Previous studies demonstrated a role for the presupplementary motor area (preSMA) in motor sequence chunking, but did not clearly differentiate between motor chunking per se and hand switching. The present study overcomes this limitation by using a novel motor sequence task that counterbalanced different types of hand switches across between- and within-chunk transitions. Results show that temporary interference with preSMA processing disrupts performance when both chunking and hand switching are required, but not under either condition alone.
Keywords: presupplementary motor area, transcranial magnetic stimulation, chunking, hand switching, motor sequence learning
Abstract
Motor chunking, the grouping of individual movements into larger units, is crucial for sequential motor performance. The presupplementary motor area (preSMA) is involved in chunking and other related processes such as task switching, response selection, and response inhibition that are crucial for organizing sequential movements. However, previous studies have not systematically differentiated the role of preSMA in motor chunking and hand switching, thus leaving its relative contribution to each of these processes unclear. The aim of this study is to demonstrate the differential role of preSMA in motor chunking and hand switching. We designed motor sequences in which different kinds of hand switches (switching toward the right or left hand or continuing with the right hand) were counterbalanced across between- and within-chunk sequence points. Eighteen healthy, right-handed participants practiced four short subsequences (chunks) of key presses. In a subsequent task, these chunks had to be concatenated into one long sequence. We applied double-pulse transcranial magnetic stimulation (TMS) over left preSMA or left M1 areas at sequence initiation, between chunks, or within chunks. TMS over the left preSMA significantly slowed the next response when stimulation was given between chunks, but only if a hand switch toward the contralateral (right) hand was required. PreSMA stimulation within chunks did not interfere with responses. TMS over the left M1 area delayed responses with the contralateral hand, both within and between chunks. Both preSMA and M1 stimulation decreased response times at sequence initiation. These results suggest that left preSMA is not necessary for chunking per se, but rather for organizing complex movements that require chunking and hand switching simultaneously.
NEW & NOTEWORTHY
Previous studies demonstrated a role for the presupplementary motor area (preSMA) in motor sequence chunking, but did not clearly differentiate between motor chunking per se and hand switching. The present study overcomes this limitation by using a novel motor sequence task that counterbalanced different types of hand switches across between- and within-chunk transitions. Results show that temporary interference with preSMA processing disrupts performance when both chunking and hand switching are required, but not under either condition alone.
chunking is an important strategy for organizing complex motor behavior into structured sequences of movements. In motor sequence learning, chunking refers to a process by which certain elements of movements are grouped together into subsequences, or chunks. Motor chunks are typically characterized by longer response times (RTs) between chunks and shorter RTs for the movements within a chunk (Rosenbaum et al. 1983). Also, an increased proneness to errors at the onset of a chunk compared with its remainder has been noted (Rosenbaum et al. 1983). Chunking patterns for a specific sequence can arise spontaneously with different chunk sizes and chunk points for different individuals (Kennerley et al. 2004; Ruitenberg et al. 2014; Sakai et al. 2003), or they can be imposed on the sequence; for example, by using salient patterns such as repetitions or inversions (Koch and Hoffmann 2000), or by inserting a temporal delay between the response-to-stimulus interval (Stadler 1993). Chunk boundaries can be independent of physical parameters such as the distance between response locations (Sakai et al. 2003), and they are critical for sequence retrieval in that shuffling a learned sequence has been shown to be less detrimental to performance if chunk boundaries are preserved during shuffling (Koch and Hoffmann 2000; Sakai et al. 2003).
The presupplementary motor area (preSMA) is located in the medial frontal cortex and has often been found to be involved in learning motor sequence chunks. In monkeys, neurons in the preSMA were found to change their firing pattern in relation to the learning state of a sequence, with an increasing concatenation of individual elements into chunks being reflected in an overall decrease in activity, which marks only the initiation of a chunk (Nakamura et al. 1998). Also in humans, the preSMA has been found to be active during motor sequencing and chunking tasks (Hikosaka et al. 1996; Kennerley et al. 2004; Sakai et al. 1998, 1999). However, the preSMA seems to be involved in a number of tasks that could be considered necessary components of chunking, but not necessarily chunking per se. Activity in the preSMA has been associated with tasks requiring an inhibition of movement (Chen et al. 2009; Obeso et al. 2013; Picazio et al. 2014), task switching (Rushworth et al. 2002), action reprogramming (Mars et al. 2009), and initiation of movement in a time-sensitive manner (Mita et al. 2009). Hand switching and transitioning between chunks might thus involve some common processes, but they are likely to operate on different levels because hand switches do not necessarily mark a chunk boundary, and motor chunks can establish in the absence of hand switches.
