Abstract
We investigated the associations among gestational factors including prenatal marijuana exposure (PME), child behavior at age 3, early age of onset of marijuana use (EAOM, < 15 years), and adult roles at 22 years. Participants were drawn from the Maternal Health Practices and Child Development (MHPCD) Project, a longitudinal study of prenatal substance exposure in offspring who have been studied for over 22 years since the prenatal phase. Data from the prenatal, birth, 3-, and 22-year phases (N=608) were used in the present study. Age of onset of offspring substance use was determined based on data from the 14-, 16-, and 22-year phases. The subjects were of lower socioeconomic status, 43% were Caucasian and the remaining were African-American, and 48% were males. Early childhood behavior was significantly (p < 0.05) related to EAOM after controlling for PME, birth and childhood environmental risk factors, and Conduct Disorder. EAOM was significantly associated with negative adult roles including increased risk of being arrested (p < 0.001), lower educational attainment (p < 0.001), having a child without being married (p < 0.05), and unemployment at 22 years (p < 0.001). The correlations between PME and negative adult roles and between early childhood behavior and negative adult roles were also statistically significant. Pathway analysis demonstrated that EAOM significantly mediated the associations between PME and fulfillment of adult roles and between early childhood behavior and adult roles. There are a number of intervention points that could be targeted that would have a long-term impact on lowering the probability of EAOM and less success in adult roles.
Keywords: early childhood behavior, prenatal marijuana exposure, early adolescent marijuana use, adult roles
1. Introduction
In an earlier publication, we demonstrated that adolescents with prenatal marijuana exposure (PME) were significantly more likely to have an early age of onset of marijuana use (EAOM; defined as < 15 years), taking into account adolescents’ depression, aggression, use of other drugs, peer use, intelligence, home environment, and parental supervision (Day et al., 2006). Porath and Fried (2005) also reported a significant relation between PME and initiation of marijuana use during adolescence among males, after controlling for prenatal demographic characteristics. However, few studies have investigated the relation between early childhood behavior and EAOM or the effects of EAOM on fulfillment of and functioning in adult roles. Further, few studies have investigated the roles played by prenatal predictors and early childhood behavior on adult roles or explored the pathways to EAOM and subsequently to functioning in young adulthood.
While several studies have shown an association between childhood aggression and unemployment in adulthood (Kokko & Pulkkinen, 2000; Tremblay et al., 2004), the pathway from early childhood behavior to adulthood role maladjustment through adolescent marijuana use has not been fully investigated. Several studies have shown a significant relation between school age child behavior problems and EAOM (e.g., King, Iacono, & McGue, 2004). Brook and colleagues (1992) have shown a significant relation between childhood (ages 5–10) aggression and adolescent drug use. However, in that study, drug use included alcohol use, marijuana, and other illicit drugs. In a study in the Netherlands, a linear growth curve fitted to physical aggression assessed at ages 4/5, 10/11, and 18 years was found to be significantly related to adolescent cannabis use (Timmermans, van Lier, & Koot, 2008), and a Finnish study reported a significant association between externalizing problems at age 8 and cannabis use at age 15 (Miettunen et al., 2014). There have been no reports on the relation between preschoolers’ behavior problems and EAOM. If this relation is detected, then intervention programs aimed at an earlier stage of life would be more effective than interventions later when the behavior is more strongly ingrained.
Multiple reports have established the negative consequences of EAOM on adolescent brain development and subsequent psychiatric morbidity (Day et al., 2015; Fergusson & Horwood, 1997; Pope et al., 2003; Renard et al., 2014; Rubino et al., 2012). EAOM is associated with a number of significant behavior problems. Hall and Degenhardt (2007) reported an association between EAOM and problem behaviors including poor school performance, affiliation with drug-using peers, and antisocial behavior. Frequent cannabis use before the age of 16 predicted significantly higher rates of substance use, delinquency, unemployment, and educational difficulties among teens (Fergusson & Horwood, 1997). By contrast, Pardini et al. (2015) found no association between EAOM (defined as <17 years) and adolescent attention problems or academic performance, although this might reflect the fact that their subjects had low to moderate levels of use and were past the age when marijuana use becomes normative.
