Abstract
Study Design
Retrospective, observational study.
Objective
To determine the utilization of various treatment modalities in the management of degenerative spondylolisthesis within Medicare beneficiaries.
Summary of Background Data
Degenerative lumbar spondylolisthesis is a condition often identified in symptomatic low back pain. A variety of treatment algorithms including physical therapy and interventional techniques can be used to manage clinically significant degenerative spondylolisthesis.
Methods
This study utilized the 5% national sample of Medicare carrier claims from 2000 through 2011. A cohort of beneficiaries with a new ICD-9 diagnosis code for degenerative lumbar spondylolisthesis was identified. Current procedural terminology codes were used to identify the number of procedures performed each year by specialty on this cohort.
Results
A total of 95,647 individuals were included in the analysis. Average age at the time of initial diagnosis was 72.8 ± 9.8 years. Within this study cohort, spondylolisthesis was more prevalent in females (69%) than males and in Caucasians (88%) compared to other racial demographics. Over 40% of beneficiaries underwent at least one injection, approximately one third (37%) participated in physical therapy, one in five (22%) underwent spinal surgery, and one third (36%) did not utilize any of these interventions. Greater than half of all procedures (124,280/216,088) occurred within 2 years of diagnosis. The ratio of focal interventions (transforaminal and facet interventions) to less selective (interlaminar) procedures was greater for the specialty of Physical Medicine and Rehabilitation compared to the specialties of Anesthesiology, Interventional Radiology, Neurosurgery, and Orthopedic Surgery. The majority of physical therapy was dedicated to passive treatment modalities and range of motion exercises rather than active strengthening modalities within this cohort.
Conclusion
Interventional techniques and physical therapy are frequently used treatment modalities for symptomatic degenerative spondylolisthesis. Understanding utilization of these techniques is important to determine relative clinical efficacies and to optimize future health care expenditures.
Keywords: Degenerative spondylolisthesis, low back pain, prevalence, epidural steroid injection, physical therapy, treatment utilization, spinal interventional techniques, interventional pain management, Medicare
Introduction
Degenerative lumbar spondylolisthesis is a spinal instability that results from progressive degeneration of the facet joints, intervertebral discs, and ligamentous structures without disruption of the posterior vertebral ring1. The condition is defined as the anterior subluxation of one vertebra in relation to the next caudal vertebra and occurs most frequently at the L4–5 level2–4. Degenerative spondylolisthesis is often asymptomatic but can present clinically as symptomatic axial low back pain, radicular pain, or neurogenic claudication as it is associated with lumbar stenosis. Prevalence within the United States of America has been reported to range widely from 20–25% of females and 4–31% of males5–10.
A variety of treatment algorithms including physical therapy and other interventional techniques can be used to manage clinically significant degenerative spondylolisthesis. However, the most recent North American Spine Society Clinical Guidelines could not adequately determine the effectiveness of medical and other non-surgical interventions to treat degenerative spondylolisthesis due to insufficient evidence-based studies11. A lack of consensus on the appropriate nonsurgical treatment algorithm for this condition has resulted in highly varied practices among providers9. Physical therapy prescriptions may focus on passive therapeutic modalities such as heat, ultrasound, massage, and manual traction or may alternatively stress active modalities of muscular strengthening and stabilization training. Spinal injection procedures include focal options to diagnostically isolate and treat the putative pain generators (transforaminal epidural injections, facet interventions) and less-specific treatment options such as interlaminar and caudal epidural injections. Some research suggests that using transforaminal injections and active physical therapy modalities may be especially beneficial and delay the need for surgical intervention to treat spondylolisthesis12,13. However, surgical interventions including decompression or arthrodesis procedures are available if non-operative modalities are unsuccessful.
The aims of this study were (1) to evaluate the incidence of degenerative spondylolisthesis within Medicare beneficiaries from 2000 through 2011; and (2) to describe the utilization of spinal interventional techniques, surgical interventions, and physical therapy within this newly diagnosed population.
Methods
To allow for feasible data analysis, this study utilized an initial cohort comprised of the standard 5% sample of all patients with ICD-9 codes for back pain recorded in the Medicare Carrier files, from 2000 to 2011. This database has been utilized previously to track subjects over time and is considered a good representation of the overall Medicare population14,15. Medicare covers the cost of health care services, including medically indicated surgeries, to US citizens that are at least 65 years in age, have been permanently disabled for at least two years, or are on dialysis. Beneficiaries of all age groups were included. This research was granted an exemption from institutional review board review.
