Abstract
The adult brain is capable of adapting to internal and external stressors by undergoing structural plasticity, and failure to be resilient and preserve normal structure and function is likely to contribute to depression and anxiety disorders. While the hippocampus has provided the gateway for understanding stress effects on the brain, less is known about the amygdala, a key brain area involved in the neural circuitry of fear and anxiety. Here, in mice more vulnerable to stressors, we demonstrate structural plasticity within the medial and basolateral regions of the amygdala in response to prolonged 21day chronic restraint stress (CRS). Three days before the end of CRS, treatment with the putative, rapidly acting antidepressant, acetyl-L-carnitine (LAC) in the drinking water opposed the direction of these changes. Behaviorally, the LAC treatment during the last part of CRS enhanced resilience, opposing the effects of CRS, as shown by an increased social interaction and reduced passive behavior in a forced swim test. Furthermore, CRS mice treated with LAC show resilience of the CRS-induced structural remodeling of medial amygdala (MeA) stellate neurons. Within the basolateral (BLA) amygdala, LAC did not reduce, but slightly enhanced, the CRS-increased length and number of intersections of pyramidal neurons. No structural changes were observed in MeA bipolar neurons, BLA stellate neurons, or in lateral amygdala (LA) stellate neurons. Our findings identify MeA stellate neurons as an important component in the responses to stress and LAC action and show that LAC can promote structural plasticity of the MeA. This may be useful as a model for increasing resilience to stressors in at risk populations.
INTRODUCTION
The adult brain possesses remarkable epigenetic structural and functional plasticity in response to environmental influences, such as stress1, 2. Stress-induced structural remodeling of neuronal architecture is a sign of adaptation; however, failed resilience contributes to the onset and recurrence of psychiatric disorders, such as depression3, 4. Such structural plasticity is evident in the hippocampus, where 21 days of CRS or 10 days of the more severe chronic immobilization stress (CIS) in the rat and mouse lead to shrinkage of hippocampal CA3 and DG neuron dendrites as well as loss of spines in hippocampal CA1 neurons5. Similar shrinkage of dendrites after chronic stress is also observed in neurons of the medial prefrontal cortex (PFC)6. Hippocampal and PFC atrophy has been detected in depressed human subjects using magnetic resonance imaging7, 8.
Growing out of findings in the hippocampus, a landmark study found that another limbic brain structure, the amygdala, shows a different type of structural plasticity in response to stress9. The amygdala, which consists of a group of nuclei with different cytoarchitecture and connectional features, is an essential component of the neural circuitry of stress8 and Pavlovian fear conditioning10 and is also critical for the processing of emotions and social interactions11-13. Pyramidal and stellate neurons in the BLA exhibit enhanced dendritic arborization in response to chronic immobilization stress (CIS), in contrast to the dendritic shrinkage seen within the hippocampus9. Opposite patterns of structural plasticity have also been observed within other nuclei of the amygdala complex: repeated 21 days of CRS leads to synapse spine formation in the BLA, while it causes a loss of spines in the MeA, an effect dependent on the extracellular matrix proteases (tPA), which is not involved in stress-induced structural effects within the BLA8, 14-16.
Thus, the amygdala has a substantial cellular and anatomical complexity that warrants further studies toward the understanding of how stress and antidepressant action may affect this brain region, which is critical for behaviors associated with psychiatric disorders such as alterations in social behavior and anxiety. Furthermore, this diversity of stress-induced plasticity within the sub-regions of the amygdala complex raises the question as to whether the different nuclei of the amygdala may contribute to different features in the pleiotropic spectrum of psychiatric disorders and whether some of the structural changes triggered by stress in one limbic area may influence changes in other brain areas, such as the ventral hippocampus and PFC which have a dense connectivity with the amygdala17, 18.
Here, we found that the input and output nuclei of the BLA and MeA showed differential responses to the same CRS paradigm, which also did not affect the neurons of the LA. Stress increased elongation and complexity of BLA pyramidal neurons and induced shrinkage of MeA stellate neurons, whereas no effect was observed in BLA stellate neurons and MeA bipolar neurons. We also report that oral treatment in drinking water with the novel antidepressant candidate acetyl-L-carnitine (LAC)19-21 3 days prior to the end of CRS, enhanced behavioral resilience to stress in at-risk individuals defined by increased anxiety-like avoidance in a light-dark test22, 23. Also, CRS mice treated with LAC show recovery of dendritic remodeling of MeA stellate neurons but continued to show the increased elongation and complexity of BLA pyramidal neurons, suggesting that a counter-regulatory mechanism between the microcircuit of MeA and BLA may be involved in the rapid antidepressant-like actions of LAC.
Materials and Methods
All experimental procedures were carried out in accordance with the National Institutes of Health and The Rockefeller University Institutional Animal Care and Use Committee guidelines. All efforts were made to minimize animal suffering.