In adulthood, many reports have shown that EAOM predicts substance use and abuse (Chen & Kandel, 1995; Ehlers et al., 2007; Lynskey et al., 2003, 2006; Palmer et al., 2009; Sonon et al., 2015). However, studies showing effects of EAOM on the roles of adulthood, including occupational status, family, problems with the law, and education, are sparse and inconclusive. In a twin study, Verweij and colleagues (2013) reported a significant association between EAOM and early school departure but found this was due to shared environmental factors, rather than to EAOM. In a longitudinal study among African American young adults, those who initiated marijuana use before age 16 were less likely to be employed or in school at age 21 than were non-users, although the difference was not statistically significant after covariates were considered (Roldos, 2014). Other studies have reported a significant relation between adolescent marijuana use and adult roles, but their independent variable was either frequent marijuana use or increased use during adolescence and not EAOM per se (Brook et al., 1999; Fergusson & Boden, 2008).
There is biological evidence that EAOM affects brain development. Early adolescence is a time of plasticity when changes occur in the distribution of the cannabinoid receptors in the prefrontal cortex and limbic regions (Giedd et al., 2006; Renard et al., 2014; Spear, 2000). At this time, working memory (Luna et al., 2004), executive functioning, and emotional capacity (Yurgelun-Todd, 2007) are maturing. CB1 receptors are particularly present in the hippocampus, basal ganglia, the cerebral cortex, amygdala, and cerebellum (Di Forti et al., 2007). Exogenous cannabinoids, such as tetrahydrocannabinol (THC), which is one of the active ingredients in marijuana, can interfere with the endocannabinoids’ stimulation of the CB1 receptors and normal development of the endogenous cannabinoid system and affect the maturation of the cortical neuronal networks (Bossong & Niesink, 2010). This may further result in a disruption in glutamate release, leading to abnormal synaptic connections. Thus, marijuana use during this vulnerable period can alter working memory, procedural learning, behavior and decision making, and emotional reactions.
The aims of the current study address multiple questions: 1) Can childhood behavior as early as age 3 predict EAOM while taking into account PME, other prenatal exposures, and environmental characteristics at birth and at early childhood? 2) Is the association between EAOM and adult roles significant? 3) Is there a significant association between PME and adult roles, and does EAOM mediate the association between early risk factors (PME and childhood behavior) and adult roles? To our knowledge, no other study has examined the relation between early childhood behavior and early onset of marijuana use, or the relation between PME and adulthood role adjustment. These questions are important since intervention during pregnancy and as early as preschool could prevent problems during later stages of life. These analyses will use data from the Maternal Health Practices and Child Development (MHPCD) Project, a longitudinal study of prenatal marijuana exposure, in which offspring were assessed at multiple points in childhood and adolescence through adulthood.
2. Methods
2.1. Study Design
This study is based on data from the MHPCD Project. Pregnant women who were at least 18 years of age were recruited from a prenatal clinic in the fourth or fifth prenatal month. Data collection for the initial phase of this project began in 1983 and was completed in 1986. The 22- year phase was conducted from 2006 through 2009. The study was approved by the Institutional Review Board of the University of Pittsburgh and the Human Experimentation Committee of the Magee-Womens Hospital. From 1360 women initially recruited, two samples were selected to study the effects of prenatal marijuana and alcohol exposure on the offspring. Sample selection was based on the women’s use of marijuana and alcohol. The marijuana cohort included all women who used two or more joints of marijuana per month and a random sample who used less than this amount or not at all. The alcohol cohort included all women who drank three or more alcoholic beverages per week and a random sample of women who drank less than this amount or did not drink. The two cohorts were combined for these analyses.
The women were interviewed again in their seventh prenatal month and at delivery. Subsequent interviews were conducted at 8 and 18 months, and at 3, 6, 10, 14, 16, and 22 years. Measures of offspring growth, cognitive development, and behavior were assessed at each follow-up, and drug use was assessed at 14, 16, and 22 years. Demographic information and measures of offspring and maternal psychological, social, and environmental factors were gathered at each interview.
The combined birth cohort consisted of 763 live singleton infants. At the 22-year phase, 80% (n=608) of the birth sample was interviewed. Missing were 30 offspring who refused to participate, 3 were adopted,18 were institutionalized (jail or rehabilitation), 56 were lost to follow-up, 29 moved out of the Pittsburgh area, 11 died, and 8 were unable to participate due to low cognitive functioning. There were no significant differences between those included in the 22-year analyses (n=608) and those who were not (n=155) in maternal socioeconomic status, race, prenatal marijuana, alcohol, or tobacco exposures.