A previously validated coding definition model was utilized to determine all inclusion and exclusion ICD-9 codes15. Beneficiaries with a new diagnosis of degenerative lumbar spondylolisthesis were isolated through a two-step process. First, all subjects assigned a primary or secondary ICD-9 diagnosis code for degenerative lumbar spondylolisthesis (738.4, 756.12) were extracted from the initial cohort. Second, this subpopulation was refined to determine the initial use of these diagnosis codes by eliminating all subjects with an association of a spondylolisthesis ICD-9 code to a billable office visit, therapy session, imaging study, or procedure during the previous 365 day period. Subjects with a concurrent diagnosis of lumbar trauma (805.0, 806.0–806.39, 952.0–952.20), infection (323, 324.1, 324.1, 336, 336.1, 730.08) or malignancy (170.6, 192.2, 192.3, 195.3, 199.0, 199.1) were excluded.
Current procedural terminology (CPT) codes for lumbar interventional techniques from 2000 to 2011 were used to identify the number of procedures performed each post-diagnosis year, including a sub-analysis of utilization by billing provider specialty. Several procedures underwent coding modifications and required extraction of multiple procedure codes. The following procedures were evaluated in terms of billing frequency by physician specialty: interventional techniques 20552 and 20553 (trigger point procedures), 27096 (injection procedure for SI joint; arthrography and/or anesthetic steroid), 62263 (percutaneous lysis of epidural adhesions using solution injection or mechanical means including radiologic localization, multiple adhesiolysis sessions, 2 or more days), 62264 (one day), 62282 (interlaminar epidural, lumbar, sacral), 62290 (discogram), 62311 (interlaminar epidural lumbar, sacral), 64475 (lumbar or sacral transforaminal, single level), 64476 (lumbar or sacral transforaminal, each additional level), 64483 (lumbar or sacral transforaminal, single level), 64484 (lumbar or sacral transforaminal, each additional level), 64493 (paravertebral facet joint injection, lumbar or sacral, single level), 64494 (paravertebral facet joint injection, lumbar or sacral, second level), 64495 (paravertebral facet joint injection, lumbar or sacral, third and each additional level), 64622 (destruction by neurolytic agent, paravertebral facet joint nerve; lumbar or sacral, single level), 64623 (lumbar or sacral, each additional level), 64635 (destruction by neurolytic agent, paravertebral facet joint nerve; lumbar or sacral, single level with image guidance), 64636 (lumbar or sacral, each additional level with image guidance). Additionally, the utilization of a −50 modifier (bilateral procedure) was recorded for each interventional technique.
Similarly, CPT codes were utilized to identify surgical interventions performed on this subset of beneficiaries. The frequency of three types of surgical intervention was investigated. Dorsal column stimulator placements were isolated using CPT code 63650. Standalone decompression procedures were isolated using the following CPT codes without additional CPT codes for instrumentation or fusion substrate within 14 calendar days: 63005 (lumbar laminectomy without facetectomy, foraminotomy or discectomy, 1 or 2 segments), 63017 (more than 2 segments), 63012 (lumbar laminectomy for spondylolisthesis with removal of abnormal facet or pars interarticularis), 63030 (lumbar laminotomy including partial facetectomy, foraminotomy and/or excision of herniated disc), 63035 (each additional interspace), 63042 (re-exploration lumbar laminotomy including partial facetectomy, foraminotomy and/or excision of herniated disc), 63044 (each additional interspace), 63047 (lumbar Laminectomy, facetectomy and foraminotomy, single level), 63048 (each additional segment). The frequency of arthrodesis procedures was identified through either designation of a specified lumbar fusion CPT code or by the presence of a standalone decompression CPT code plus an additional CPT code for lumbar instrumentation or fusion substrate within 14 calendar days. Lumbar arthrodesis CPT codes utilized were: 22558 (anterior lumbar interbody fusion, first interspace), 22585 (anterior lumbar interbody fusion, additional interspace), 22612 (lumbar posterolateral fusion, first level), 22614 (each additional segment), 22630 (posterior lumbar interbody fusion, first interspace), 22632 (each additional interspace), 22633 (combined fusion, posterolateral fusion, with posterior interbody fusion, first segment), 22634 (each additional segment). Instrumentation/ fusion substrate CPT codes were: 22840 (posterior non-segmental instrumentation), 22842 (posterior segmental instrumentation, 3–6 segments), 22843 (7–12 segments), 22844 (13+ segments), 22845 (anterior instrumentation, 2–3 segments), 22846 (4–7 segments), 22847 (8 or more segments), 22851 (application of biomechanical device, first interspace), 20930 (allograft, morselized), 22931 (allograft, structural), 20936 (autograft, same incision), 20937 (autograft morselized, separate incision), 20938 (autograft structural, separate incision).