Animals
Charles River C57BL/6N male mice (6wk old, 20-25g) were used in this study. Mice were housed five per cage under controlled conditions (12-h light/dark cycle, 22 °C, food and water ad libitum) and were allowed to recover from any potential stress for a week after their arrival before any experiments were performed. Stress began when the animals were 7wks old. LD screening was performed on a n of 45 mice; from 6 to 9 biological replicates were used for the behavioral experiments; from 8 to 10 biological replicates were used for assessment of stress effects on adrenal glands; from 4 to 6 biological replicates were used for the structural analyses.
Stress challenge procedure
We devised a CRS paradigm by restraining 7-week-old male mice two hours per day in the morning (9-11AM) for 21 consecutive days. The restraint device contained two 0.4 cm air holes and allowed mice to stretch the legs but did not permit locomotion within the tube. Naïve age- and sex-matched animals were used as controls. Control mice were left undisturbed in their home cage and were not subjected to any type of stress. The whole stress procedure is schematized in Figure 1a. Behavioral and structural stress effects were evaluated 24hrs after the last restraint stressor. The weight of the animals was recorded once a week and, as expected, the stressed mice show a lower gain weight than control mice, reflecting the progression of the stress procedure. Adrenal glands were collected 24hrs after the last episode of restraint stress for the CRS group and according to a matched time for the control group.
Figure 1. Rapidity and efficacy of LAC oral prophylactic treatment in enhancing resilience to stress.
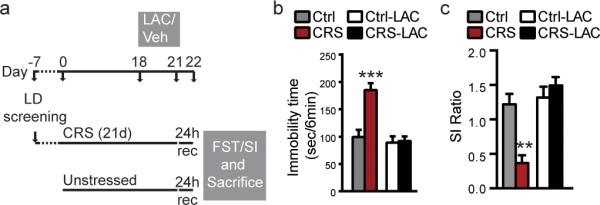
(a) Time course and design of LD screening to identify at-risk individuals and CRS paradigm, LAC prophylactic treatment and behavioral outcomes. (b,c) An oral treatment with LAC, 3 days prior to the end of the chronic stress, decreased immobility time in CRS mice at the forced swim test (b) and increased social interaction ratio in CRS mice (c), thus exerting a pro-resilient effects in blocking the occurrence of a depressive-like phenotype and social interaction impairments associated with prolonged 21day CRS. (FST: F1,24=20.03, p<0.001 (treatment), F1,24=14.87, p<0.001 (stress); SI: F1,28=19.9, p<0.001 (treatment), F1,28=6.09, p<0.05 (stress)). Bars represent mean+s.e.m., *indicate significant comparisons with all of the other groups, **P<0.01.***P<0.001.
Screening method for inherent susceptibility
At-risk individuals used for assessment of CRS and LAC effects have been identified using a recent devised screening method22 (Fig.SI1), which employs a modified version of the light dark test (LDT) and allow identification of at-risk individuals with an array of behavioral and molecular features of increased endogenous susceptibility to depressive-like behaviors22, 23. Details are available elsewhere22. Briefly, naïve male mice were videotaped and the time spent by each mouse in the light chamber was measured and scored by two independent blind observers. Mice were considered to have entered a chamber when all four paws were positioned into the chamber. No pretest (habituation session) was carried out before the test session. At-risk mice with an anxiety-prone phenotype are characterized by a molecular signature (i.e: high MR hippocampal levels) and by a behavioral signature of susceptibility (i.e.: low time spent in the light chamber, less than 110sec, in the modified version of the light-dark box24). At-risk mice were used for the CRS experiment with and without LAC treatment.
Prophylactic treatment
LAC (acetyl-L-carnitine, Sigma Aldrich) was orally administered, at a concentration of 0.3% dissolving the drug in the drinking water, for 3 days prior to the end of CRS. Additional animal groups were used to assure that the animals’ fluid intake and hydration state were not altered by the oral LAC administration. This was carried out evaluating the skin turgor, body weight and daily food and fluid consumption for 3 days. C57black 6N male mice in the LAC treatment group showed a fluid intake with an average of 4,5ml per day per mouse as non-stressed control animals. For example, when housed 4 per cage, the fluid intake per cage show an average of about 18ml regardless of the stress and LAC treatment.
Behavioral assessment after CRS and LAC treatment
Forced swim test (FST) was performed as previously described19, 22. Mice were placed individually into a vertical glass cylinder (25 cm in height and 12 cm in diameter) filled with 12-cm deep water at 23–24°C and videotaped for 6min. Two blind observers, who were not aware of the groups, scored the duration of immobility for 6min. Mice were considered ‘immobile’ if they showed only minimal movements to keep the head above water or floating.
Social interaction test was performed as previously described25 under red light. Mice were placed in an open-field arena (42×42×42cm) with a small wire animal box (10×6.5×42cm) placed at one side of the arena. Two blind observers scored exploratory behavior for 2.5min, respectively, in the absence and presence of a CD-1 mouse in the small wire animal box with one minute interval between the sessions. Social interaction behavior was calculated as the ratio of the time spent in an interaction zone near the novel animal divided by the time spent in the same area near the empty box. Impairment in social interaction is defined by a ratio below 1, namely animals spent less time near the empty cage than the novel animal.