2.2. Sample Characteristics
In the first trimester, the mean age of the mothers was 23 (range 18–42) years. The sample consisted of lower socioeconomic status women, with 61% reporting a family income of less than $400/month in 1983–1986. Their average education was 11.8 years and 26% of the women had less than 12 years of education. Thirty-three percent of the women were primigravidous, 32% were married, and 25% worked outside of the home. The prevalence of marijuana, alcohol, tobacco, cocaine, and other illicit drug use in the first trimester of pregnancy was 41.0%, 64.5%, 53.3%, 3.6%, and 8.6%, respectively.
At the 22-year assessment, the offspring were, on average, 22.8 years of age (range 21–26), 48% were males, and 57% were African American. Sixty percent of the offspring worked full or part-time, 27% attended school, and 4% served in the military. Their average education was 12.8 years (range 8–18). Twenty-eight percent lived with a significant other, 6% were married, and 37% had at least one child.
2.3. Measures
Prenatal substance exposure
The quantity and frequency of substance use during the first trimester of pregnancy were weighted according to the pattern of use from conception to pregnancy recognition, recognition to pregnancy confirmation, and from confirmation to the end of the first trimester (refer to Day et al., 1985 for further details). Marijuana, sinsemilla, and hashish use were expressed as joints and were weighted according to the amount of delta-9-THC (sinsemilla = 2 joints; hashish = 3 joints) in each (Gold, 1989; Julien, 1992). PME was expressed as average daily joints (ADJ) during the first trimester of pregnancy. Fifty-nine percent of the women did not use marijuana, 28% smoked less than a joint/day, and 13% smoked one or more joints/day. The women reduced their use as their pregnancy progressed. During the second and third trimesters, 22% and 18.6% used marijuana, and 4.9% and 5.3% used at the rate of one or more joints/day, respectively. Therefore, first trimester exposure was used in this study. Prenatal alcohol exposure was measured similarly to marijuana exposure and was based on consumption of all types of alcoholic beverages. The average daily volume (ADV) was calculated separately for each trimester of pregnancy and was used as a continuous variable. Prenatal tobacco exposure was measured by the number of cigarettes smoked per day and was a continuous variable for each trimester of pregnancy.
Behavior at age 3
Offspring behavior problems at age 3 were measured by the Toddler Behavior Checklist (TBC; Larzelere, Martin, & Amberson, 1989), a 103-item questionnaire completed by the mother. The total behavior score is based on five subscales: 1) oppositional - a measure of disobedience and anger expression; 2) physical aggression - fighting and not getting along with other children; 3) emotional instability - acting unpredictably or excessively; 4) immaturity - age-inappropriate behavior such as wetting and throwing objects; and 5) shyness - clinging to adults and shying away. The internal consistency of the subscales was 0.91, 0.83, 0.80, 0.90, and 0.67, respectively.
Measures of the environment at birth and at age 3
A cumulative risk model was developed combining risks from multiple domains. At birth, risk factors included: less than 12 years of maternal education, family income of less than $200/month, not living in a nuclear family, more than 5 people in the household, low social support (lower 10th percentile), and upper 10th percentile for depression, anxiety, and hostility, and number of life events. Similar environmental risk factors were included at age 3: family income of less than $300/month, more than 5 people in the household, lower 10th percentile in social support, and upper 10th percentile for depression, anxiety, and hostility, and number of life events. At both phases, maternal depression was measured by the Center for Epidemiological Studies- Depression scale (Radloff, 1977), anxiety and hostility were assessed with the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970), and life events with the PERI life events scale (Dohrenwend et al., 1978). At 3 years postpartum, each maternal substance used was assessed since the last interview at 18 months, using the same questions as in the prenatal questionnaires.
Offspring substance use
Starting at the 14-year phase, the offspring were asked at what age they first smoked marijuana, whether they had used marijuana over the past year, the frequency and quantity of their use, and with how many people they shared. These questions were repeated at the 16- and 22-year phases. To avoid recall bias, age of initiation was recorded only for those who had not initiated at a previous phase. Early initiation was defined as initiation prior to age 15 consistent with our previous publication (Day et al., 2015). All of the subjects who did not report onset of use prior to age 15 had a negative THC urine test at 14 years. Alcohol and tobacco use were assessed similarly, and to be consistent with marijuana use, early initiation was defined as use before age 15. Offspring substance use at 22 years was assessed using the same instrument developed by the MHPCD for the mothers (Day & Robles, 1989).