Current billing codes for several modalities of physical therapy from 2000–2011 were used to identify the number of procedures performed each year on this cohort, using the CMS Carrier file. The utilization of multiple billing codes, including 97001 (initial evaluation), 97002 (repeat evaluation), 97113 (water therapy, 15 minutes), and 97535 (activities of daily living training, 15 minutes) were assessed. Codes designated to be passive modalities of physical therapy were 97032 (electrical stimulation, 15 minutes), 97035 (ultrasound, 15 minutes), 97124 (massage, 15 minutes), and 97140 (manual traction, 15 minutes). Range of motion and flexibility therapy utilization was extracted with 97110 (15 minutes). Active modalities of physical therapy were designated to be 97112 (neuromuscular re-education, 15 minutes), 97116 (gait training and stair climbing, 15 minutes), and 97530 (dynamic modalities to improve functional performance, 15 minutes). Numbers are reported as 15 minute billing units unless otherwise stated.
Results
A total of 95,647 individuals with a new diagnosis of degenerative spondylolisthesis were included in the analysis after exclusion criteria were applied. Average age at the time of initial diagnosis was 72.8 ± 9.8 years (range 21–104 years). Within this population, the male to female ratio of a new diagnosis was 1:2.2. Caucasians represented 87.8% of newly diagnosed beneficiaries compared to 7.6% for self-reported African Americans (Table 1). Greater than 40% of beneficiaries included in this analysis underwent at least one injection over the study period. Approximately one third (37%) participated in physical therapy, one in five (21%) underwent spinal surgery, and one third (36%) did not utilize any of these interventions (Table 2).
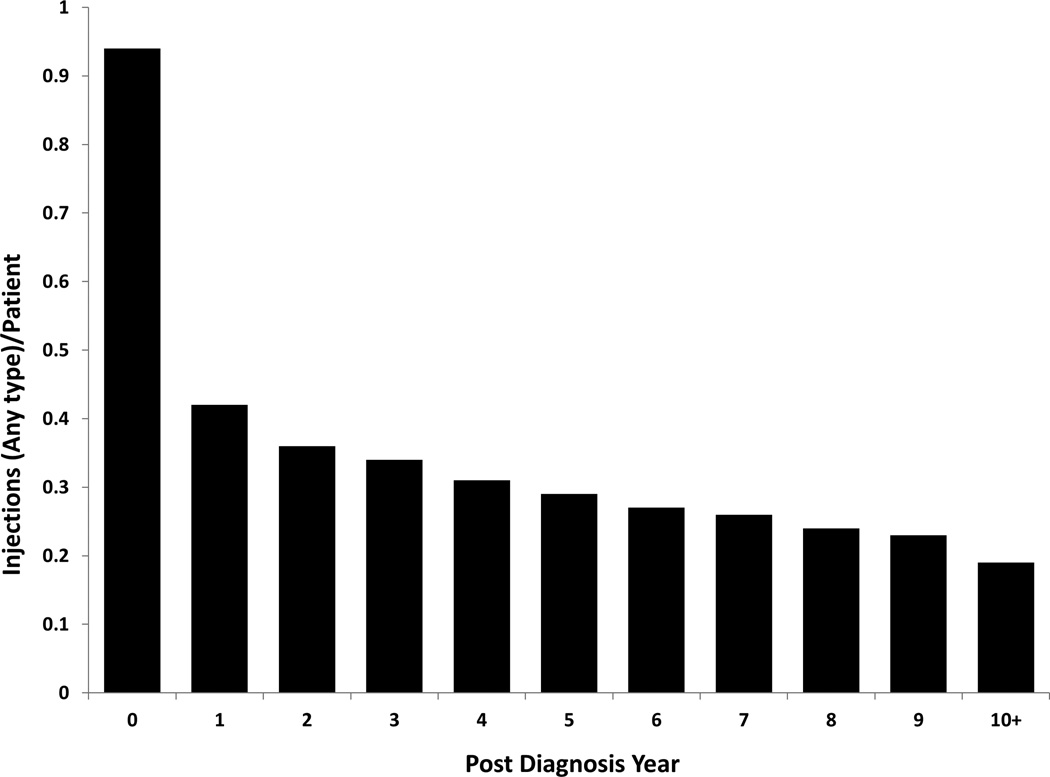
There were 216,088 total procedures performed on 53,297 beneficiaries within this cohort over the study period (4.1 procedures per treated individual). Injection therapy was most frequently utilized (0.94 injections performed per patient) during the initial year of a degenerative spondylolisthesis diagnosis. Utilization sharply decreased to 0.42 injections per patient the second year after diagnosis and continued to downtrend over each subsequent year (Figure 1A). Overall, greater than half of all procedures (58%) occurred within 2 years of diagnosis. The utilization of each injection technique by post-year diagnosis is illustrated in Table 3.
Figure 1.
