Abstract
The ability to regulate emotion is crucial to promote well-being. Evidence suggests that the medial prefrontal cortex (mPFC) and adjacent anterior cingulate (ACC) modulate amygdala activity during emotion regulation. Yet less is known about whether the amygdala-mPFC circuit is linked with regulation of the autonomic nervous system and whether the relationship differs across the adult lifespan. The current study tested the hypothesis that heart rate variability (HRV) reflects the strength of mPFC-amygdala interaction across younger and older adults. We recorded participants’ heart rates at baseline and examined whether baseline HRV was associated with amygdala-mPFC functional connectivity during rest. We found that higher HRV was associated with stronger functional connectivity between the amygdala and the mPFC during rest across younger and older adults. In addition to this age-invariant pattern, there was an age-related change, such that greater HRV was linked with stronger functional connectivity between amygdala and ventrolateral PFC (vlPFC) in younger than in older adults. These results are in line with past evidence that vlPFC is involved in emotion regulation especially in younger adults. Taken together, our results support the neurovisceral integration model and suggest that higher heart rate variability is associated with neural mechanisms that support successful emotional regulation across the adult lifespan.
Keywords: heart rate variability, emotion regulation, ageing, functional connectivity, amygdala, mPFC
1. Introduction
Encountering emotionally arousing information changes not only subjective emotional states, but also physiological arousal reactions via the autonomic nervous system. For instance, as physiological arousal increases, activity of the sympathetic nervous system becomes dominant, which results in increased heart rates. In contrast, when individuals are relaxed, the vagal (parasympathetic) nervous system is dominant and maintains a decreased heart rate. Given the link between subjective and physiological states, successful emotion regulation should involve regulation of both subjective emotional feelings and physiological states.
Previous research suggests that heart rate variability (HRV) provides an index of how strongly individuals can regulate emotion and autonomic responses in the body (for a review see Appelhans and Luecken, 2006). For example, Thayer and colleagues proposed the neurovisceral integration model and argue that HRV reflects the activity of an integrative neural network which flexibly regulates physiological, cognitive, and emotional responses (Thayer and Lane, 2000, 2009). In this model, cortical and subcortical regions in the brain interact with each other, allowing the prefrontal cortex (PFC) to exert inhibitory control over subcortical regions and the autonomic nervous system. The model also posits that HRV at rest reflects the PFC’s ability to inhibit subcortical circuits; thus higher levels of resting HRV are linked with better emotion regulation (Williams et al., 2015).
Consistent with this model, lower levels of HRV at baseline are associated with a variety of psychopathological symptoms, such as anxiety disorders (Chalmers et al., 2014; Friedman, 2007; Kemp et al., 2014), depression (Kemp et al., 2010), chronic alcohol abuse (Ingjaldsson et al., 2003), and post-traumatic stress disorder (Cohen et al., 2000). Individuals with low resting HRV also show lower marriage quality (Smith et al., 2011) and exaggerated startle responses to the threat of shock (Melzig et al., 2009). In addition, phasic HRV reactions induced by stimuli increase during successful emotion regulation (Butler et al., 2006; Ingjaldsson et al., 2003).
Neuroimaging research provides further evidence for the association between HRV and emotion regulation (e.g., Matthews et al., 2004; for a review see Thayer et al., 2012). Emotion regulation is known to rely on the interaction between the medial prefrontal cortex (mPFC) and the amygdala. Substantial evidence indicates that the mPFC and adjacent anterior cingulate cortex (ACC) are involved in down-regulation of the amygdala (for a review see Etkin et al., 2011). Thus, according to the neurovisceral integration model, HRV should be associated with ACC/mPFC function (Thayer et al., 2012). Indeed, increases in HRV are accompanied by increased dorsal ACC activity during performance of cognitive and motor tasks (Critchley et al., 2003). MPFC activity and HRV fluctuation were also positively correlated while people watched emotional clips (Lane et al., 2009). Furthermore, individuals with social phobia exhibited a positive correlation between HRV and ACC activity after completing a stressful public speech task (Åhs et al., 2009). Recent research also reveals that temporal fluctuations in HRV levels are positively correlated with functional connectivity strength between amygdala and dorsal ACC (Chang et al., 2013; see also Smith et al., 2015).
However, since most previous neuroimaging work is based on younger adults, it’s unclear whether HRV is related to the mPFC-amygdala circuit in older adults. Normal aging is linked with a decline in cardiovascular control (Colosimo et al., 1997) and substantial research shows that HRV decreases with age (Agelink et al., 2001; Bonnemeier et al., 2003; O’Brien et al., 1986; Russoniello et al., 2013; Santillo et al., 2012; Schwartz et al., 1991; Wrzus et al., 2013). The age-related reduction in HRV has been observed not only in cross-sectional comparisons but also in a longitudinal study (Sinnreich et al., 1998). But the steady decline of HRV with age does not necessarily mean that the relationship between emotion regulation and HRV changes with age. Indeed, one study which tested participants aged 20–59 found that high HRV is linked with low anxiety irrespective of age (Dishman et al., 2000). Another study also reported that older adults were able to regulate negative emotion more easily in simple situations than in complex situations; and simple situations induced higher HRV reactions relative to complex situations in older adults (Wrzus et al., 2013). These results suggest that high HRV is linked with successful emotion regulation even in older adults.
In addition, ventromedial PFC brain regions involved in emotion regulation are relatively preserved in normal aging, despite marked decline in dorsal and lateral regions (for reviews see Mather, 2012, 2016). Older adults tend to recruit the mPFC spontaneously when presented with negative stimuli (for a review see Nashiro et al., 2012), and the spontaneous mPFC activity is linked with reduced subjective negative emotional responses to stimuli in older adults (Dolcos et al., 2014). In studies that compare younger and older adults’ responses to positive versus negative stimuli, regions within mPFC show an age by valence interaction, typically with more activity in response to positive than negative stimuli in younger adults and vice versa in older adults (Leclerc and Kensinger, 2008, 2010, 2011). In older adults, mPFC activity during processing emotional stimuli has been linked with having more of a bias towards positive than negative stimuli in memory (Leclerc and Kensinger, 2011; Sakaki et al., 2013) and both mPFC and ACC activity during emotional tasks have been linked with better profiles of diurnal cortisol response (Urry et al., 2006) and better emotional stability (Brassen et al., 2011; Williams et al., 2006).
In contrast, there are some indications that, consistent with the greater structural decline seen in lateral PFC (Fjell et al., 2009), lateral PFC regions are less involved in emotion regulation among older adults than among younger adults. For instance, in fMRI studies in which participants are instructed to reappraise stimuli, there are many overlapping areas of activation among the two age groups, but the age differences seen thus far have been in dorsal and lateral PFC, where older adults show less activation during reappraisal than younger adults do (Allard and Kensinger, 2014; Opitz et al., 2012; Winecoff et al., 2011).