Tissue processing
Mice were sacrificed immediately after the end of a 6-min FST to minimize any effects due to the minimal stress manipulation (Fig.1a). Fresh brains were extracted and processed for morphological analysis following the FD Rapid GolgiStain protocol (PK401, FD Neurotechnologies, Inc, Columbia, US) with few changes. When extracted, brains were coded for quantitative analysis. Both hemispheres were used to obtain 100μm sections using a vibratome. Sections were collected serially, dehydrated, cleared in xylene, and then cover-slipped.
Structural Analysis
Structural analysis was carried out by two observers who were blind to the sample conditions. We examined three sub-regions of the amygdala complex: lateral amygdala (LA, bregma −1.155 to −2.155), basolateral amygdala (BLA, bregma −1.155 to −2.255) and medial amygdala (MeA, bregma −1.155 to −2.055) using the Allen Mouse Brain Atlas26. We used the parahippocampal formation, the optic tract, and the endorhinal sulcus as anatomical references to localize the amygdala complex. To be selected for Golgi analysis, we established several criteria for Golgi-impregnated neurons: 1) no truncated dendrites are present on the neuron, 2) have neuronal cell bodies located within the boundaries of the MeA, LA and BLA, respectively 3) consistent staining along the entire length of the dendrites, 3) neurons had to be relatively isolated from other neurons to avoid interference during tracing with other dendrites. Neurons of the amygdala were also distinguished as pyramidal cells or stellate cells based on their morphology. Neurolucida tracings were obtained at 40x objective. We report that very few bipolar neurons have been depicted in the BLA to be included in the structural throughput.
Statistics
Statistical analyses were performed using a 2-way analysis of variances (ANOVA) followed by Sidak test for the post hoc analysis or unpaired t-Test.
Results
Prolonged 21day stress decreases dendritic elongation and arborization in MeA stellate, but not bipolar, neurons
We established the efficacy of our CRS paradigm (Fig.1a) to increase adrenal to body weight ratio compared with non-stressed mice (Fig.SI2) that is consistent with elevated glucocorticoid production contributing to dendritic remodeling in CA3 hippocampus27 and with the occurrence of depressive-like behavior as suggested by the increased immobility time in the forced swim test (FST) (Fig.1b) and decreased social interaction (Fig.1c). These were done on at-risk mice (Fig.SI1) identified by the LD screening (see Methods and SI) as susceptible individuals, which, in previous reports, have been characterized by behavioral and molecular signatures of susceptibility (e.g.: light avoidance, high expression of mineralocorticoid receptors MR in hippocampus, reduced ability to cope with stress)22. Morphological analysis of Golgi impregnated tissue revealed novel findings regarding stress-induced structural changes in the MeA (Fig.3). The MeA consists of two principal cell-types: stellate and bipolar neurons. The stellate neurons have a round cell body with more than two primary dendrites with no clear separation of basal and apical dendrites, whereas the bipolar neurons have an ovoid soma with two primary dendrites that start from two opposite poles of the cell body (Fig.2).
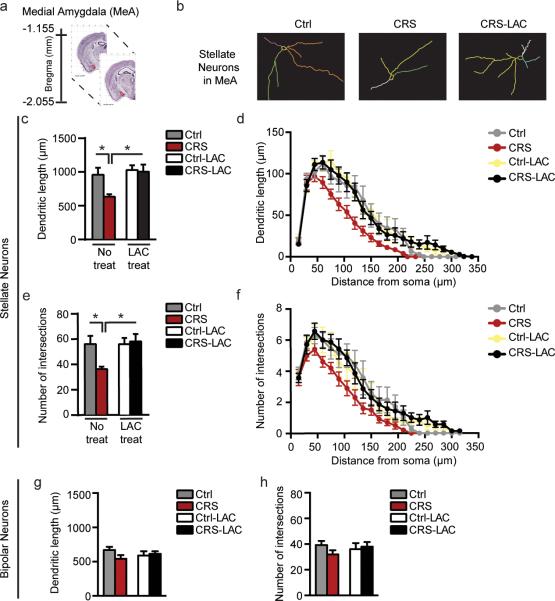
Figure 3. LAC oral prophylactic treatment rapidly promotes structural plasticity of MeA stellate, but not bipolar, neurons.
(a) Schematic of brain regional details along the dorso-ventral axis of the mouse brain with the bregma coordinates for the medial amygdala (MeA: highlighted in red), available from 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas26. (b) Representative tracing of Golgi-impregnated stellate neurons in the MeA. (c,e) CRS induced retraction and decreased the number of intersections of MeA stellate neurons, whereas 3 days of oral treatment with LAC, administered prior to the end of CRS, promoted structural plasticity of MeA stellate neurons (Length: F1,24=6.7, p<0.05 (treatment); F1,24=4.26, p<0.05 (stress); Intersections: F1,24=4.58, p<0.05 (treatment); F1,24=3.03, p=0.09 (stress)). (d,f) Morphometric Sholl analysis in incremental steps of 15μm from the soma shows that MeA stellate neurons underwent the most pronounced decrease in length and number of intersections in CRS mice starting from a distance of 105μm from the soma (see also SIFig.3). To note, Sholl analysis reveals that 3 days of LAC oral treatment promotes dendritic plasticity of MeA stellate neurons at the closer distance (75μm) from the soma (see also SIFig.3), suggesting that LAC prophylactic treatment induced growth of novel primary and secondary dendrites rather than reversing a pathological phenotype by elongation of CRS-shrink dendrites. (g,h) MeA bipolar neurons were not affected by CRS and LAC treatment as the total dendritic length and number of intersections appeared similar to non-stressed control mice. Bars represent mean+s.e.m.,*indicate significant comparisons with all of the other groups, *P<0.01.**P<0.001.