A. Mean number of spinal injection procedures performed per beneficiary by post-diagnosis year
B. Mean number of physical therapy billing units per beneficiary by post-diagnosis year
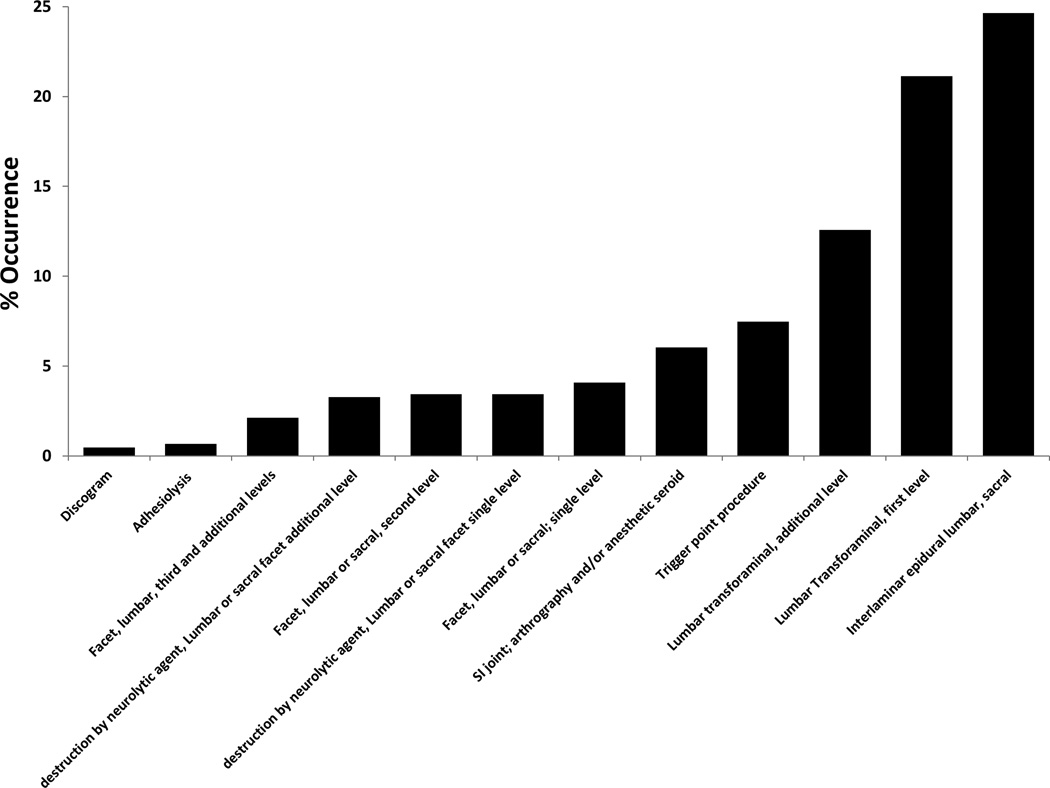
An interlaminar or caudal injection technique was performed on 24.6% of the beneficiaries over the course of this study. A transforaminal epidural approach was performed on 21.1% of beneficiaries with 12.6% undergoing a multi-level transforaminal procedure within 10 years of a degenerative spondylolisthesis diagnosis. Trigger point injections (7.5%), sacroiliac injections (6.0%), facet or medial branch blocks (4.1% initial level, 3.4% multi-level), and radiofrequency ablations (3.4% initial level, 3.3% multi-level) were also frequently utilized in this patient population (Figure 2).
Figure 2.
Percentage of beneficiaries that underwent each type of procedure after initial degenerative spondylolisthesis diagnosis.