Lifetime Conduct Disorder
Lifetime psychiatric disorders among the offspring were assessed using the Diagnostic Interview Schedule-IV at age 16 (Robins et al., 2000). An experienced clinician trained lay interviewers to administer the computerized DIS-IV. The interviews were audiotaped and reliability checks occurred to maintain administration standards. The adolescents reported their own history of symptoms.
Adult roles at 22 years
At the 22-year phase, the subjects were asked about their adult roles including current marital status, the last year of school completed, current working status (either full- or part-time), military service, having any children, and whether they had ever been arrested.
2.4. Statistical Analyses
The bivariate relations between EAOM and offspring characteristics at birth and at 22 years, components of adult roles at 22 years, and the TBC at 3 years were tested using chi-square and Mann-Whitney rank-sum tests.
A logistic regression was applied to examine the relation between the TBC total score at age 3 and EAOM, controlling for birth and early childhood environmental risk factors, prenatal substance exposures, race, and gender. The environmental risk factors were combined and represented as cumulative risk sums to reduce the number of covariates and avoid model saturation. The regression was done in forwarding steps and the changes in significance between covariates, TBC scores, and the outcome variable at each step were observed. The final model included variables that were significant at an alpha level of 0.05.
The indirect effects of PME and early childhood behavior on adult roles through EAOM were assessed using path analysis. For this analysis, the negative adult role measure was represented as a combination of four factors: arrests (yes=1/no=0), work/military status (yes=0/no=1), more than 12 years of education (yes=0/no=1), and unmarried with children (yes=1/no=0). The covariates considered for inclusion in this model were race, gender, prenatal exposure to alcohol and tobacco, maternal substance use at age 3, birth and childhood environmental risk factors, initiation of alcohol or tobacco use prior to age 15, and age of the offspring at 22-year assessment. The statistical package Mplus (Muthén & Muthén, 2007) was used to estimate the parameters of the model and the significance of the mediation.
3. Results
Thirty-eight percent (234/608) of the young adults in the cohorts reported onset of marijuana use before the age of 15 years (EAOM) and 67.5% of those continued to report marijuana use at the 22-year assessment. At 22 years, offspring with EAOM were significantly less likely to have more than 12 years of education (37.2% vs. 54.5%, p < 0.001), and were less likely to work or serve in the military than their peers (52.1% vs. 67.6%, p < 0.001) (Table 1). The EAOM subjects were also less likely to be married and more likely to have children by the 22-year assessment. The percentage of those with EAOM who were unmarried with children was 42.7% compared to 28.9% among the rest of the offspring (p < 0.001). The EAOM subjects were also more likely to have been arrested (56% vs. 27.3%, p < .001). EAOM was significantly associated with prenatal marijuana and tobacco exposure. EAOM was not statistically significantly related to race, gender, prenatal alcohol exposure, or maternal substance use at age 3 (Table 1).
Table 1.
Characteristics Associated With Early Onset of Marijuana Use (< 15 years)
| Early Initiators N= 234 |
All others N= 374 |
Pa | |
|---|---|---|---|
| 22 year characteristics | |||
| Current use of marijuana % | 67.5 | 38.8 | < 0.001 |
| Average joints/day [SD] | 2.3 [8.2] | 0.6 [2.5] | < 0.001 |
| Education > 12 years % | 37.2 | 54.5 | < 0.001 |
| Work or serve in military % | 52.1 | 67.6 | < 0.001 |