In the current study, we addressed the relationship between resting HRV and the amygdala-PFC connectivity across younger and older adults. Based on past findings reviewed above, we hypothesized that HRV levels at rest would be associated with stronger amygdala-mPFC coupling across the adult lifespan–even in older adults. While most neuroimaging studies on HRV examined the effects of concurrent HRV fluctuations rather than the effects of baseline HRV levels, according to the neurovisceral integration model, HRV at rest is also an important index of the prefrontal inhibitory mechanisms. Thus, we examined whether HRV levels at rest were associated with functional connectivity between the amygdala and mPFC across younger and older adults. During the study, we first obtained a baseline HRV assessment before scanning participants. Participants then completed a resting-state scan that provided us with the functional connectivity measure between the amygdala and mPFC.
We first took a region-of-interest (ROI) approach, focusing on the connectivity between right amygdala and mPFC. The amygdala was defined anatomically. Since mPFC is relatively large, it was defined based on a prior study on the same sample (Sakaki et al., 2013). In this prior study, stronger connectivity between mPFC and right amygdala during rest was predictive of subsequent memory positivity in older adults, suggesting the connectivity was associated with selective attention and memory processes contributing to emotion regulation (Carstensen, 1992; Mather and Carstensen, 2005). We predicted that higher HRV levels at baseline would predict stronger functional connectivity between this mPFC sub-region and right amygdala irrespective of age.
Second, we performed a whole-brain analysis to examine age-related similarity and differences in a neural network relevant to HRV. While mPFC has been implicated in emotion regulation, the mPFC ROI in the current study was defined based on previous findings on older adults’ memory positivity; thus it was not entirely clear whether this region would be related to emotion regulation in younger adults. Furthermore, based on the results from the prior study (Sakaki et al., 2013), our ROI analysis focused on the right amygdala’s connectivity. Yet previous research on emotion regulation suggests that emotion regulation is related to the PFC’s regulation not only of the right amygdala but also of the left amygdala (Urry et al., 2006; Winecoff et al., 2011), indicating the importance of the functional connectivity of the bilateral amygdalae in emotion regulation. In addition, past research on emotion regulation revealed enhanced activity not only in mPFC but also in ventrolateral PFC (vlPFC) during successful emotion regulation especially in younger adults compared with older adults (Opitz et al., 2012; Winecoff et al., 2011). To probe the potential age-related differences in the neural network relevant to HRV, a whole-brain functional connectivity analysis was performed using the left and right amygdalae as seed regions. Based on the previous findings of age-related differences in lateral brain regions related with emotion regulation, we expected that higher HRV levels at baseline would be associated with stronger functional connectivity between the amygdala and vlPFC in younger than in older adults.
2. Materials and Methods
2.1. Participants
Participants included 21 older adults (10 males; age range = 61–78) and 20 younger adults (12 males; age range = 19–37). Data from four of these participants were excluded before any analyses: one older adult due to a prior stroke identified by a neuroradiologist who reviewed all structural scans for incidental findings and three younger adults who did not provide the resting scan on the session day. In addition, two older adults were identified as outliers: according to Tukey’s (1977) test at the 3 interquartile range criterion, an HRV value from one older adult was identified as an outlier and another older adult was identified as an outlier with the same criterion applied to the amygdala-mPFC connectivity ROI signal. These two outliers were excluded from our main analyses reported in the paper (see Supplementary Results for results when the outliers were included: S-Tables 1–3, S-Figures 3 & 4), resulting in 18 older adults (9 males, age range=61–78 years; 5 African American, 2 Asian, 8 Caucasian and 3 other) and 17 younger adults (9 males, age range 19–37 years; 8 Asian, 7 Caucasian, 5 other).
2.2. Procedures
After participants gave informed consent, they completed questionnaires about their subjective emotional states, including the state positive affect and negative affect scale (PANAS; Watson et al., 1988) and the CES-D scale for depression (Radloff, 1977). Participants also completed the Wechsler Test of Adult Reading (WTAR: Wechsler, 2001) to provide a measure of verbal intelligence. They then entered the scanner. The experimenters attached electrodes to monitor participants’ heart rates. Participants lay quietly during a prescan (the mean duration = 3 minutes), which provided a baseline measure of heart rate variability. Next, resting fMRI BOLD (blood oxygenation level-dependent) data were acquired. The resting fMRI scan lasted 5.2 min. Participants were told to stay still with their eyes closed, not to think anything particular, and not to fall asleep.1
2.3. FMRI data acquisition and preprocessing
MRI scanning was performed on a 3.0-T Siemens MAGNETOM Trio scanner with a 12-channel matrix head coil at the University of Southern California Dana and David Dornsife Neuroimaging Center. The imaging parameters were TR = 2000 ms, TE = 25 ms, slice thickness = 3 mm, interslice gap = 0 mm, and FA = 90°. Data preprocessing were performed using FMRIB’s Software Library (FSL; www.fmrib.ox.ac.uk/fsl), including skull stripping of structural images with BET, motion correction with MCFLIRT, and smoothing with full-width half-maximum 5 mm. Noise components were identified using MELODIC Independent Component Analysis (ICA Beckmann and Smith, 2004) and removed. Registration was performed with FLIRT; each functional image was registered to the participant’s high-resolution brain-extracted structural image and the standard Montreal Neurological Institute (MNI) 2-mm brain. We applied both high- (Gaussian-weighted least-squares straight line fitting, with sigma=50 sec) and low- (Gaussian low-pass temporal filtering with a HWHM=2.8 sec) pass temporal filters to the functional data from the resting scan (Roy et al., 2009).
2.4. HRV collection and analysis
The electrocardiogram (ECG) was obtained by BIOPAC MP150 data acquisition system at the University of Southern California Dana and David Dornsife Neuroimaging Center with three leads. A LEAD108 setup with EL508 MRI-compatible/radio translucent electrodes was used for recording. The raw signal was 0.05–35 Hz bandpass-filtered and amplified using an ECG100C amplifier. The signal was continuously digitized at a sampling rate of 4000 Hz. During the resting scan, we also monitored participants’ respiratory signals using a respiratory belt in the BIOPAC system.
The recorded ECG data were processed with Acqknowledge software (Biopac systems Inc.) for noise reduction. Because the ECG was recorded in the MRI scanner, the recorded ECG signal included noise signals from the scanner’s strong static magnetic field (Dietrich et al., 2008; Tse et al., 2014). Therefore, we performed noise removal through the following three steps. First, a comb band stop filter was applied to filter out the MRI fundamental frequency. Second, an ICA was performed to separate the ECG signal, respiration and scanner signal. Third, the data were transformed by applying the template-correlation function in Acqknowledge.