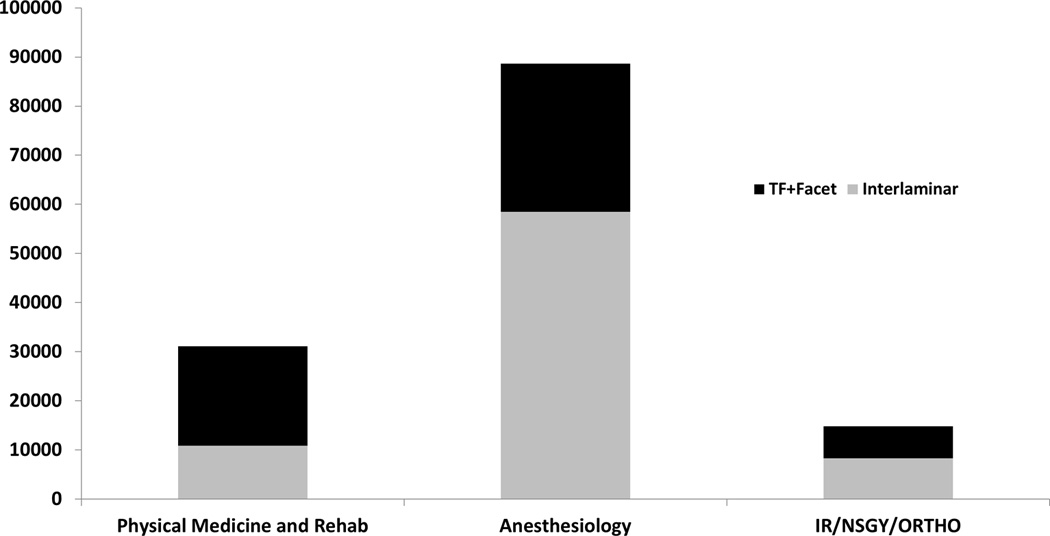
A subanalysis of utilization by injection type was performed on three distinct groups of billing providers. Physical Medicine and Rehabilitation specialists performed 35,141 (16%) total procedures and Anesthesiologists performed 77,353 (36%) total procedures on the beneficiaries. The third group, which was comprised of Interventional Radiologists, Neurosurgeons, and Orthopedic Surgeons (INOS) performed 15,527 (7%) total injections. The ratio of focal interventions targeted toward putative pain generators (transforaminal and facet) to less specific, (interlaminar/caudal) procedures was 2.4 times greater for the specialty of Physical Medicine and Rehabilitation compared to INOS and 2.9 times greater for Physical Medicine specialists compared to Anesthesiologists (Figure 3).
Figure 3.
Focal (transforaminal epidural injections and facet interventions) versus less specific (interlaminar) interventions by physician specialty.
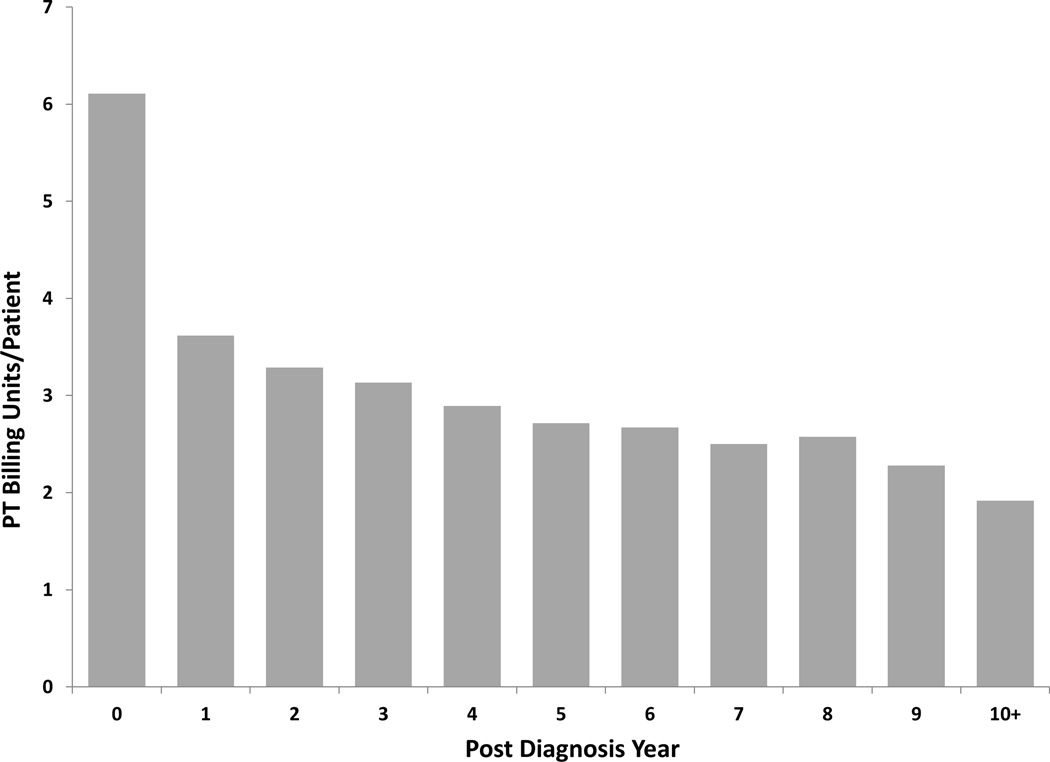
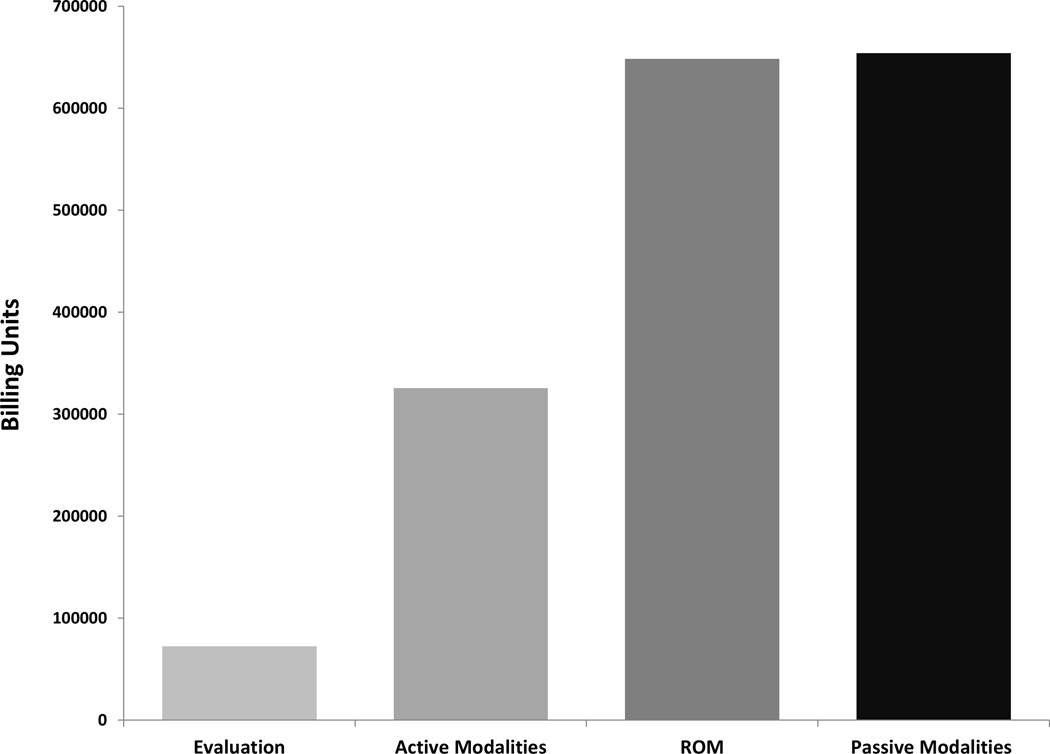
There were 57,596 initial evaluations for physical therapy performed within this cohort (Table 4). Range of motion and flexibility exercises accounted for 648,346 (37%) of the total physical therapy billing units. Active modalities of strengthening accounted for 325,487 (19%) of the total units (32,518 (2%) units gait training and stair climbing; 139,237 (8%) units dynamic functional performance; 153,732 (9%) units neuromuscular re-education, Figure 4). There were 654,130 (37%) units of passive modalities (39,190 (2%) units massage therapy; 74,344 (4%) units electrical stimulation; 157,866 (9%) units of ultrasound; 382,730 (22%) units of manual traction, Figure 4) and 29,510 (2%) total billing units of water therapy (Table 4). Physical therapy was most frequently utilized during the initial year of diagnosis (6.1 billing units/beneficiary). Similar to interventional techniques, utilization of physical therapy down trended with each subsequent year after the first year of diagnosis (Figure 1B).
Figure 4.
Overall billing units for each type of physical therapy modality. Active modalities includes stair climbing, dynamic modalities to improve functional performance, and neuromuscular re-education. Passive modalities include massage, electrical stimulation, ultrasound, and manual traction.