| Married % | 2.6 | 7.8 | < 0.01 |
| Have children % | 44.4 | 32.6 | < 0.01 |
| Have children & not married % | 42.7 | 28.9 | < 0.001 |
| Arrested % | 56.0 | 27.3 | < 0.001 |
| Race (% Caucasian) | 43.2 | 43.6 | NS |
| Birth characteristics | |||
| Gender (% male) | 51.3 | 45.2 | NS |
| 1st trimester marijuana (ADJb) | 0.6 [1.2] | 0.3 [0.7] | < 0.005 |
| 1st trimester alcohol (ADVc) | 0.6 [1.3] | 0.5 [1.1] | NS |
| 1st trimester tobacco (average cigarettes/day) |
9.8 [11.9] | 7.2 [10.9] | <0.01 |
| Sum environmental risks | 1.5 [1.2] | 1.2 [1.1] | < 0.01 |
| Three year characteristics | |||
| Behavior at 3 (TBC) | 122.2 [31.6] | 115.0 [30.4] | < 0.005 |
| Sum environmental risks | 0.7 [1.0] | 0.5 [0.9] | NS |
| Maternal marijuana (ADJ) | 0.3 [0.8] | 0.2 [0.9] | NS |
| Maternal alcohol (ADV) | 1.0 [1.9] | 0.8 [1.6] | NS |
| Maternal tobacco (cigarettes/day) | 13.8 [14.0] | 12.1 [14.4] | NS |
| Lifetime Conduct Disorder % | 22.8 | 5.1 | <0.001 |
Significance based on χ2 test for dichotomous variables and Mann-Whitney rank-sum test for continuous variables
Average daily joints
Average daily drinks
The bivariate relation between the TBC and EAOM is presented in Table 1. On average, the TBC scores of those with EAOM were 7 points higher than other subjects in the cohort (p < 0.005). The 25th percentile, median, and 75th percentile TBC scores among EAOM were 102, 124, and 142, respectively compared to 96, 117, and 132 among non-EAOM subjects. We also explored the relation of EAOM to each subscale of TBC. Although the EAOM group scored higher than non-EAOM on all subscales, the differences were statistically significant for the physical aggression (19.4 vs. 17.5, p < 0.001) and immaturity (15.7 vs. 14.3, p < 0.05) subscales.
A stepwise logistic regression was applied to test whether the TBC was significantly associated with EAOM, controlling for birth and childhood environmental risks, PME, race, and gender (Table 2). Although gender and race were not significantly correlated with EAOM in our cohort, they were included in the analysis based on reports by other studies. TBC at age 3 was significantly related to EAOM controlling for the other covariates: ten point higher scores on the TBC increased the odds of being in the EAOM group by 6 percent. In addition to the TBC, other predictors of EAOM were PME, prenatal exposure to tobacco, and cumulative environmental risks at birth.
Table 2.
Significant Predictors of EAOM Using Logistic Regressiona
| Variables | Coefficient | Adjusted OR (95% CI) | Significance |
|---|---|---|---|
| 1st trimester marijuana exposure (ADJb) |
0.34 | 1.40 (1.14–1.73) | 0.001 |
| 1st trimester tobacco (cigs/day) exposure |
0.018 | 1.02 (1.002–1.03) | 0.02 |
| Behavior at age 3 (TBCc) | 0.006 | 1.01 (1.001–1.01) | 0.03 |
| Sum environmental risks at birth | 0.17 | 1.18 (1.02–1.38) | 0.03 |
| Results with Lifetime Conduct Disorder added to the model | |||
| 1st trimester marijuana exposure (ADJ) |
0.33 | 1.40 (1.13–1.72) | 0.002 |
| 1st trimester tobacco (cigs/day) exposure |
0.018 | 1.02 (1.002–1.03) | 0.02 |
| Behavior at age 3 (TBC) | 0.006 | 1.01 (1.001–1.01) | 0.04 |
| Sum environmental risks at birth | 0.15 | 1.16 (0.99–1.35) | 0.07 |
| Lifetime Conduct Disorder | 1.57 | 5.04 (2.61–8.91) | 0.000 |
Variables in the model included race, gender, prenatal exposure to marijuana, tobacco, and alcohol, and cumulative environmental risks at birth and at 3 years.
Average daily joints
Toddler Behavior Checklist
Because other studies (e.g., Fergusson, Lynskey, Horwood, 1993) found a significant relation between Conduct Disorder and EAOM, the logistic regression analysis was repeated adding lifetime Conduct Disorder to the model. TBC remained a significant predictor of EAOM controlling for Conduct Disorder (Table 2).