Figure 2. MeA and BLA neuronal cell-types.
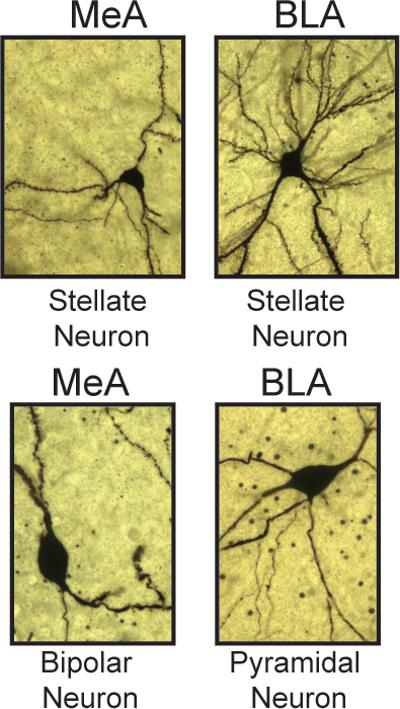
Neurons within the sub-regions of the amygdala were distinguished as pyramidal, stellate or bipolar cells based on the morphology of the cell body and arborization of dendrites. 40x z-stack photomicrographs of Golgi-impregnated neurons show MeA stellate neurons (top-left: round soma with more than two principal dendrites and no clear separation of basal and apical dendrites), MeA bipolar neurons (bottom-left: ovoid soma with two primary dendrites originating from two opposite poles of the cell body), BLA stellate neurons (top-right: cortical-like stellate cells with a round cell body and a complex dendritic tree with several radiating primary and secondary dendrites that extend in all directions), BLA pyramidal neurons (bottom-right: pyramidal-like shape soma with a thick principle dendrite that arises from the apex of the cell body, and multiple thinner principal dendrites that arises from the base and the sides of the soma). We note that stellate neurons in the BLA and MeA differ each other, whereby the first show a dendritic tree with a higher complexity as reflected by the longer dendrites and higher number of intersections.
Golgi analysis revealed that MeA stellate neurons from CRS male mice have shorter total dendritic length and decreased number of total intersections compared to non-stressed controls (Fig.3c,e). In-depth morphometric analysis with the Sholl method, where densities and length of dendrites arising from the soma are determined within concentric three-dimensional shells of increasing diameter (15μm) centered around the cell body, showed that the dendrites of stellate neurons from the CRS group were less elaborate starting from a distance of 105μm from the soma compared to the control group (Fig. 3d,f; SIFig.3).
In contrast to the stellate neurons, the bipolar neuronal subpopulation was unaffected by CRS, as the dendritic length and number of intersections remained at baseline with the control group (Fig.3g,h). Hence, structural CRS effects in the MeA were specific to the more elaborate stellate neurons that showed a dendritic shrinkage reminiscent of previous studies reporting that prolonged stress leads to a loss of spines in the output neurons of the MeA16.
Effects of acetyl-L-carnitine (LAC)
Recent work showed that the novel antidepressant candidate, L-acetylcarnitine (LAC, i.p.), rapidly corrected hippocampal deficits along with behavioral abnormalities (e.g.: sucrose preference and immobility at FST) in a genetic animal model of depression and in animals that develop depressive-like behaviors after chronic unpredictable stress (CUS)19-21. Therefore, we next tested the effects on behavior and the responses of the MeA to LAC treatment, orally administered 3 days prior to the end of the CRS (Fig.1a). We found that, after LAC treatment, dendrites of the MeA stellate neurons were more complex and longer as compared to the CRS group that received vehicle (Fig.3c,e). Behaviorally, LAC reduced passive behavior in a FST and increased social interaction ratio in the social avoidance test, opposing the effects of CRS, indicating an action of LAC to enhance resilience to stress (Fig.1b,c). In agreement with previous findings28, LAC had no effect upon the immobility time or social interaction ratio of non-stressed control animals (Fig.1b,c).
Additional Sholl analysis in incremental steps of 15μm from the soma showed that dendritic length and number of intersections of CRS LAC-treated MeA neurons underwent the most pronounced reduction of the stress-induced dendritic shrinkage within a distance of 75μm from the soma (Fig.3d,f, SIFig.3), indicating that LAC prophylactic treatment leads to the growth of novel primary and secondary dendrites rather than reversing a pathological phenotype by elongation of CRS-shrink dendrites. This is consistent with previous work showing that recovery from chronic stress involves morphological changes different from the initial dendritic shrinkage29. Furthermore, even in segments that did not exhibit a statistically significant difference after CRS as compared to non-stressed male mice, LAC-treated stellate neurons tended to have higher average values relative to CRS-untreated neurons (Fig.3d,f, Fig.SI3). Representative camera lucida drawings of control and CRS MeA stellate neurons along with LAC-treated CRS MeA stellate neurons are depicted in Figure 3b.