Lumbar arthrodesis was performed on 14,563 beneficiaries. Approximately 69% (11,185) of these individuals underwent arthrodesis within one year of initial diagnosis. A standalone lumbar decompression procedure (without arthrodesis) was performed on 5,646 subjects and a dorsal column stimulator was placed in 958 newly diagnosed individuals over the study period.
Discussion
An initial diagnosis of degenerative spondylolisthesis was commonly assigned to Medicare beneficiaries. The aggregate number of beneficiaries from 2000 through 2011 with a newly diagnosed spondylolisthesis can be estimated to be approximately 1.9 million individuals after multiplying the frequency of diagnosis in the 5% sample by a factor of 20. Since average Medicare enrollment was 48 million individuals over the period of this study, approximately 4% of all beneficiaries received a new diagnosis of degenerative spondylolisthesis16.
The incidence of degenerative spondylolisthesis within this cohort was more than two-fold higher in females compared to males. Several epidemiologic studies of degenerative spondylolisthesis have reported higher disease prevalence in the female population6,17–20. These studies report male to female anterolisthesis prevalence ratios that range from 1:1.6 to 1:6.4. Proposed mechanisms for this gender disparity include relative generalized joint laxity in females compared to males as well as a possible association with pregnancy, oophorectomy, and post-menopausal status6,17,19,20. Other studies have implicated that paraspinal muscular development may contribute to increased prevalence among females18. Kalichman et al. compared lumbar paraspinal muscle density in individuals with a degenerative lumbar spondylolisthesis and compared the value to individuals that did not have a lumbar deformity within the Framingham Study. These authors reported female sex, increased BMI, and advanced age were all associated with decreased multifidus and erector spinae density. Furthermore, they described a significant relationship between decreased multifidus density and an increased rate of anterolisthesis18.