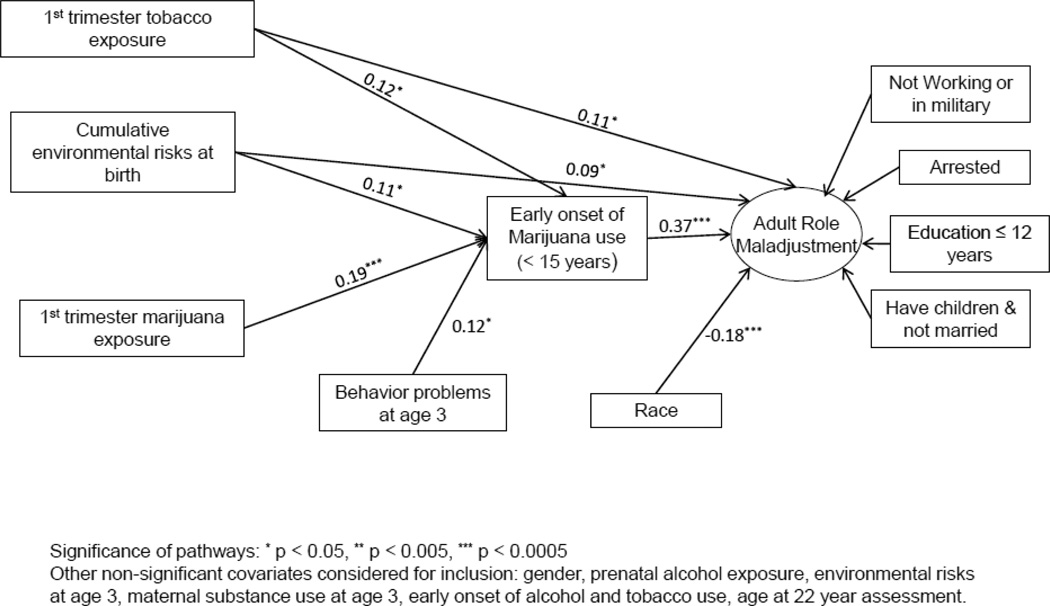
The composite score for adult roles had five categories ranging from 0 (no negative roles) to 4 (4 negative roles). The rate of EAOM in each category was 17.3%, 32.6%, 42.7%, 54.9%, and 66.0%, respectively (p < 0.001). The zero order correlations between the TBC and negative adult roles (0.09, p < 0.05) and PME and adult roles (0.15, p < 0.001) were also significant. As shown in Figure 1, using path analysis and controlling for race, environmental risk factors at birth, and prenatal exposure to tobacco, EAOM significantly mediated the path from childhood behavior problems to negative adult roles (standardized estimated coefficient of mediation= 0.05, p < 0.01). The indirect effect of PME on adult roles via EAOM was also significant (Std coef. = 0.07, p < 0.001), as well as the indirect effect of prenatal tobacco exposure (Std coef. = 0.04, p < 0.05). The paths in Figure 1 are marked by standardized estimated coefficients to express the relative significance of each predictor. The direct associations between PME and adult roles and between the TBC and adult roles were not significant after the indirect pathway through EAOM was added to the model. Prenatal exposure to alcohol, gender, age at the 22 year assessment, childhood environmental risk factors, maternal substance use at age 3, and early initiation of alcohol and tobacco use were not significantly related to the cumulative negative adult roles measure after controlling for EAOM.
Figure 1.
Path analysis model illustrating mediating effects of early onset of marijuana use from prenatal marijuana exposure and childhood behavior problems to negative adult roles.
Given the high rate of frequent and continued marijuana use among the EAOM offspring, it is possible that the association between EAOM and adult roles represented the effects of current high frequency of use. We re-examined the relation between EAOM and adult roles excluding subjects in the upper quartile of current (age 22) marijuana use, and EAOM continued to be statistically significantly related to negative adult roles (p < 0.001), even after exclusion of current frequent users.
4. Discussion
In this longitudinal study, we demonstrated that there are two significant pathways to unsuccessful attainment of roles in young adulthood. One pathway was from PME to EAOM to adult roles at 22 years of age; the second pathway was from 3-year behavior problems to EAOM to adult roles.
While other studies have shown EAOM to be related to later substance use disorders, juvenile offending, psychotic symptoms, and poorer cognitive performance (Day et al., 2015; Fergusson & Horwood, 1997; Kokkevi, Gabhainn, & Spyropoulou, 2006; Pope et al., 2003), this report illustrates the cumulative and significant relation between EAOM and difficulty in successfully adopting adult roles. These analyses go further, to describe the effects of PME and early childhood behaviors on EAOM and the subsequent combined effects of these variables on negative adult roles. These associations were significant even after controlling for environmental factors and the potential biological effects of PME. Thus, while there is a well-known association between EAOM and negative outcomes in young adulthood, assessment of this relation should include earlier factors that are also significant in the pathway.