Responses of the BLA to 21 days of stress and to the novel antidepressant LAC
The BLA contains two principal cell-types: pyramidal and stellate neurons (Fig.2). These cell-types are similar to cortical pyramidal neurons and cortical spiny stellate cells, respectively, and no clear separation into basal and apical dendrites is evident. Pyramidal neurons are characterized by a pyramidal-shape soma with several principal dendrites originating from all directions of the cell body (Fig.2)9. Interestingly, BLA stellate neurons show a more complex tree than MeA stellate neurons (Fig.2). It is noteworthy that stellate neurons from naïve mice in the BLA appear longer and with a more sophisticated complexity than stellate neurons in the MeA, and this observation may be related to the connectivity of the BLA with the hippocampus, whereas the shorter dendrites of the MeA output nuclei may play a role in integrating the responses of the amygdala complex via their projections to the nearby BLA30.
Previous studies have shown opposite effects of a chronic immobilization stress paradigm within the sub-regions of the amygdala complex as indicated by increased spine density in the BLA and spine loss in the MeA8. Here, we also found opposite structural effects within the sub-regions of the amygdala in which CRS resulted in a significant increase in total dendritic length and number of intersections in BLA pyramidal neurons compared to mice that were not subjected to stress (Fig.4c,e). Sholl-segmental analysis in incremental steps of 15μm from the soma revealed that dendrites from the CRS group were more elaborate at a close distance to the soma compared to the non-stressed group (Fig.4d,f), revealing that BLA pyramidal neurons underwent the most pronounced increase within a distance of 60μm from the soma (Fig. 4d,f).
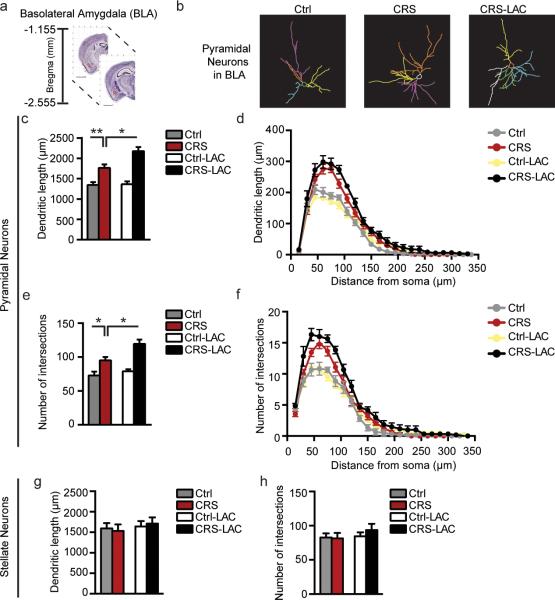
Figure 4. BLA pyramidal, but not stellate, neurons show dendritic elongation in responses to prolonged 21day chronic stress and LAC antidepressant treatment.
(a) Schematic of brain regional details along the dorso-ventral axis of the mouse brain with the bregma coordinates for the basolateral amygdala (BLA: highlighted in red), available from 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas26. (b) Representative tracing of Golgi-impregnated pyramidal neurons in the BLA. (c) CRS induced elongation of BLA pyramidal neurons and LAC treatment, administered prior to the end of CRS, further increased the total length of CRS pyramidal neurons in the BLA while promoting plasticity of the CRS-shrink MeA stellate neurons, as shown in Fig.3. (Length: F1,26=4.78, p<0.05 (treatment); F1,26=38.86, p<0.0001 (stress)). (e) CRS resulted in increased number of total dendritic intersections in the BLA pyramidal neurons and this increase was further boosted by LAC oral treatment. (Intersections: F1,26=6.21, p<0.05 (treatment); F1,26=26.51, p=0.0001 (stress)). (d,f) Sholl-segmental analysis in incremental steps of 15μm from the soma shows that BLA pyramidal neurons underwent the most pronounced increase in length and number of intersections after CRS at a distance of 60μm from the soma. In CRS mice treated with LAC no increase was observed in the dendritic length at the various incremental shells, whereas the number of dendritic intersections showed an increase at a close distance of 30μm from the soma. (g,h) BLA stellate neurons were not affected by CRS and LAC treatment as the total dendritic length and number of intersections appeared similar to non-stressed control mice. Bars represent mean+s.e.m., *indicate significant comparisons with all of the other groups, *P<0.05, **P<0.01, ***P<0.001.
Interestingly, after LAC treatment, BLA pyramidal neurons continued to show increased length and complexity. Indeed, CRS mice treated with LAC showed slightly increased total length and number of intersections of pyramidal neurons as compared to CRS mice (Fig.4c,e). Additional Sholl-segmental analysis to evaluate LAC treatment effects showed no increase in the dendritic length at the various incremental shells from the soma, but revealed a consistent increase in the number of intersections at 30μm from the soma, suggesting that LAC treatment led to branching of dendrites at a close distance from the cell body. Representative camera lucida drawings of control and CRS BLA pyramidal neurons along with LAC-treated CRS BLA pyramidal neurons are shown in Figure 4b.