Heart rate and heart rate variability calculations were then performed on the corrected data using Kubios version 2.2 (Tarvainen et al., 2014). Inter-beat-intervals (RR intervals) were derived from the ECG signal and checked for artifacts and corrected. The time domain analyses led to estimates of the root mean square successive differences (RMSSD in ms: see Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, 1996). In the current study, RMSSD was used as an index of HRV (see Supplemental Results for results from other measures).
2.5. Functional connectivity analysis: ROI analysis
Based on our hypothesis, we first focused on the resting-state connectivity between amygdala and mPFC. Our ROIs were right amygdala and a region within mPFC; they were determined based on a paper published previously on the same participant sample (Sakaki et al., 2013). The right amygdala was anatomically defined in each participant’s T1 image (for details see Sakaki et al., 2013). The mPFC area (Figure 1) was defined based on coordinates reported in the same study as a neural correlate of older adults’ memory positivity; from the significant cluster, we chose voxels only from ACC and paracingulate gyrus in the Harvard Oxford atlas with the probability of 25% (the number of voxels = 263; the center of the gravity: x = −1, y = 47, z = 8).
Figure 1.
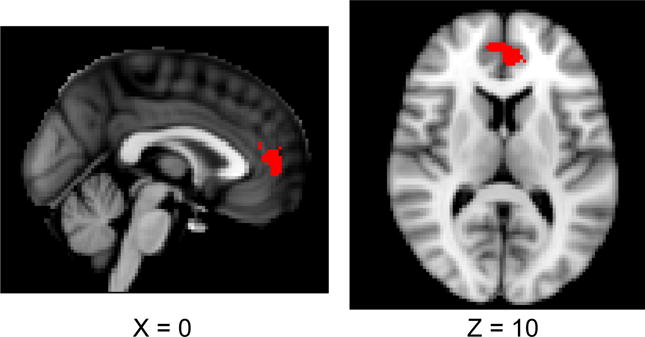
MPFC ROI cluster used in the ROI analysis.
To obtain the connectivity measure between the right amygdala and mPFC, we obtained mean time series from the right amygdala for each participant during the resting scan with an FSL command line tool called fslmeants. Using this amygdala time course, a multiple regression analysis was performed for each participant using FSL FEAT; the regression model included the amygdala time series, six motion parameters, global signal, signal from a ventricular ROI (number of voxels = 126; center of the cluster: x = +/− 12, y = −16, z = 21), and signal from a white-matter ROI (number of voxels = 54; center of the cluster: x = +/− 30, y = −18, z = 28; Zhang et al., 2008). A beta value was then extracted from the mPFC cluster in this amygdala connectivity map by FMRIB’s Featquery as an index of the connectivity strength between these two regions.2
2.6. Functional connectivity analysis: Whole brain analysis
Next, a whole-brain functional connectivity analysis was performed. The seed regions were left and right amygdala. Each individual’s amygdala was defined in his/her anatomical space.
For each seed region, we obtained mean time series from each participant during the resting scan by averaging across all voxels involved in the region. As in the ROI analysis, we then performed a multiple regression analysis for each participant for left and right amygdala separately using FSL FEAT; the regression model included the amygdala time series, six motion parameters, global signal, signal from a ventricular ROI, and signal from a white-matter ROI (Zhang et al., 2008). Group-level analyses were then conducted using FSL’s FEAT. The model included the age group (old vs. young), baseline HRV levels (continuous measures after centering), and the interaction between HRV and age group. We employed cluster-based corrections for multiple comparisons with Gaussian random field theory (Z = 2.3; cluster significance: p = .05-corrected). Locations reported by FSL were converted into Talairach coordinates by the MNI-to-Talairach transformation algorithm (Lancaster et al., 2007). These coordinates were used to provide labels of the nearest gray matter using the Talairach Daemon (Lancaster et al., 2000).
This whole brain analysis allowed us to test the age-by-HRV interaction but did not indicate the precise interaction pattern. To understand the interaction pattern and consider the potential effects of outliers, when we found significant clusters in areas where we had a-priori hypotheses (i.e., mPFC and vlPFC), we extracted beta values from these clusters by FMRIB’s Featquery. When clusters included the mPFC, we extracted the averaged beta values only from ACC and paracingulate gyrus in the Harvard Oxford atlas with the probability of 25%. For the vlPFC, the averaged beta values were extracted from significant voxels within inferior frontal gyrus, orbitofrontal cortex, and frontal operculum cortex in the Harvard Oxford atlas with the probability of 25%.
3. Results
Mean age, RR interval, RMSSD, mPFC-amygdala connectivity, and self-reported questionnaires by age group are reported in Table 1. The groups differed on age, F (1, 33) = 534.92, p < .01, d = 8.06, and RMSSD, F (1, 33) = 13.00, p < .01, d = 1.26. There was also a trend for group differences in RR interval, F (1, 33) = 3.88, p = .06, d = 69, and positive affect, F (1, 33) = 3.18, p = .08, d = 62. The two groups did not differ on connectivity (p = .38), the WTAR vocabulary score (p = .63), CES-D depression score (p = .32) and negative affect (p = .59). RMSSD was not significantly correlated with the vocabulary score, depression, positive and negative affect in either age group or across groups (ps > .16; Table 2).
Table 1.
Descriptive statistics for age, HRV, mPFC-amygdala connectivity and self-report questionnaires
| Young | Old | Cohen’s d | |
|---|---|---|---|
| age | 25.82 (5.63) |
68.22 (5.22) |
8.06* |
| RMSSD (ms) | 28.62 (10.83) |
17.68 (6.77) |
1.26* |
| RR interval (ms) | 848.21 (90.79) |
938.91 (168.06) |
0.69 |
| connectivity | 0.14 (0.30) |
0.09 (0.41) |
0.17 |
| WTAR | 42.88 (3.59) |
43.72 (6.61) |
0.16 |
| CES-D | 0.51 (0.36) |
0.39 (0.31) |
0.35 |
| Positive affect | 2.95 (0.67) |
3.48 (1.02) |
0.62 |
| Negative affect | 1.26 (0.36) |
1.20 (0.35) |
0.19 |
Note:
p < .05
Table 2.