The percentage of degenerative anterolisthesis among white and black racial groups in this study is proportionally consistent with the racial composition of the United States geriatric population reported by the 2006 Census21. However, several epidemiologic studies have reported an increased prevalence of degenerative anterolisthesis in black individuals. Rosenberg et al. reported the ratio of degenerative anterolisthesis in black women compared to white women was 3 to 1 within their study cohort. These authors proposed two possible mechanisms for this disparity. First, they described a comparatively higher frequency of L5 sacralization in black individuals. This variation increases lumbosacral stability and results in distribution of greater magnitude biomechanical forces to the L4–5 level. Second, these authors proposed that increased prevalence of anterolisthesis among black individuals could be a result of more frequent absence of accessory processes for ligamentous attachments at the fourth lumbar vertebral level and less overall slope between anterior and posterior aspects of the vertebral body (2.1mm) compared to non-black comparison groups (6mm)5. The lower than expected proportion of newly diagnosed degenerative spondylolisthesis among reported black beneficiaries indicates that these individuals may have less access to medical resources or may face other cultural and socioeconomic barriers to seeking medical care.
Medicare-wide expenditures on spinal injection techniques increased 186% per 100,000 beneficiaries from 2000 to 200822. The results of our study demonstrate that a large volume of Medicare resources are apportioned specifically to treating symptomatic degenerative spondylolisthesis. Overall, 41,582 individuals (44% of initial cohort) received physical therapy or spinal injections without progression to surgical intervention. This may reflect the clinical benefits of physical therapy and spinal interventional procedures within this patient subset. Treatment utilization was most intense within the first year of initial diagnosis with the majority of both physical therapy and interventional procedures occurring during this period. Medical provider specialty was strongly associated with the interventional treatment pathway. Physical Medicine and Rehabilitation specialists were markedly more likely to perform transforaminal and facet injections than Anesthesiologists, Interventional Radiologists, Neurosurgeons, and Orthopedic Surgeons.
Many studies have described the clinical benefit of strengthening the lumbar paraspinal and truncal musculature in patients presenting with low back pain12,23–26. However, the majority of physical therapy was dedicated to passive treatment modalities and range of motion exercises within this cohort. This finding may indicate that patients were unable to tolerate the increased demand of active therapy modalities secondary to low back pain or radiating symptoms. Additionally, this finding could reflect a deficiency of provider education or interdisciplinary communication between the prescribing physician and physical therapist.
There are a number of inherent limitations to this study. First, this is a descriptive analysis that relies on billing metrics and demographic information within the Medicare database. Although previous studies have determined that degenerative spondylolistheses are rare before the sixth decade of life, only a few younger individuals were included in the cohort5,6. There is limited sociodemographic information captured within the database and we could not assess how factors such as smoking status or educational level impacted our findings. Furthermore, the availability of health related quality of life data would allow more sensitive analysis of clinical outcomes after each category of intervention. Second, the accuracy of our analysis is dependent on proper and consistent coding among providers. Analytical errors could have been introduced if a provider improperly associated the degenerative spondylolisthesis diagnosis code with a procedure that was actually performed for an unassociated adjacent level pathology. Additionally, the spondylolisthesis code may have been omitted and substituted with another diagnosis code (such as lumbar spondylosis) when procedures were performed predominantly for axial low back pain. These omissions would lead to a misclassification bias that yields an underestimation of the true incidence of degenerative spondylolisthesis within the Medicare population. Third, the ICD-9 code for degenerative spondylolisthesis does not distinguish subluxation severity. As a result, the data could not be stratified to distinguish the rate of surgery based on deformity progression.
Overall, this study demonstrates that spinal injection techniques and physical therapy are frequently used as a treatment modality for symptomatic degenerative spondylolisthesis. Anatomically selective intervention is utilized with varying frequency depending on provider specialty. Future studies will investigate the clinical impact of injection selectivity and the various modalities of physical therapy. Understanding utilization of these techniques is important to determine relative clinical efficacies and to optimize future health care expenditures.
Supplementary Material
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s).
The Intramural Research Program of the National Institutes of Health funds were received in support of this work.
Relevant financial activities outside the submitted work: employment, patents, stocks, grants.