CRS structural effects appeared to be specific to the BLA pyramidal neurons as the BLA stellate neurons were unaffected by CRS and LAC treatment as shown by the finding that dendritic length and number of intersections remained at baseline with the control group (Fig.4g,h).
Responses of the LA to prolonged 21day stress: divergent effects with the BLA and MeA
We extended our analysis on the effects of CRS to the lateral amygdala (LA, Fig.5a), which anatomically is next to the BLA, although the two subregions show a different cytoarchitectonic organization. In the LA, we restricted our structural analysis to stellate neurons, which appeared with a round-to ovoid-shaped soma with variable size and dendrites staring from different parts of the cell body and no clear distinction of basal and apical dendrites was observed. As for the stellate neurons depicted within the BLA, we found that CRS mice showed no change in the dendritic length and number of intersections in LA stellate neurons. No structural change was observed in LA stellate neurons of CRS mice treated with LAC (Fig.5b,c). Thus, CRS resulted in a specific structural plasticity of the BLA pyramidal neurons, which are excitatory neurons9, while not affecting the neurons of the near LA9.
Figure 5. LA stellate neurons are unaffected by prolonged 21day CRS and LAC treatment.
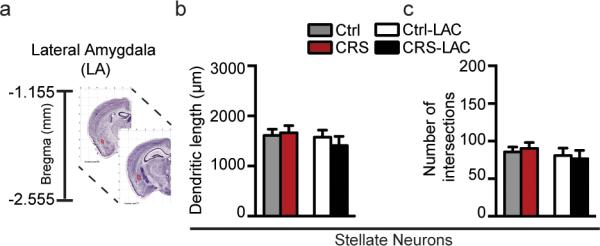
(a) Schematic of brain regional details along the dorso-ventral axis of the mouse brain with the bregma coordinates for the lateral amygdala (LA: highlighted in red), available from 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas26. (b,c) The dendritic length and number of intersections of LA stellate neurons were unaffected by 21day CRS and LAC treatment.
DISCUSSION
Despite the well-known role of the hippocampus and basolateral amygdala in behavioral, autonomic and endocrine responses to epigenetic environmental influences8, less is known about the role of the medial amygdala in these processes. Here, we have uncovered contrasting signs of structural plasticity within the three sub-regions of the amygdala - MeA, BLA and LA - in response to prolonged 21day CRS and a prophylactic treatment with LAC. Moreover, LAC also promoted pro-resilient effects in at-risk individuals as suggested by better outcomes of CRS mice on social interaction and forced swim tests.
Structural contrast between MeA and BLA
Prolonged 21day CRS leads to growth of BLA pyramidal neurons in agreement with previous studies showing that 10 day chronic immobilization stress (CIS) leads to dendritic expansion of BLA pyramidal neurons15. No effect of CRS was observed in the BLA stellate neurons in contrast to effects of CIS, suggesting that, although both stress paradigms induce arborization of BLA pyramidal neurons and increased production of adrenal steroids8, other processes may be involved in mediating the structural effects of stress on the brain, although this may also be a difference between rats and mice.
In contrast to the dendritic growth of BLA pyramidal neurons, chronic stress leads to dendritic shrinkage of MeA stellate neurons with a non-significant trend in the less complex MeA bipolar neurons. Such contrasting structural plasticity between the MeA and BLA is in line with published findings showing that stress induced spine loss in the MeA and spine formation in the BLA along with leading to opposite effects on the serine protease tissue-plasminogen activator (tPA), a key mediator of morphological plasticity and synaptic re-organization16. No structural effect was found in the stellate neurons of the LA, which is anatomically localized close to the BLA.
Recently, we have shown that the same CRS paradigm opens windows of epigenetic plasticity in hippocampus and alters the responses to familiar and novel stressors31. We showed these behavioral outcomes were accompanied by a glutamatergic hyperactivity of the ventral hippocampus based on the continued down-regulation of the regulator of glutamate release, mGlu2 receptors, which normally inhibit glutamate release32. Therefore, stress-induced structural shrinkage in the MeA may be a homeostatic response to the increased glutamatergic overflow from the nearby hippocampus, which directly projects to the MeA33. Previous studies have also shown bidirectional connectivity between the BLA and ventral hippocampus, a target of antidepressants34. Hence, it is possible that the stress-induced elongation of BLA pyramidal neurons may be a sign of adaptive plasticity to limit this dissociation effect between the BLA and MeA in the attempt to reestablish direct and indirect synaptic connectivity with the stress-induced shorter dendrites of the MeA. Such potential adaptive plasticity of the BLA is also supported by the lack of a reversal effect of LAC on CRS-induced increased dendritic length and complexity of BLA pyramidal neurons.