Correlation with RMSSD in each age group and across groups (p-values in parentheses).
| Young | Old | Across groups | |
|---|---|---|---|
| Positive affect | .04 (.87) |
−.10 (.70) |
.18 (.31) |
| Negative affect | .16 (.53) |
−.10 (.70) |
.10 (.57) |
| Depression | .36 (.16) |
−.31 (.20) |
.18 (.29) |
| WTAR | .10 (.70) |
−.20 (.44) |
−.09 (.61) |
3.1. ROI analysis on relationship between HRV and amygdala-MPFC connectivity
To examine whether RMSSD was related to connectivity strength between mPFC and right amygdala obtained by the ROI approach, we employed a General Linear Model (GLM) analysis on the mPFC-amygdala connectivity strength. Independent variables were RMSSD, age group and an interaction between RMSSD and age group. This analysis revealed a significant main effect of RMSSD, F (1, 31) = 8.02, p < .01, η2 = .17, suggesting that greater RMSSD is associated with stronger connectivity between MPFC and amygdala (Figure 2). Neither the main effect of age group (p = .97), nor the age-by-RMSSD interaction was significant (p = .64). Indeed, RMSSD was positively correlated with the amygdala-MPFC connectivity both in younger (r = .52, p < .05) and older adults (r = .42, p < .05). These results suggest that RMSSD predicts mPFC-amygdala connectivity similarly across the two age groups.
Figure 2.
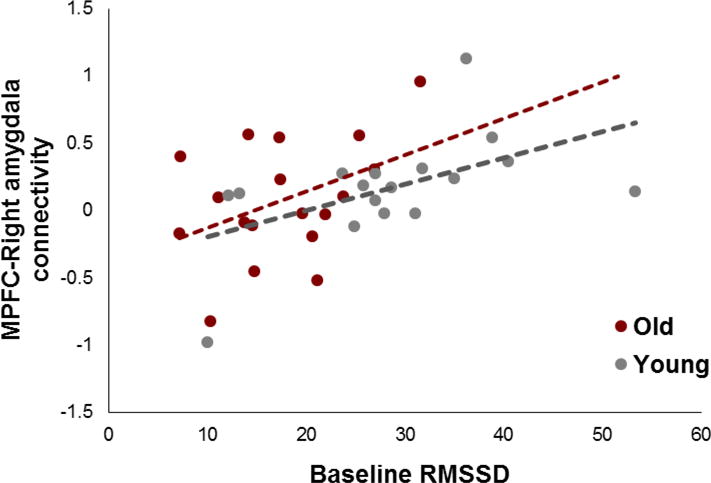
Effects of RMSSD on beta values for the amygdala-MPFC connectivity during rest.
3.2. Whole brain analysis
The whole brain connectivity analysis of the right amygdala replicated results of the ROI analysis: across age groups, higher HRV levels at rest were associated with stronger functional coupling between right amygdala and mPFC/ACC (Figure 3A); this mPFC/ACC area overlapped with the one used in the ROI analysis. Beta values extracted from the mPFC area are plotted in Figure 3D, suggesting a similar pattern to the previous ROI analysis. In addition, there was a significant interaction between age and HRV in vlPFC (Figure 3B), such that higher HRV levels were associated with stronger connectivity between right amygdala and vlPFC in younger adults but not in older adults (Figure 3E). Other areas that showed significant results were indicated in Table 3 and S-Figure 1 in the Supplementary Materials.
Figure 3.
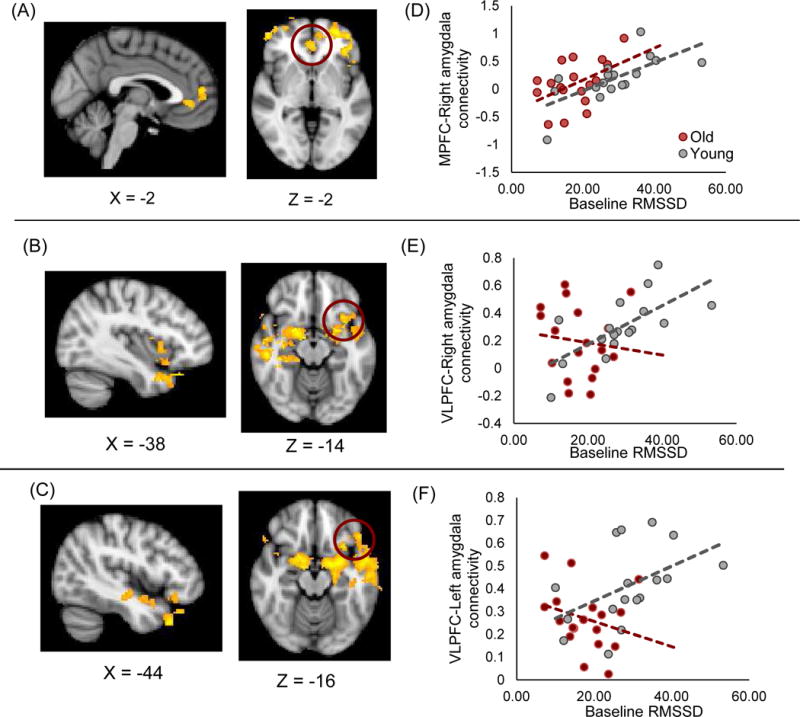
(A) Across age groups, MPFC/ACC showed stronger connectivity with the right amygdala as HRV levels increased. (B) Higher HRV was associated with stronger connectivity between right amygdala and vlPFC in younger than older adults. (C) The same interaction was observed with the left amygdala. Beta values extracted from the circled area plotted on the right side (D, E and F).
Table 3.