The effects of EAOM stem from both environmental and biological influences. EAOM has been shown to be related to poorer home environment and peers’ drug use (Fergusson & Horwood, 1997; Von Sydow et al., 2002), which consequently leads to continuation of drug use, depressive and anxious symptoms, and less desire to pursue a career and life partner. On the other hand, MRI studies have shown that cortical gray matter peaks during adolescence (Ashtari & Cyckowski, 2011; Giedd et al., 1999) and exposure to external cannabinoids may disrupt the function of the endocannabinoid system during this crucial period (Bossong & Niesink, 2010; Filbey et al., 2015; Rubino, Zamberletti, & Parolaro, 2012).
In a longitudinal study by Brook and colleagues (1999), they showed a significant relation between frequent marijuana use during adolescence up to young adulthood and negative adult roles. In our study, early onset of use was found to be related to negative young adult roles, even though 32% of the EAOM group did not use any marijuana in the year prior to the assessment. Further, the association between EAOM and adult roles was statistically significant even after excluding subjects in the upper quartile of current marijuana use. These findings imply that this association is not solely related to progression to more frequent and problematic use, but that there is the possibility of irreversible insult regardless of duration of use.
While Fergusson and Horwood (1997) showed a significant relation between childhood behavior at age 8 and EAOM, the data from the MHPCD study indicate that the relation is present at an even earlier stage of life. We found a significant relation between child behavior problems at age 3 and EAOM, controlling for birth and childhood environmental risk factors and Conduct Disorder. Among subscales of the Toddler Behavior Checklist, physical aggression and immaturity were the most significant predictors of EAOM. Thus, the pathway to EAOM and to adult role difficulties begins at an early age and the effects of gestational factors and childhood behavior are mediated through EAOM. This is an important finding because we have identified significant points for targeting intervention and prevention programs.
There are strengths and weaknesses of these data. The MHPCD sample was from a general prenatal clinic for low income women and the findings may not generalize to other populations. However, strengths of the sample are that all of the women were receiving prenatal care, all were relatively homogeneous with respect to socioeconomic status, we recruited a large population of women, half of whom were white and half were African-American, we had multiple, prospective measures across the lifespan, and we were able to control for other prenatal and current substance use.
There are clearly other pathways to EAOM and to difficulties adapting to adult roles. We have identified two new pathways to adult roles from PME and from early childhood behaviors that are mediated through early use of marijuana. Further research is needed to identify other pathways from PME to young adult roles, and the association of these models with the pathways we have identified. However, the simplicity of the model in this study allowed us to focus more closely on the relations among PME, early childhood behavior problems, EAOM, and adult roles at 22 years, and hence, identify time points that are appropriate to interrupt and reduce risks of negative adaptations to adult roles.
Highlights.
A longitudinal study of prenatal substance exposure was used to investigate influences on adult roles at 22 years.
Behavior problems at age 3 were significantly related to early onset of marijuana use (< 15 years).
Early onset of marijuana and maladjustment to adulthood roles were highly correlated.
Prenatal marijuana exposure and early behavior problems were significantly related to adult roles.
These associations with adult roles were mediated through early onset of marijuana use.
Acknowledgments
This study was supported by the National Institute on Drug Abuse DA03874 and National Institute on Alcoholism and Alcohol Abuse AA06666 (N. Day, Principal Investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashtari M, Cyckowski L. Brain development during adolescence. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in Health and Disease. New York, NY: Springer; 2011. [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Progress in Neurobiology. 2010;92(3):370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Brook JS, Richter L, Whiteman M, Cohen P. Consequences of adolescent marijuana use: Incompatibility with the assumption of adult roles. Genet Soc Gen Psych. 1999;125:193–207. [PubMed] [Google Scholar]
- Brook JS, Whiteman M, Finch S. Childhood aggression, adolescent delinquency and drug use: a longitudinal study. J Gen Psychol. 1992;151:369–383. doi: 10.1080/00221325.1992.10753733. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Pub Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, Day R, Larkby C, Richardson GA. Prenatal marijuana exposure, age of marijuana initiation, and the development of psychotic symptoms in young adults. Psychol Med. 2015;45(8):1779–1787. doi: 10.1017/S0033291714002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101(9):1313–1322. doi: 10.1111/j.1360-0443.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- Day NL, Robles N. Methodological issues in the measurement of substance use. Ann NY Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- Day NL, Wagener DK, Taylor PM. Measurement of substance use during pregnancy: methodological issues. In: Pinkert T, editor. Current Research on the Consequences of Maternal Drug Abuse. Vol. 59. Rockville, MD: NIDA Res Monog; 1985. pp. 36–40. [Google Scholar]