Kennerley et al. (2004) found that short (0.5-s) bursts of 10 Hz repetitive transcranial magnetic stimulation (rTMS) over the preSMA disrupted motor sequence performance when delivered at the beginning, but not in the middle, of a motor chunk. However, the motor sequence they used involved frequent transitions between left- and right-hand key presses, and chunk points differed between participants. Chunk boundaries were identified post hoc for each individual and thus could not be controlled for whether they contained hand switches or not. Therefore, it remains unclear whether rTMS to the preSMA in that study interfered with motor chunking per se, or whether it disrupted processes more related to hand switching or stopping or activating movement in one hand.
Ruitenberg et al. (2014) used offline rTMS at an inhibitory frequency (1 Hz) over the preSMA to interfere with motor chunking. Contrary to the method used by Kennerley et al. (2004), Ruitenberg et al. (2014) used unimanual sequencing tasks, thus avoiding the possible confound between chunking and hand switching. However, the two sequences that participants performed were relatively short (six elements), and not all participants developed a clear chunking pattern. Furthermore, RTs of chunk points from within the sequence were pooled together with RTs of the first sequence element. Therefore, it was not possible to dissociate between effects on initiating a sequence as a whole and initiating a new chunk within an ongoing sequence.
In the present study, our aim was to investigate the role of the left preSMA in performing motor sequences that require both hand switching and chunking independent of each other. To dissociate between hand switching and chunking, we designed motor sequences that would 1) evoke consistent and predictable chunk points across individuals and 2) contain comparable hand switches both within and between chunks. To interfere transiently with neural processing in the left preSMA and left primary motor cortex (M1) we used double-pulse TMS (dTMS). We selected seven sequence positions at which we compared the effect of dTMS with baseline performance. First, we investigated the effect on sequence initiation. Additionally, to dissociate between chunking and hand switching, we evaluated the effect of dTMS at three different hand-switch conditions (switch toward right hand, switch away from right hand, and stay on right hand) at both between- and within-chunk transitions. We hypothesized that if the left preSMA is necessary for sequence chunking, then TMS over this area would interfere with processing of the next chunk (i.e., delay the next response in all hand switch conditions), but only if stimulation was delivered between chunks. On the other hand, if the role of the left preSMA is more related to hand switching, we would expect TMS to delay responses in all conditions involving a hand switch, independent of the chunk boundary. For the control site, left M1, we expected dTMS to delay all responses with the right hand (i.e., to interfere with contralateral finger movements irrespective of whether they involve a hand switch or a chunk boundary).
METHODS
Participants.
Eighteen healthy volunteers (9 women, mean age = 29 yr, SD = 4.2) participated in the experiment. All participants were right-handed (mean score = 94.4, SD = 11.7) according to the Edinburgh Handedness Inventory of Manual Preference (Oldfield 1971), and had normal results upon neurological examination. Subjects were excluded from the study if they had contraindications to TMS (Rossi et al. 2009), took any medication affecting the central nervous system, practiced piano for more than 20 h/wk, or were professional musicians. Participants were asked to abstain from alcohol for 48 h and from caffeine for 6 h before the experiment. The study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Neuroscience Institutional Review Board of the National Institutes of Health. All participants gave written informed consent before the experiment.
Apparatus and procedure.