Brain regions and local maxima for the whole brain connectivity with the right amygdala (MNI coordinates)
| Area | H | BA | x | y | z | Z stat | |
|---|---|---|---|---|---|---|---|
| HRV+ (across age) | |||||||
| Superior Frontal Gyrus | L | 9 | −24 | 48 | 22 | 3.82 | |
| L | 10 | −26 | 62 | 8 | 3.51 | ||
| Middle Frontal Gyrus | L | 10 | −28 | 56 | −2 | 3.73 | |
| L | 8 | −30 | 48 | 36 | 3.67 | ||
| L | 47 | −46 | 40 | −12 | 3.54 | ||
| R | 46 | −50 | 48 | 18 | 3.49 | ||
|
|
|||||||
| Superior Frontal Gyrus | R | 8 | 26 | 46 | 42 | 4.65 | |
| R | 10 | 42 | 60 | 2 | 3.98 | ||
| R | 8 | 24 | 54 | 34 | 3.75 | ||
| R | 10 | 30 | 60 | −14 | 3.51 | ||
| Superior Frontal Gyrus | R | 9 | 22 | 62 | 24 | 3.43 | |
| Middle Frontal Gyrus | R | 46 | 52 | 50 | −2 | 3.45 | |
|
| |||||||
| HRV− (across age) | |||||||
| Inferior Parietal Lobe | R | 40 | 40 | −36 | 56 | 4.92 | |
| R | 40 | 36 | −32 | 56 | 3.36 | ||
| Precentral Gyrus | R | 6 | 38 | −10 | 64 | 4.07 | |
| R | 4 | 28 | −14 | 72 | 3.9 | ||
| R | 6 | 28 | −10 | 62 | 3.79 | ||
| R | 4 | 40 | −6 | 56 | 3.6 | ||
|
| |||||||
| HRV+: Young > Old | |||||||
| Globus Pallidus | R | 16 | −2 | −10 | 5.37 | ||
| Hyothalamus | R | 12 | −6 | −14 | 4.71 | ||
| Superior Temporal Gyrus | R | 22 | 52 | −26 | −12 | 4.75 | |
| R | 38 | 28 | 16 | −36 | 4.6 | ||
| R | 38 | 34 | 22 | −42 | 4.46 | ||
| Parahippocampal Gyrus | R | 28 | 20 | −24 | −22 | 4.44 | |
|
|
|||||||
| Inferior Frontal Gyrus | L | 13 | −30 | 6 | −16 | 4.14 | |
| Insula | L | 13 | −32 | 16 | −16 | 4.02 | |
| Insula | L | 13 | −34 | 12 | −14 | 3.96 | |
| Superior Temporal Gyrus | L | 38 | −42 | 20 | −32 | 3.94 | |
| L | 38 | −28 | 14 | −44 | 3.92 | ||
| Parahippocampal Gyrus | L | 34 | −24 | 2 | −14 | 3.84 | |
|
|
|||||||
| Cingulate Gyrus | R | 24 | 14 | −6 | 38 | 3.8 | |
| R | 24 | 10 | 0 | 36 | 3.52 | ||
| R | 23 | 10 | −22 | 36 | 3.41 | ||
| R | 24 | 6 | 6 | 40 | 2.96 | ||
| L | 23 | 0 | −16 | 30 | 3.51 | ||
| L | 24 | 0 | 12 | 30 | 3.14 | ||
|
| |||||||
| HRV+: Old > Young | |||||||
| Superior Parietal Lobe | L | 7 | −32 | −66 | 54 | 4.59 | |
| Cuneus | L | 19 | −22 | −88 | 42 | 4.56 | |
| Precuneus | L | 19 | −8 | −84 | 48 | 4.24 | |
| L | 7 | −2 | −76 | 46 | 4.22 | ||
| L | 19 | −16 | −80 | 50 | 4.18 | ||
| L | 19 | −26 | −76 | 50 | 4.02 | ||
Next, we performed a similar analysis with the left amygdala. As seen in the right amygdala, there was a significant interaction between HRV and age in left vlPFC (Figure 3C): higher HRV levels were associated with stronger functional coupling between vlPFC-amygdala in younger than in older adults (Figure 3F). Thus, while the results were weaker in the left amygdala connectivity than the right amygdala, it appears that the HRV is relevant for not only the right amygdala’s functional network for emotion regulation but also the left amygdala’s network for emotion regulation. Other areas that showed significant results were indicated in Table 4 and S-Figure 2 in the Supplementary Materials.
Table 4.
Brain regions and local maxima for the whole brain connectivity analysis with the left amygdala (MNI coordinates)
| Area | H | BA | x | y | z | Z stat | |
|---|---|---|---|---|---|---|---|
| HRV+ (across age) | |||||||
| No significant results | |||||||
|
| |||||||
| HRV− (across age) | |||||||
| Cerebellum | L | −2 | −68 | 4 | 4.49 | ||
| L | −10 | −54 | −10 | 3.88 | |||
| Cerebellum | R | 2 | −80 | −6 | 3.66 | ||
| Cuneus | L | 30 | −2 | −68 | 10 | 3.86 | |
| R | 30 | 2 | −68 | 10 | 3.75 | ||
| Lingul Gyrus | L | 18 | −22 | −76 | −2 | 3.8 | |
|
|
|||||||
| Precuneus | L | 7 | −12 | −64 | 58 | 3.46 | |
| L | 7 | −26 | −54 | 62 | 3.43 | ||
| Superior Parietal Lobe | L | 7 | −10 | −60 | 68 | 3.32 | |
| L | 7 | −12 | −64 | 68 | 3.27 | ||
| L | 7 | −12 | −68 | 68 | 3.24 | ||
| L | 7 | −36 | −48 | 68 | 3.19 | ||
|
| |||||||
| HRV+: Young > Old | |||||||
| Amygdala* | L | −28 | 0 | −14 | 4.65 | ||
| Parahippocampal Gyrus | L | 28 | −20 | −22 | −20 | 4.94 | |
| L | 34 | −24 | 0 | −14 | 4.7 | ||
| L | −16 | 0 | −16 | 4.75 | |||
| Superior Temporal Gyrus | L | 38 | −48 | 24 | −34 | 4.71 | |
| Inferior Frontal Gyrus | L | 47 | −38 | 24 | −26 | 4.11 | |
|
|
|||||||
| Superior Temporal Gyrus | L | 38 | −54 | 6 | −18 | 4.12 | |
| L | 38 | −52 | −2 | −16 | 3.7 | ||
| Middle Temporal Gyrus | L | 21 | −60 | 2 | −20 | 4.05 | |
| L | 21 | −58 | −14 | −14 | 3.61 | ||
| L | 21 | −66 | −6 | −14 | 3.55 | ||
| Inferior Frontal Gyrus | L | 47 | −48 | 28 | −16 | 3.46 | |
|
|
|||||||
| Putamen | R | 20 | 4 | −14 | 4.76 | ||
| Parahippocampal Gyrus | R | 34 | 16 | 4 | −16 | 4.37 | |
| R | 34 | 12 | −6 | −18 | 4.16 | ||
| Inferior Frontal Gyrus | R | 47 | 40 | 30 | −26 | 4.32 | |
| R | 47 | 36 | 14 | −26 | 4.06 | ||
|
|
|||||||
| Inferior Temporal Gyrus | L | 37 | −64 | −58 | −6 | 3.95 | |
| Middle Temporal Gyrus | L | 22 | −60 | −46 | 4 | 3.85 | |
| L | 37 | −64 | −62 | 2 | 3.66 | ||
| Superior Temporal Gyrus | L | 22 | −56 | −48 | 10 | 3.53 | |
| L | 22 | −70 | −16 | 2 | 3.47 | ||
| L | 22 | −62 | −24 | 6 | 3.44 | ||
|
|
|||||||
| Cingulate Gyrus | R | 23 | 4 | −16 | 32 | 4.6 | |
| R | 24 | 10 | −12 | 36 | 3.55 | ||
| R | 31 | 12 | −32 | 48 | 3.48 | ||
| R | 24 | 22 | −12 | 38 | 3.25 | ||
| R | 24 | 12 | 0 | 38 | 3.13 | ||
| R | 24 | 28 | −16 | 40 | 2.97 | ||
|
| |||||||
| HRV+: Old > Young | |||||||
| Precentral Gyrus | L | 6 | −64 | 6 | 28 | 4.33 | |
| L | 6 | −52 | 0 | 24 | 3.97 | ||
| L | 6 | −44 | −10 | 26 | 3.85 | ||
| L | 6 | −44 | −4 | 24 | 3.68 | ||
| L | 6 | −66 | 2 | 28 | 3.5 | ||
|
|
|||||||
| Middle Frontal Gyrus | R | 8 | 44 | 34 | 34 | 3.62 | |
| R | 9 | 38 | 38 | 30 | 3.4 | ||
| Medial Frontal Gyrus | R | 32 | 14 | 18 | 42 | 3.43 | |
| Precentral Gyrus | R | 9 | 38 | 28 | 30 | 3.38 | |
| Superior Frontal Gyrus | R | 8 | 22 | 36 | 42 | 3.41 | |
| R | 6 | 20 | 20 | 48 | 3.3 | ||
|
|
|||||||
| Inferior Parietal Lobe | L | 40 | −48 | −56 | 40 | 3.99 | |
| Superior Temporal Gyrus | L | 39 | −50 | −56 | 34 | 3.67 | |
| Supramarginal Gyrus | L | 40 | −44 | −46 | 34 | 3.21 | |