Effects of acetyl-L-carnitine in at-risk individuals
Thus, as an approach to promote resilience to stress, we found that three days of oral administration with acetyl-L-carnitine (LAC) - a novel antidepressant that was shown to be more rapidly effective than fluoxetine and chlorimipramine in humans and animal models19-21, 35, 36 – enhances behaviors impaired by 21 days CRS and promotes structural plasticity of a key limbic brain region, the medial amygdala, that is in a direction opposite to the effects of CRS.
LAC is a nutritional supplement and is also synthesized from lysine and methionine in the brain, liver and kidney. Among its beneficial effects on the brain and the body35, 37, LAC regulates mitochondrial homeostasis by facilitating transfer of fatty acids from the cytoplasm to the mitochondrial matrix for subsequent β-oxidation38. Thus, it is expected that LAC has other beneficial effects, the extent of which remains to be fully elucidated. Also, it has been shown that LAC exerts antidepressant-like effects in several animal models, similar to what SSRIs do more slowly in the same animal model39. Here, we show that LAC prophylactic treatment - administered 3 days prior to the end of chronic stress - exerted pro-resilient effects upon at-risk individuals selected for a reduced ability to cope with stress22. Using a recently developed screening method22 built upon a modified version of a simple light-dark test24 that allows identification of mice with behavioral and molecular signatures of susceptibility (e.g.: high hippocampal mineralocorticoid receptors MR levels, light avoidance and reduced ability to cope with stress due to a robust down-regulation of mGlu2 receptors in hippocampus22), we demonstrated that the windows of epigenetic plasticity opened by CRS in the immediate aftermath of stress32 in at-risk individuals can be manipulated by LAC pharmacological intervention. We propose that LAC increases resilience by reestablishing balanced neural circuitry in limbic brain regions, doing so by promote dendritic growth of the MeA stellate neurons while not shortening dendrites of the BLA pyramidal neurons in at-risk individuals that are increased by CRS.
Thus, regulation of MeA stellate neuron structural plasticity is one sign of increased ability to cope with stress following LAC treatment that also reduces the occurrence of anxiety- and depressive-like behaviors. The rapid LAC effects to increase MeA dendrite branching and length show again that the cytoskeleton can rapidly depolymerize and repolymerize when needed. This has been shown in the hippocampus of hibernating animals where rapid shrinkage of CA3 dendrites is seen within hours of the onset of hibernation and growth of those dendrites occurs within hours of termination of hibernation40-42. Importantly, we found that LAC increased the growth of novel primary and secondary dendrites in MeA rather than reversing CRS-induced shrinkage of distal dendrites. This has been found previously for neurons in the PFC during recovery from chronic stress29.
Function of the medial amygdala
Here, we found that CRS mice, which show dendritic shrinkage of MeA stellate neurons, have a reduced social interaction ratio and that such behavior along with CRS-increased passive behavior at the FST was not found in CRS mice administered with LAC for 3d at the end of CRS. Such emerging role of the MeA in social and passive behaviors12, hallmarks of psychiatric disorders as depression, anxiety and autism43 is in agreement with previous brain imaging studies showing a positive correlation between the volume of the amygdala and social interactions in humans44. Of note, mice here identified as at-risk individuals, using the LD screening, that show MeA impairments after CRS are the same mice that show high vulnerability to social defeat stress45, which results in susceptible and unsusceptible subpopulations, with the susceptible mice showing impairments in social interactions46. Therefore, future studies using the social defeat stress model of susceptibility and resilience are warranted to further investigate the role of the MeA in resilience vs. susceptibility to stress, and particularly for social interactions.
Dysfunction of MeA is increasingly considered to be an integral component of the olfactory system, so important in mice for social interactions, that includes also olfactory bulb and piriform cortex. Interestingly, the accessory olfactory bulb and piriform cortex form dense synaptic contacts with the MeA, while the basolateral nuclei of the amygdala do not receive projections from the olfactory bulb and form weak connections with the piriform cortex47, 48. In animal models, for example, exposure to a predator odor results in increasing freezing behaviors associated with amygdaloidal changes in the expression of the sub-unit α2-δ1 that regulate calcium-signaling24 reminiscent of lesions studies showing that ablation of the medial amygdala reduce freezing in response to predator odor exposure49.
In conclusion, the use of a screening test to identify at-risk individuals and the rapid effects of LAC upon such at-risk individuals are important as translational models because antidepressants are typically used to treat depressive symptoms, and a therapeutic treatment that has rapid, pro-resilient effects to restore or maintain normal function can be useful to reduce a reoccurrence of depressive episodes in at-risk individuals. Future research is needed to uncover the molecular signaling that regulates the cytoskeletal assembly and disassembly processes that drive structural plasticity within the MeA, a limbic brain region here identified as a novel target of structural and functional remodeling by stressors.
Supplementary Material
Acknowledgments
We thank Anjali Ferris for her commitment in blind analyses for structural and behavioral experiments. This work was supported by the AFSP (American Foundation for Suicide Prevention), HDRF (Hope for Depression Research Foundation), NIH Grant RO1 MH41256 and by Grant UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
Footnotes
Authors declare no conflict of interest.