References
- 1.Watters WC, Bono CM, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. The Spine Journal. 2009;9:609–614. doi: 10.1016/j.spinee.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 2.He L-C, Wang Y-XJ, Gong J-S, et al. Prevalence and risk factors of lumbar spondylolisthesis in elderly Chinese men and women. European radiology. 2014;24:441–448. doi: 10.1007/s00330-013-3041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald J, Newman P. Degenerative spondylolisthesis. Journal of Bone & Joint Surgery, British Volume. 1976;58:184–192. doi: 10.1302/0301-620X.58B2.932080. [DOI] [PubMed] [Google Scholar]
- 4.Frymoyer JW. Degenerative spondylolisthesis: diagnosis and treatment. Journal of the American Academy of Orthopaedic Surgeons. 1994;2:9–15. doi: 10.5435/00124635-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg N. Degenerative spondylolisthesis. Predisposing factors. The Journal of Bone & Joint Surgery. 1975;57:467–474. [PubMed] [Google Scholar]
- 6.Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine. 2007;32:120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 7.Vogt MT, Rubin D, San Valentin R, et al. Lumbar olisthesis and lower back symptoms in elderly white women: the study of osteoporotic fractures. Spine. 1998;23:2640–2647. doi: 10.1097/00007632-199812010-00020. [DOI] [PubMed] [Google Scholar]
- 8.Kauppila LI, Eustace S, Kiel DP, Felson DT, Wright AM. Degenerative Displacement of Lumbar Vertebrae: A 25-year Follow-up Study in Framingham. Spine. 1998;23:1868–1873. doi: 10.1097/00007632-199809010-00014. [DOI] [PubMed] [Google Scholar]
- 9.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34:199. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denard PJ, Holton KF, Miller J, et al. Lumbar spondylolisthesis among elderly men: prevalence, correlates and progression. Spine. 2010;35:1072. doi: 10.1097/BRS.0b013e3181bd9e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matz PG, Meagher RJ, Lamer T, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2015 doi: 10.1016/j.spinee.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan PB, Phyty GDM, Twomey LT, Allison GT. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22:2959–2967. doi: 10.1097/00007632-199712150-00020. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan EE, Demir-Deviren S, Sencan S, et al. Comprehensive Nonsurgical Treatments Decrease the Need for Spine Surgery in Patients with Spondylolisthesis. The Spine Journal. 2015;15:S140–S141. [Google Scholar]
- 14.Chan L, Houck P, Prela CM, MacLehose RF. Using medicare databases for outcomes research in rehabilitation medicine. American journal of physical medicine & rehabilitation. 2001;80:474–480. doi: 10.1097/00002060-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32:1754–1760. doi: 10.1097/BRS.0b013e3180b9f96e. [DOI] [PubMed] [Google Scholar]
- 16.Medicare Enrollment from the Chronic Conditions CMS Data Warehouse. [Accessed March 7, 2016]; Available at: https://www.ccwdata.org/web/guest/medicare-charts/medicare-enrollment-charts. [Google Scholar]
- 17.Imada K, Matsui H, Tsuji H. Oophorectomy predisposes to degenerative spondylolisthesis. Journal of Bone & Joint Surgery, British Volume. 1995;77:126–130. [PubMed] [Google Scholar]
- 18.Kalichman L, Hodges P, Li L, Guermazi A, Hunter DJ. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. European Spine Journal. 2010;19:1136–1144. doi: 10.1007/s00586-009-1257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter R, Hibbert C. Vertebral displacement in spondylolisthesis. Clinical Biomechanics. 1989;4:58–63. doi: 10.1016/0268-0033(89)90069-7. [DOI] [PubMed] [Google Scholar]
- 20.Sanderson PL, Fraser RD. The influence of pregnancy on the development of degenerative spondylolisthesis. Journal of Bone & Joint Surgery, British Volume. 1996;78:951–954. doi: 10.1302/0301-620x78b6.1291. [DOI] [PubMed] [Google Scholar]
- 21.US Census Bureau. [Accessed November 22, 2015]; Available at: http://www.agingstats.gov/Main_site/Data/2008_Documents/OA_2008.pdf.
- 22.Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Growth of spinal interventional pain management techniques: analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine. 2013;38:157–168. doi: 10.1097/BRS.0b013e318267f463. [DOI] [PubMed] [Google Scholar]
- 23.Cassisi JE, Robinson ME, O'Conner P, MacMillan M. Trunk strength and lumbar paraspinal muscle activity during isometric exercise in chronic low-back pain patients and controls. Spine. 1993;18:245–251. doi: 10.1097/00007632-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Richardson C, Jull G, Hodges P, Hides J, Panjabi MM. Churchill Livingstone Edinburgh; 1999. Therapeutic exercise for spinal segmental stabilization in low back pain: scientific basis and clinical approach. [Google Scholar]
- 25.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 26.Manniche C, Bentzen L, Hesselsøe G, Christensen I, Lundberg E. Clinical trial of intensive muscle training for chronic low back pain. The Lancet. 1988;332:1473–1476. doi: 10.1016/s0140-6736(88)90944-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.