| Inferior Parietal Lobe | L | 40 | −40 | −46 | 44 | 3.1 | |
| Middle Temporal Gyrus | L | 39 | −40 | −64 | 34 | 3.45 | |
| L | 30 | −34 | −70 | 34 | 2.97 | ||
4. Discussion
The neurovisceral integration model suggests that HRV at rest is linked with one’s emotion regulation ability (Thayer et al., 2012; Thayer and Lane, 2009). Based on this model, the current paper addressed the hypothesis that higher baseline HRV would be associated with stronger functional coupling between the amygdala and mPFC across younger and older adults. In line with the hypothesis, we found that higher HRV at rest was associated with stronger connectivity between the right amygdala and mPFC during rest irrespective of age. A subsequent whole-brain analysis also confirmed the same pattern. Given the importance of mPFC in emotion regulation both in younger and older adults (Goldin et al., 2008; Urry et al., 2006; van Reekum et al., 2007), our results suggest that resting HRV is linked with the brain mechanisms underlying emotion regulation irrespective of age. Interestingly, a recent study identified a very similar mPFC region, common to both the default mode network and the salience network, as being associated with HRV (Jennings et al., 2015). These authors suggested that this region may help coordinate autonomic cardic control and the engagement and disengagement with the environment. In fact, a similar mPFC region was also implicated in the modulation of heart rates (Wong et al., 2007). Taken together these studies support the idea that HRV may index a set of neural structures important for producing context appropriate responses to environmental challenges and thus to self-regulation including emotion regulation.
In addition to the age-invariant patterns, we also found age-related changes in the amygdala’s functional connectivity associated with HRV. Functional connectivity between the amygdalae and vlPFC was more strongly correlated with HRV levels in younger adults than in older adults. In fact, previous research suggests that while neural mechanisms underlying emotion regulation are relatively preserved with normal aging, older adults show less vlPFC involvement during emotion regulation than do younger adults (e.g., Winecoff et al., 2011). It is also known that the lateral PFC declines more rapidly with normal aging compared with medial PFC (Fjell et al 2009). Our results are consistent with these previous notions and suggest that HRV at rest is related to the PFC’s top-down control of the amygdala connectivity both in younger and older adults.
Both animal and human studies support the idea that the amygdala and the mPFC are structurally as well as functionally connected (Kim and Whalen, 2009; Thayer, 2006). In addition, research suggests that this connectivity is critical in emotion regulation, such that greater connection strength between these two areas allows for greater prefrontal inhibition of amygdala activity during emotion regulation (e.g., Urry et al., 2006). The present finding that greater amygdala-mPFC connectivity was associated with greater resting HRV suggests that resting HRV may reflect the efficiency of the prefrontal cortex to regulate amygdala activity in the service of emotion regulation. Thus resting HRV may reflect the degree to which this prefrontal-amygdala circuit can be recruited to produce context appropriate emotional responses (Thayer et al., 2012; Thayer and Lane, 2000).
Resting HRV has been repeatedly found to be associated with successful emotion regulation (for reviews see Appelhans and Luecken, 2006; Thayer et al., 2012; Thayer and Lane, 2000, 2009). For example, Butler et al. (2006) reported that greater resting HRV was associated with successful emotion regulation via either reappraisal or suppression. Ruiz-Padial and colleagues also reported that higher resting HRV was associated with more context appropriate emotion-modulated startle responses (Ruiz-Padial et al., 2003; Ruiz-Padial and Thayer, 2014). In addition, Williams et al. (2015) demonstrated that greater resting HRV was associated with fewer difficulties with the regulation of everyday emotions. Similarly, connectivity of the amygdala and the prefrontal cortex has been associated with successful emotion regulation in many studies (for a review see Kim et al., 2011b). Moreover, we have recently reported that task-related HRV was associated with task-related connectivity between the mPFC and the dorsal pons such that greater resting HRV was associated with greater connectivity (Smith et al., 2015). The current results extend these previous findings by showing that HRV is linked with the brain mechanisms for emotion regulation irrespective of age groups.
Previous studies have found that state and trait anxiety were negatively associated with greater amygdala-mPFC resting state connectivity (Kim et al., 2011a) and that lower anxiety was associated with greater amygdala-mPFC connectivity when viewing fearful stimuli (Pezawas et al., 2005). Likewise, depression is associated with reduced amygdala-mPFC connectivity (Anand et al., 2009) and greater resting state amygdala-mPFC connectivity has been associated with better treatment response in depression (Dichter et al., 2015). As discussed earlier, lower resting HRV has been associated with depression (Kemp et al., 2010), as well as state and trait anxiety (Kemp et al., 2014; Thayer et al., 1996). The present results suggest that these past findings may be related, such that greater resting state connectivity between the amygdala and the mPFC is associated with greater resting HRV, providing a functional basis for the association of lower anxiety/depression with greater resting HRV. However, compared with studies of task related connectivity (e.g., Ochsner and Gross, 2008; Wager et al., 2008; Winecoff et al., 2011), surprisingly few studies have examined resting state connectivity and emotion regulation. The present results suggest that future research in this area might prove fruitful.