References
- 1.McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences. 2010;1204:38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwen BS. Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiology of Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Southwick SM, Charney DS. The Science of Resilience: Implications for the Prevention and Treatment of Depression. Science. 2012;338(6103):79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. STRESS AND HIPPOCAMPAL PLASTICITY. Annual Review of Neuroscience. 1999;22(1):105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.JE L. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. 1992:106. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 11.Vinkers CH, Bijlsma EY, Houtepen LC, Westphal KG, Veening JG, Groenink L, et al. Medial amygdala lesions differentially influence stress responsivity and sensorimotor gating in rats. Physiology & behavior. 2010;99(3):395–401. doi: 10.1016/j.physbeh.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhao S, Wu Z, Feng Y, Zhao C, Zhang C. Oxytocin in the regulation of social behaviours in medial amygdala-lesioned mice via the inhibition of the extracellular signal-regulated kinase signalling pathway. Clinical and experimental pharmacology & physiology. 2015;42(5):465–474. doi: 10.1111/1440-1681.12378. [DOI] [PubMed] [Google Scholar]
- 13.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in neurosciences. 1997;20(11):517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 14.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress- induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144(1):8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 17.Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(2):586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tejeda HA, O'Donnell P. Amygdala Inputs to the Prefrontal Cortex Elicit Heterosynaptic Suppression of Hippocampal Inputs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(43):14365–14374. doi: 10.1523/JNEUROSCI.0837-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4804–4809. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo SJ, Charney DS. Next generation antidepressants. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4441–4442. doi: 10.1073/pnas.1301593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flight MH. Antidepressant epigenetic action. Nat Rev Neurosci. 2013;14:226. doi: 10.1038/nrn3466. [DOI] [PubMed] [Google Scholar]
- 22.Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Molecular psychiatry. 2015;20(6):755–763. doi: 10.1038/mp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen BS, Nasca C, Hodes GE, Kana V, Nestler EJ, Russo SJ. Glucocorticoids-IL6 crosstalk allows identification of inherent individual differences that predict and promote vulnerability to social stress. 2015 SfN Abstract. [Google Scholar]
- 24.Nasca C, Orlando R, Marchiafava M, Boldrini P, Battaglia G, Scaccianoce S, et al. Exposure to predator odor and resulting anxiety enhances the expression of the alpha2 delta subunit of voltage-sensitive calcium channels in the amygdala. Journal of neurochemistry. 2013;125(5):649–656. doi: 10.1111/j.1471-4159.2012.07895.x. [DOI] [PubMed] [Google Scholar]
- 25.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 27.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 28.Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proceedings of the National Academy of Sciences. 2013;110(12):4804–4809. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164(2):798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sah P, Faber ES, Loped De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 31.Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nisticò R, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA. 2015;112:14960–14965. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasca C, Zelli Danielle, Bigio Benedetta, Piccinin Sonia, Scaccianoce Sergio, Nisticò Robert, McEwen Bruce S. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. The Journal of comparative neurology. 2006;496(3):349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- 34.O'Leary OF, Cryan JF. A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends in pharmacological sciences. 2014;35(12):675–687. doi: 10.1016/j.tips.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression. Molecular psychiatry. 2000;5(6):616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Lu Y, Xue Z, Li C, Wang C, Zhao X, et al. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience. 2015;285:281–291. doi: 10.1016/j.neuroscience.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Schaevitz LR, Nicolai R, Lopez CM, D'Iddio S, Iannoni E, Berger-Sweeney JE. Acetyl-L-carnitine improves behavior and dendritic morphology in a mouse model of Rett syndrome. PloS one. 2012;7(12):e51586. doi: 10.1371/journal.pone.0051586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz IB, McEwen BS. Effects of carnitine on fatty-acid oxidation by muscle. Science. 1959;129:334–335. doi: 10.1126/science.129.3345.334. [DOI] [PubMed] [Google Scholar]
- 39.Nasca C, Zelli D, Lau T. McEwen BS mGlu2 is a key mediator in the responses to next-generation antidepressant treatments: epigenetic mechanisms of neuronal plasticity. 2014 SfN Abstract 2014. [Google Scholar]
- 40.McEwen BS. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain research. 2015 doi: 10.1016/j.brainres.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 41.Magariños AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(49):18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(18):6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steger MF, Kashdan TB. Depression and Everyday Social Activity, Belonging, and Well-Being. Journal of counseling psychology. 2009;56(2):289–300. doi: 10.1037/a0015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala Volume and Social Network Size in Humans. Nature neuroscience. 2011;14(2):163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McEwen BS, Nasca C, Hodes GE, Kana V, Nestler EJ, Russo SJ. Glucocorticoids-IL6 crosstalk allows identification of inherent individual differences that predict and promote vulnerability to social stress. 2015 SfN Abstract. [Google Scholar]
- 46.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;8:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 48.Mouly AM, Sullivan R. Memory and plasticity in the olfactory system: from infancy to adulthood. In: Menini A, editor. The Neurobiology of Olfaction. CRC Press; Boca Raton, FL, USA: 2010. Chap 15. [PubMed] [Google Scholar]
- 49.Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behavioral neuroscience. 2004;118(2):324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.