Subjects sat comfortably on a chair with their head fixed to a headrest. They wore a swim-cap throughout the course of the experiment on which the coil positions were marked. Based on observations that chunking patterns transfer more easily from the nondominant to the dominant hand, Sakai et al. (2003) suggested that the dominant hemisphere might be more important for chunking. Therefore, we chose to focus on the left hemisphere in right-handed participants only. The motor hotspot was identified as the site over the left primary motor cortex that consistently elicited the largest motor-evoked potentials (MEPs) in the right first dorsal interosseous (FDI) muscle. MEPs were recorded using disposable surface Ag-AgCl electrodes placed in a belly-tendon montage. The electromyography (EMG) signal was recorded, amplified, and band-pass filtered between 20 and 2,000 Hz using a conventional EMG machine (Nihon Kohden, Tokyo, Japan). Signal software version 5.09 (Cambridge Electronic Design, Cambridge, UK) was used to digitize (5 kHz) and visualize the EMG signal in real time. Resting motor threshold (RMT) was determined by placing the coil over the motor hotspot with the handle pointing backward and 45° away from the midline of the skull. The stimulation intensity that produced MEPs larger than 50 μV in 5 out of 10 successive trials at rest was taken as the RMT (Rossini et al. 1994). Monophasic TMS pulses were generated by a Magstim 2002 stimulator (Magstim, Whitland, Dyfed, UK) connected to a figure eight-shaped coil (70 mm external loop diameter) and a bistim module.
Each subject's anatomical magnetic resonance image (MRI) was used to localize the preSMA on the left hemisphere, just anterior to the vertical line passing through the anterior commissure (AC) and perpendicular to the AC-PC plane (Mayka et al. 2006; Picard and Strick 1996). Anatomical MRIs were obtained from either MPRAGE or 3D-T1 images and were left in the native subject-space. Brainsight TMS navigation (Rogue Research, Cardiff, UK) software was used to identify and mark the location for preSMA stimulation. The average MNI coordinates for the preSMA stimulation site was x = −3.67 (SD = 2.74), y = 23.26 (SD = 6.93), and z = 52.48 (SD = 4.74). Coil orientation was lateromedial with the handle pointing toward the left and 90° away from the midline of the skull (Arai et al. 2012; Janssen et al. 2015).
The experiment consisted of two parts, a sequence practice session (without TMS), and a sequence testing session (with TMS). During the practice session, participants practiced the four subsequences in two different computer tasks as described below. In the testing session, participants had to retrieve the subsequences from memory, concatenate them into a long sequence and type it as quickly and accurately as possible. The order of the subsequences within the long sequence varied between trials. All the tasks just described were programmed in E-Prime 2.0 on a personal computer running Windows 7 (Microsoft). Stimuli were presented on a 19-inch monitor positioned at eye level and at an approximate distance of 60 cm. Responses were collected using a standard qwerty keyboard placed on a pillow on the participant's lap.
Tasks.
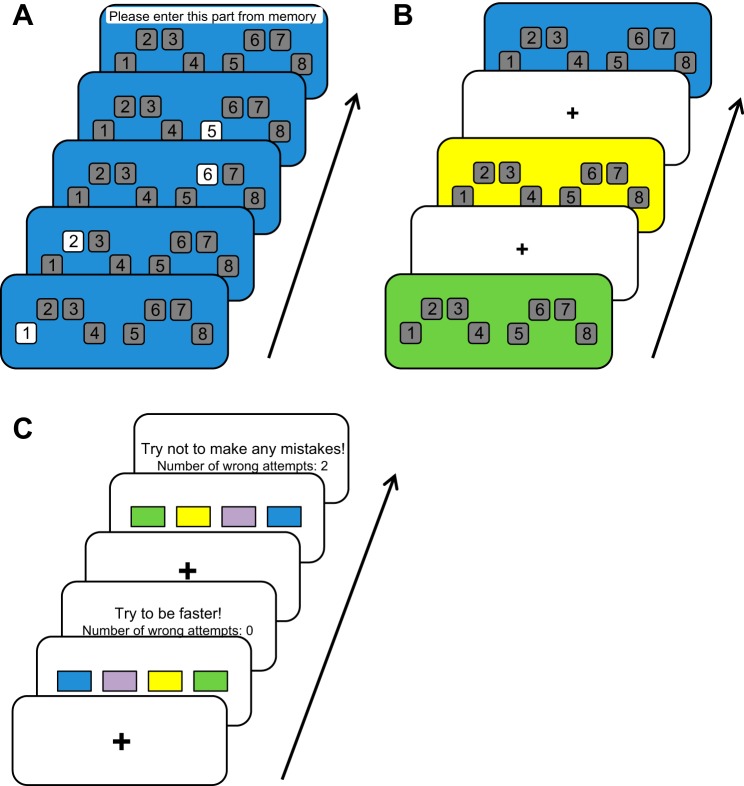
Participants performed the tasks by following instructions displayed on the computer screen. They placed the four fingers of their left hand (little-ring-middle-index) on response keys S, E, R, and G; and the four fingers of their right hand (index-middle-ring-little) on keys H, U, I, and L, respectively. For the practice session, participants were instructed to learn four different subsequences of key presses, each of which was associated with a specific color. The background color of the screen (blue, purple, yellow, or green) indicated which of the four subsequences was being practiced. In the first practice task, participants saw eight gray boxes numbered 1 through 8 corresponding to the response keys S, E, R, G, H, U, I, and