The effects of age on the neural concomitants of emotion regulation have also been a topic of investigation. For example, during presentation of negative emotional stimuli, older adults spontaneously recruit the mPFC (Nashiro et al., 2012). Importantly it has been reported that, in addition to mPFC, younger adults also recruit vlPFC during emotion regulation whereas recruitment of vlPFC in older adults is reduced (Winecoff et al., 2011). Consistent with this finding we report an age-related differential association of vlPFC-amygdala connectivity with HRV in younger versus older adults. Specifically, in addition to the age invariant mPFC-amygdala connectivity association with HRV we also found that younger adults showed a vlPFC-amygdala connectivity association with HRV that was absent in the older adults. These findings suggest that more cognitive control regions are recruited and linked to HRV in younger adults than in older adults.
In a prior study with the same sample (Sakaki et al., 2013), the participants with higher levels of amygdala-mPFC connectivity during rest (the participants shown here to also have higher HRV) had stronger mPFC recruitment when younger and older participants saw negative stimuli. Importantly, in this prior study, participants were not explicitly asked to regulate emotion. Thus, it appears that individuals with higher HRV tend to spontaneously recruit the emotion regulation network when exposed to negative stimuli irrespective of age. However, the recruitment of the emotion regulation network could result in different outcomes across age groups. The results from the current study suggest that relative to younger adults, older adults rely less on emotion regulation strategies that depend on the vlPFC. In fact, recent research shows that younger adults with higher emotion regulation self-efficacy tend to use reappraisal (Tamir et al., 2007) which is demanding and requires lateral PFC (Gyurak et al., 2011), whereas older adults with higher emotion regulation self-efficacy tend to use less effortful emotion regulation strategies, such as situation selection (Rovenpor et al., 2013). Further studies are needed to more fully explore these possibilities.
There are several important limitations to the present study that should be noted. First, there are documented gender differences in both emotion regulation and HRV (Snieder et al., 2007; Wager et al., 2003). Despite the present groups being relatively balanced with respect to gender, the current sample size was not sufficient to allow the examination of potential gender differences in this study. Similarly, there are documented ethnic differences in HRV (Hill et al., 2015) and the neural correlates of HRV (Allen et al., 2015). Again the current sample size was not sufficient to allow examination of potential ethnic differences in the present study. Furthermore, in the current study, we did not observe any significant correlations between HRV and self-reported measures related to wellbeing, such as depression or positive affect. Future studies with larger sample sizes are needed to more fully investigate such potential gender, ethnic and other individual difference relationships.
5. Conclusions
In conclusion, this is the first study to demonstrate the association between resting HRV and resting state connectivity between the prefrontal cortex and the amygdala. Consistent with the importance of the mPFC in emotion regulation across age groups, the medial PFC and amygdala connectivity association with resting HRV was found to be largely age invariant. In addition, in line with the age-related decline in the lateral PFC, the lateral PFC and amygdala connectivity association with resting HRV was observed only in younger adults. These results support the idea, proposed by the Neurovisceral Integration model, that prefrontal-amygdala connectivity should be related to resting HRV and suggest that resting HRV reflects the function of emotion-regulation circuit irrespective of age.
Supplementary Material
Highlights.
Revealed a link between HRV and a brain circuit underlying emotion regulation.
The sample included both healthy younger and older adults.
Age-related changes and age-invariant patterns were revealed.
In both groups, higher HRV correlated with more amygdala-MPFC functional connectivity.
But HRV was linked with stronger amygdala-vlPFC connectivity only in younger adults.
Acknowledgments
This work was supported by grants from the National Institute on Aging (RO1AG025340) and from the European Commission (FP7-PEOPLE-2013-CIG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We did not obtain the ECG data outside the scanner due to the time constrains and to prevent participants’ fatigue given that all participants needed to complete two scanning sessions each of which took approx. 2 hours (Sakaki et al., 2013). However, in addition to the baseline period in the scanner before scanning, we also recorded the ECG data during the resting scan. However, during the resting scan, there were additional scanner-related artifacts (Dietrich et al., 2008; Tse et al., 2014) and we were not able to effectively remove artifacts by the described methods. Among 37 participants who provided ECG data, we were able to obtain RMSSD values only from 23 participants when we looked at the ECG data during the resting scan; this sample size was not sufficient to address the age-related difference in relationships between HRV and resting-state functional connectivity. In addition, the RMSSD value during the resting-state scan was not significantly correlated with the RMSSD value during baseline among these 23 participants (r = .32, p = .15). Therefore we focused on the ECG data during baseline in the current study.
Recent research suggests that regressing out a global signal and other signals correlated with a global signal (e.g., white matter signal) can alter the functional connectivity results (Murphy et al., 2009; Saad et al., 2012). However, in our data, removing these regressors (i.e., global signal, signal from a ventricular ROI, and signal from a white-matter ROI) from the model did not change the results either in the ROI analysis or in the whole brain analysis (see Supplemental Materials for the results without those regressors).