- Di Forti M, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cognitive disorders: the chicken or the egg? Curr Opin Psychiatry. 2007;20:228–234. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- Dohrenwend B, Krasnoff K, Askenasy A, Dohrenwend P. Exemplification of a method for scaling life events: the PERI life events scale. J Health Soc Behav. 1978;19:205–229. [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P. Age of first marijuana use and the occurrence of marijuana use disorders in Southwest California Indians. Pharmacol Biochem Behav. 2007;86(2):290–296. doi: 10.1016/j.pbb.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–976. doi: 10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction. 1997;92:279–296. [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Conduct problems and attention deficit behavior in middle childhood and cannabis use by age 15. Aust NZ J Psych. 1993;27:673–682. doi: 10.3109/00048679309075830. [DOI] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use on cortical architecture. Dev Cogn Neuro. 2015;12:16–22. doi: 10.1016/j.dcn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Keshavan M, Paus T. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2006;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. Marijuana. New York, NY: Plenum; 1989. [Google Scholar]
- Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Curr Opin Psychiatr. 2007;20(4):393–397. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- Julien R. A Primer of Drug Action. 6th. New York: Freeman; 1992. [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Gabhainn S, Spyropoulou M. Early initiation of cannabis use: A cross-national European perspective. J Adolescent Health. 2006;39:712–719. doi: 10.1016/j.jadohealth.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Kokko K, Pulkkinen L. Aggression in childhood and long-term unemployment in adulthood: A cycle of maladaptation and some protective factors. Dev Psychol. 2000;36:463–472. doi: 10.1037//0012-1649.36.4.463. [DOI] [PubMed] [Google Scholar]
- Larzelere R, Martin J, Amberson T. The Toddler Behavior Checklist: A parent-completed assessment of social-emotional characteristics of young preschoolers. Family Rel. 1989;38:418–425. [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs. co-twin controls. J Amer Med Assoc. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behav Genet. 2006;36(2):195–200. doi: 10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- Miettunen J, Murray GK, Jones PB, Mäki P, Ebeling H, Taanila A, Joukamaa M, Savolainen J, Törmänen S, Järvelin MR, Veijola J, Moilanen I. Longitudinal associations between childhood and adulthood externalizing and internalizing psychopathology and adolescent substance use. Psychol Med. 2014;44:1727–1738. doi: 10.1017/S0033291713002328. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Fifth. Los Angeles, CA: Muthén and Muthén; 2007. [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depen. 2009;102(1–3):78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini D, White HR, Xiong S, Bechtold J, Chung T, Loeber R, Hipwell A. Unfazed or dazed and confused: Does early adolescent marijuana use cause sustained impairments in attention and academic functioning? J Abnorm Child Psych. 2015;43:1203–1217. doi: 10.1007/s10802-015-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Porath AM, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol. 2005;27:267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Renard JJ, Krebs M-O, LePen G, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci. 2014;8:1–14. doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University School of Medicine, Department of Psychiatry; 2000. [Google Scholar]
- Roldos MI. The longitudinal effect of drug use on productivity status of nonmetropolitan African American young adults. J Drug Educ: Subst Abuse Res Prev. 2014;44(1–2):34–50. doi: 10.1177/0047237915573524. [DOI] [PubMed] [Google Scholar]
- Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharm. 2012;26:177–188. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- Sonon KE, Richardson GA, Cornelius JR, Kim KH, Day NL. Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol Teratol. 2015;47:10–15. doi: 10.1016/j.ntt.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- Timmermans M, van Lier PAC, Koot HM. Which forms of child/adolescent externalizing behaviors account for late adolescent risky sexual behavior and substance use? J Child Psychol Psych. 2008;49:386–394. doi: 10.1111/j.1469-7610.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- Tremblay RE, Nagin DS, Séguin JR, Zoccolillo M, Zelazo PD, Boivin M, Pérusse D Japel C. Physical aggression during early childhood: Trajectories and predictors. Pediatrics. 2004;114:e43–e50. doi: 10.1542/peds.114.1.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJH, Huizink AC, Agrawal A, Martin NG, Lynskey MT. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug Alcohol Depend. 2013;133(2):580–586. doi: 10.1016/j.drugalcdep.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Sydow K, Lieb R, Pfister H, Höfler M, Wittchen HU. What predicts incident use of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend. 2002;68:49–64. doi: 10.1016/s0376-8716(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;2:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]