L, respectively. At the start of each trial, the gray box representing the first subsequence element turned white and participants responded by pressing the corresponding key. Then, the box for the next sequence element would turn white (see Fig. 1A). This way the participants practiced typing each subsequence by responding to the visual cues. Each subsequence was repeated three times, after which it had to be entered from memory without any cue. In the second practice task, participants saw the gray boxes against one of the four background colors and were asked to type the corresponding sequence from memory (see Fig. 1B). In each trial, a subsequence had to be typed correctly twice. All participants performed 12 such trials for each color (48 total trials). The order in which the different subsequences were practiced was randomized.
Fig. 1.
Training and testing tasks. During training, participants practiced each subsequence separately. A: in the initial training phase participants saw eight gray boxes representing the response keys for the little to index finger of the left hand (boxes 1–4) and for the index to little finger of the right hand (boxes 5–8). Each subsequence corresponded to a different color that was represented by the background color of the screen (blue, purple, green, or yellow.) The sequence order was indicated by the order in which the boxes lit up white. Participants responded to the lit-up boxes by pressing the corresponding key. Each subsequence was four key-presses long. After three repetitions of responding to the lit-up boxes of a subsequence participants had to type it once from memory. B: in the second training phase, participants had to type the subsequence corresponding to each background color from memory. The subsequences were presented in random order. C: during testing, participants first saw a fixation cross, followed by the sequence cue consisting of four horizontally aligned rectangles. The colors of the rectangles corresponded to the previously memorized subsequences and had to be concatenated from left to right. No feedback was provided during sequence performance, but errors caused the trial to be aborted and wrong trials had to be repeated until they were error-free. Correct trials were followed by performance feedback showing the number of incorrect attempts and either an encouragement not to make any mistakes (if they had incorrect attempts), to be faster (if they had no incorrect attempts, but were slower than the mean +2 SD of their previous three trials), or a “Well done!” message. The sequence order (i.e., the order of the colored rectangles) varied pseudorandomly between trials. There were 8 blocks, each containing 21 trials.
During the testing session, the participants’ task was to concatenate the four different subsequences they had previously learned into one long sequence. The order of that sequence was indicated by the order of four horizontally arranged colored blocks, each representing one of the subsequences. First, a fixation cross was displayed for 3 s, followed by the image indicating the sequence order (see Fig. 1C). Participants were told to type the sequence as fast as possible without making errors. No feedback was given for correct key presses, but wrong key presses led to an immediate trial abortion. If a trial was aborted, the same sequence was repeated until it was performed correctly. After the sequence was typed correctly, a feedback display was shown for 3 s, stating the number of false attempts for that trial. Additionally, to maintain motivation, participants received one of three possible messages regarding their performance: “Try not to make any mistakes!” (if they had made one or more false attempts), “Try to be faster!” (if they had not made any errors but were slower than the mean +2 SD of the previous three responses), or “Well done!” (if they had not made any errors and their speed was within the mean +2 SD limit). The session consisted of 8 blocks with 21 trials each.