References
- Agelink M, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clinical Autonomic Research. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- Åhs F, Sollers JJ, III, Furmark T, Fredrikson M, Thayer JF. High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. NeuroImage. 2009;47:815–820. doi: 10.1016/j.neuroimage.2009.05.091. [DOI] [PubMed] [Google Scholar]
- Allard ES, Kensinger EA. Age-related differences in functional connectivity during cognitive emotion regulation. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2014 doi: 10.1093/geronb/gbu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Jennings JR, Gianaros PJ, Thayer JF, Manuck SB. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology. 2015;52:277–287. doi: 10.1111/psyp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Research: Neuroimaging. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10:229–240. [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. Medical Imaging, IEEE Transactions on. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bonnemeier H, Wiegand UKH, Brandes A, Kluge N, Katus HA, Richardt G, Potratz J. Circadian profile of cardiac autonomic nervous modulation in healthy subjects. Journal of Cardiovascular Electrophysiology. 2003;14:791–799. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- Brassen S, Gamer M, Buchel C. Anterior Cingulate Activation Is Related to a Positivity Bias and Emotional Stability in Successful Aging. Biological Psychiatry. 2011;70:131–137. doi: 10.1016/j.biopsych.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging. 1992;7:331–338. doi: 10.1037//0882-7974.7.3.331. [DOI] [PubMed] [Google Scholar]
- Chalmers J, Quintana DS, Abbott MJA, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Research. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- Colosimo A, Giuliani A, Mancini AM, Piccirillo G, Marigliano V. Estimating a cardiac age by means of heart rate variability. 1997 doi: 10.1152/ajpheart.1997.273.4.H1841. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. Journal of Affective Disorders. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich O, Reiser MF, Schoenberg SO. Artifacts in 3-T MRI: Physical background and reduction strategies. European Journal of Radiology. 2008;65:29–35. doi: 10.1016/j.ejrad.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. International Journal of Psychophysiology. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Dolcos S, Katsumi Y, Dixon RA. The role of arousal in the spontaneous regulation of emotions in healthy aging: An fMRI investigation. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The Neural Bases of Emotion Regulation: Reappraisal and Suppression of Negative Emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: A dual-process framework. Cognition & Emotion. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LK, Hu DD, Koenig J, Sollers J, III, Kapuku G, Wang X, Snieder H, Thayer JF. Ethnic Differences in Resting Heart Rate Variability: A Systematic Review and Meta-Analysis. Psychosomatic Medicine. 2015;77:16–25. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Sheu LK, Kuan DCH, Manuck SB, Gianaros PJ. Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology. 2015 doi: 10.1111/psyp.12586. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Brunoni AR, Santos IS, Nunes MA, Dantas EM, Figueiredo RCd, Pereira AC, Ribeiro ALP, Mill JG, Andreão RV, Thayer JF, Benseñor IM, Lotufo PA. Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: An ELSA-Brasil cohort baseline study. American Journal of Psychiatry. 2014;171:1328–1334. doi: 10.1176/appi.ajp.2014.13121605. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of Depression and Antidepressant Treatment on Heart Rate Variability: A Review and Meta-Analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex. 2011a;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research. 2011b;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The Structural Integrity of an Amygdala–Prefrontal Pathway Predicts Trait Anxiety. The Journal of Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBN-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Social Neuroscience. 2010;5:560–576. doi: 10.1080/17470910903512296. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Neural Processing of Emotional Pictures and Words: A Comparison of Young and Older Adults. Developmental Neuropsychology. 2011;36:519–538. doi: 10.1080/87565641.2010.549864. [DOI] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. The Affective Neuroscience of Aging. Annual Review of Psychology. 2016;67:213–238. doi: 10.1146/annurev-psych-122414-033540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: Implications for panic disorder. International Journal of Psychophysiology. 2009;71:109–117. doi: 10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation? Gerontology. 2012;58:156–163. doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. British Heart Journal. 1986;55:348–354. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz PC, Rauch LC, Terry DP, Urry HL. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging. 2012;33:645–655. doi: 10.1016/j.neurobiolaging.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rovenpor DR, Skogsberg NJ, Isaacowitz DM. The choices we make: An examination of situation selection in younger and older adults. Psychology & Aging. 2013;28:365–376. doi: 10.1037/a0030450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Padial E, Sollers JJ, Vila J, Thayer JF. The rhythm of the heart in the blink of an eye: Emotion-modulated startle magnitude covaries with heart rate variability. Psychophysiology. 2003;40:306–313. doi: 10.1111/1469-8986.00032. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Thayer JF. Resting heart rate variability and the startle reflex to briefly presented affective pictures. International Journal of Psychophysiology. 2014;94:329–335. doi: 10.1016/j.ijpsycho.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Russoniello CV, Zhirnov YN, Pougatchev VI, Gribkov EN. Heart rate variability and biological age: Implications for health and gaming. CyberPsychology, behavior & Social Networking. 2013;16:302–308. doi: 10.1089/cyber.2013.1505. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Nga L, Mather M. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. Journal of Cognitive Neuroscience. 2013;25:1206–1224. doi: 10.1162/jocn_a_00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santillo E, Migale M, Fallavollita L, Marini L, Balestrini F. Electrocardiographic analysis of heart rate variability in aging heart. In: Millis R, editor. Advances in Electrocardiograms - Methods and Analysis InTech. 2012. [Google Scholar]
- Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. Journal of Gerontology. 1991;46:M99–M106. doi: 10.1093/geronj/46.3.m99. [DOI] [PubMed] [Google Scholar]
- Sinnreich R, Kark JD, Friedlander Y, Sapoznikov D, Luria MH. Five minute recordings of heart rate variability for population studies: repeatability and age–sex characteristics. Heart. 1998;80:156–162. doi: 10.1136/hrt.80.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Allen JJB, Thayer JF, Lane RD. Altered functional connectivity between medial prefrontal cortex and the inferior brainstem in major depression during appraisal of subjective emotional responses: A preliminary study. Biological Psychology. 2015;108:13–24. doi: 10.1016/j.biopsycho.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Smith TW, Cribbet MR, Nealey-Moore JB, Uchino BN, Williams PG, MacKenzie J, Thayer JF. Matters of the Variable Heart: Respiratory Sinus Arrhythmia Response to Marital Interaction and Associations With Marital Quality. Journal of Personality & Social Psychology. 2011;100:103–119. doi: 10.1037/a0021136. [DOI] [PubMed] [Google Scholar]
- Snieder H, van Doornen LJP, Boomsma DI, Thayer JF. Sex Differences and Heritability of Two Indices of Heart Rate Dynamics: A Twin Study. Twin Research and Human Genetics. 2007;10:364–372. doi: 10.1375/twin.10.2.364. [DOI] [PubMed] [Google Scholar]
- Tamir M, John OP, Srivastava S, Gross JJ. Implicit Theories of Emotion: Affective and Social Outcomes Across a Major Life Transition. Journal of Personality & Social Psychology. 2007;92:731–744. doi: 10.1037/0022-3514.92.4.731. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-aho PO, Karjalainen PA. Kubios HRV – Heart rate variability analysis software. Computer Methods and Programs in Biomedicine. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Thayer JF. On the Importance of Inhibition: Central and Peripheral Manifestations of Nonlinear Inhibitory Processes in Neural Systems. Dose-Response. 2006;4 doi: 10.2203/dose-response.004.01.002.Thayer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tse ZTH, Dumoulin CL, Clifford GD, Schweitzer J, Qin L, Oster J, Jerosch-Herold M, Kwong RY, Michaud G, Stevenson WG, Schmidt EJ. A 1.5T MRI-conditional 12-lead electrocardiogram for MRI and intra-MR intervention. Magnetic Resonance in Medicine. 2014;71:1336–1347. doi: 10.1002/mrm.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Schaefer HS, Alexander AL, Davidson RJ. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, Thayer JF. Resting heart rate variability predicts self-reported difficulties in emotion regulation: A focus on emotional clarity and impulse control. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years?: Neural basis of improving emotional stability over age. The Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, LaBar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Massé N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. NeuroImage. 2007;35:698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Wrzus C, Müller V, Wagner GG, Lindenberger U, Riediger M. Affective and cardiovascular responding to unpleasant events from adolescence to old age: Complexity of events matters. Developmental Psychology. 2013;49:384–397. doi: 10.1037/a0028325. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of Neurophysiology. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


