM, Albertini R, Lopes-Martins RA, de Oliveira LV, de Carvalho PT, Ligeiro de Oliveira AP, Leal EC, Jr, Vieira RP. J Photochem Photobiol B. 2014;134:57–63. doi: 10.1016/j.jphotobiol.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Burger E, Mendes AC, Bani GM, Brigagao MR, Santos GB, Malaquias LC, Chavasco JK, Verinaud LM, de Camargo ZP, Hamblin MR, Sperandio FF. PLoS Negl Trop Dis. 2015;9:e0003541. doi: 10.1371/journal.pntd.0003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayadas TN, Cullere X. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. J Immunol. 1992;149:1326–1333. [PubMed] [Google Scholar]
- 14.Netea MG, Gijzen K, Coolen N, Verschueren I, Figdor C, Van der Meer JW, Torensma R, Kullberg BJ. Microbes Infect. 2004;6:985–989. doi: 10.1016/j.micinf.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Mahanty S, Greenfield RA, Joyce WA, Kincade PW. Infect Immun. 1988;56:3162–3166. doi: 10.1128/iai.56.12.3162-3166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond RD. Arch Med Res. 1993;24:361–369. [PubMed] [Google Scholar]
- 17.Dias MF, Mesquita J, Filgueira AL, De Souza W. Med Mycol. 2008;46:241–249. doi: 10.1080/13693780701824411. [DOI] [PubMed] [Google Scholar]
- 18.Boyum A. Scand J Clin Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- 19.Maqbool M, Vidyadaran S, George E, Ramasamy R. Med J Malaysia. 2011;66:296–299. [PubMed] [Google Scholar]
- 20.Nauseef WM. Methods Mol Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- 21.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. J Biol Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 22.Green JN, Winterbourn CC, Hampton MB. Methods Mol Biol. 2007;412:319–332. doi: 10.1007/978-1-59745-467-4_21. [DOI] [PubMed] [Google Scholar]
- 23.Dahlgren C, Karlsson A, Bylund J. Methods Mol Biol. 2007;412:349–363. doi: 10.1007/978-1-59745-467-4_23. [DOI] [PubMed] [Google Scholar]
- 24.Cohen HJ, Chovaniec ME. J Clin Invest. 1978;61:1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahlgren C, Stendahl O. Infect Immun. 1983;39:736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajecky M, Lojek A, Ciz M. Int J Lab Hematol. 2012;34:136–142. doi: 10.1111/j.1751-553X.2011.01370.x. [DOI] [PubMed] [Google Scholar]
- 27.Jancinova V, Drabikova K, Nosal R, Rackova L, Majekova M, Holomanova D. Redox Rep. 2006;11:110–116. doi: 10.1179/135100006X116592. [DOI] [PubMed] [Google Scholar]
- 28.Lundqvist H, Dahlgren C. Free Radic Biol Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 29.Lundqvist H, Kricka LJ, Stott RA, Thorpe GH, Dahlgren C. J Biolumin Chemilumin. 1995;10:353–359. doi: 10.1002/bio.1170100608. [DOI] [PubMed] [Google Scholar]
- 30.Sperandio FF, Huang YY, Hamblin MR. Recent Pat Antiinfect Drug Discov. 2013;8:108–120. doi: 10.2174/1574891x113089990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Wang M, Dai T, Sperandio FF, Huang YY, Xuan Y, Chiang LY, Hamblin MR. Nanomedicine (Lond) 2014;9:253–266. doi: 10.2217/nnm.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Huang L, Sharma SK, Jeon S, Thota S, Sperandio FF, Nayka S, Chang J, Hamblin MR, Chiang LY. J Med Chem. 2012;55:4274–4285. doi: 10.1021/jm3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperandio FF, Sharma SK, Wang M, Jeon S, Huang YY, Dai T, Nayka S, de Sousa SC, Chiang LY, Hamblin MR. Nanomedicine. 2013;9:570–579. doi: 10.1016/j.nano.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L, Wang M, Sharma SK, Sperandio FF, Maragani S, Nayka S, Chang J, Hamblin MR, Chiang LY. ECS Trans. 2013;45:1–11. doi: 10.1149/04520.0065ecst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soloviev DA, Jawhara S, Fonzi WA. Infect Immun. 2011;79:1546–1558. doi: 10.1128/IAI.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abelson JA, Moore T, Bruckner D, Deville J, Nielsen K. Pediatrics. 2005;116:61–67. doi: 10.1542/peds.2004-1605. [DOI] [PubMed] [Google Scholar]
- 37.Snydman DR. Chest. 2003;123:500S–503S. doi: 10.1378/chest.123.5_suppl.500s. [DOI] [PubMed] [Google Scholar]
- 38.Torres M, Hall FL, O’Neill K. J Immunol. 1993;150:1563–1577. [PubMed] [Google Scholar]
- 39.Hidalgo MA, Carretta MD, Teuber SE, Zarate C, Carcamo L, Concha II, Burgos RA. J Immunol Res. 2015;2015:120348. doi: 10.1155/2015/120348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell BV, Tew DG, Jones OT, England PJ. Biochem J. 1993;290(1):41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Lima FM, Aimbire F, Miranda H, Vieira RP, de Oliveira AP, Albertini R. J Lasers Med Sci. 2014;5:63–70. [PMC free article] [PubMed] [Google Scholar]
- 42.Passarella S, Ostuni A, Atlante A, Quagliariello E. Biochem Biophys Res Commun. 1988;156:978–986. doi: 10.1016/s0006-291x(88)80940-9. [DOI] [PubMed] [Google Scholar]
- 43.Pastore D, Di Martino C, Bosco G, Passarella S. Biochem Mol Biol Int. 1996;39:149–157. doi: 10.1080/15216549600201151. [DOI] [PubMed] [Google Scholar]
- 44.Pastore D, Greco M, Passarella S. Int J Radiat Biol. 2000;76:863–870. doi: 10.1080/09553000050029020. [DOI] [PubMed] [Google Scholar]
- 45.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Proc Natl Acad Sci USA. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novoselova EG, Glushkova OV, Cherenkov DA, Chudnovsky VM, Fesenko EE. Photodermatol Photoimmunol Photomed. 2006;22:33–38. doi: 10.1111/j.1600-0781.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 47.Mamalis A, Garcha M, Jagdeo J. Lasers Surg Med. 2015;47:210–215. doi: 10.1002/lsm.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kushibiki T, Hirasawa T, Okawa S, Ishihara M. Photomed Laser Surg. 2013;31:95–104. doi: 10.1089/pho.2012.3361. [DOI] [PubMed] [Google Scholar]
- 49.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. J Clin Microbiol. 2005;43:5425–5427. doi: 10.1128/JCM.43.11.5425-5427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavanti A, Davidson AD, Fordyce MJ, Gow NA, Maiden MC, Odds FC. J Clin Microbiol. 2005;43:5601–5613. doi: 10.1128/JCM.43.11.5601-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]