During the testing session, dTMS was applied over either left M1 or left preSMA. Participants had their chin and forehead fixated to minimize head movements. The site of TMS stimulation varied pseudorandomly across blocks, with the constraint that there were as many M1 stimulation blocks as preSMA stimulation blocks in each half of the session (i.e., four stimulation blocks for each site, two of which had to occur in the first four blocks). Within any given trial, maximally one pair of dTMS pulses was delivered at an interpulse interval of 40 ms. Paired TMS pulses at an interpulse interval of 40 ms have been shown to be temporally precise and yet effective in disrupting local neural processing (Pitcher 2014; Pitcher et al. 2007). The stimulation intensity was the same for both pulses. Stimulation intensity was 110% RMT over M1 and 120% RMT over preSMA. The intensity was lower for M1 stimulation (110% RMT) to avoid twitching of hand muscles interfering with the task.
Each block contained 21 trials: 15 trials with dTMS applied during sequence performance (between or within chunks), 4 trials with dTMS applied before sequence performance (at sequence initiation), and 2 trials without dTMS. For dTMS during sequence performance, TMS pulses were triggered by the previous key press with a delay of 1–2 ms. For example, if dTMS was supposed to be applied between the 4th and 5th key press, the 4th key press would trigger the dTMS. The ordinal sequence position at which dTMS was given was counterbalanced across trials. The average ordinal sequence positions at which dTMS was applied in each condition are shown in Table 1. For dTMS at sequence initiation, pulses were delivered 120 ms after the onset of the sequence cue.
Table 1.
Properties of transition points of interest
| Transition Type | Chunk/Chunk Transition | Digits (Hand) Involved in Transition* | Mean Ordinal Position Within Sequence |
|---|---|---|---|
| Sequence start | Any | 1 (L) or 6 (R) | 1 |
| Between chunks, switch toward right | Green-purple, yellow-green, yellow-purple | 2 (L) to 6 (R) | 5.75 |
| Between chunks, stay on right | Blue-purple, purple-green, blue-green | 5 (R) to 6 (R) | 5.625 |
| Between chunks, switch away from right | Blue-yellow, purple-yellow, purple-blue | 5 (R) to 1 (L) | 5.75 |
| Within chunk, switch toward right | Blue | 2 (L) to 6 (R) | 8 |
| Within chunk, stay on right | Purple | 5 (R) to 8 (R) | 8 |
| Within chunk, switch away from right | Green | 5 (R) to 1 (L) | 8 |
R, right hand; L, left hand.
Sequences.
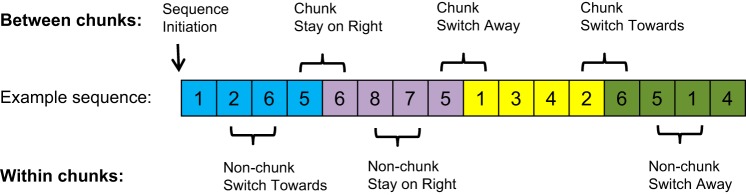
The four subsequences were constructed such that all movement transitions (i.e., switching away from the right hand, switching toward the right hand, and continuing with the right hand) could be evaluated both within and between chunks. Each subsequence was four key-presses long and contained a different within-chunk transition: switch from left to right hand, switch from right to left, stay on right, and stay on left hand (although the latter transition was not evaluated in this study). For the main experiment, the 4 subsequences were concatenated into 16 different full sequences. Each full sequence thus consisted of three transitions between the subsequences. These between-chunk transitions were the same as the within-chunk transitions, i.e., switch from left to right hand, a switch from right to left hand, stay on right hand, and stay on left hand (again, the latter condition was not evaluated). Table 1 shows all conditions that were evaluated and their corresponding color or color transition. Furthermore, each subsequence was constructed such that none of the between-chunk transitions would require a repeated key press with the same finger. Also, the finger that was involved in the transition was matched as much as possible between conditions (Fig. 2).
Fig. 2.
Sequence design. The sequences and subsequences were designed to contain the same hand transitions between chunks as within chunks. The colors represent different subsequences (chunks), and the numbers represent the finger to be used (1–4 for the little to index finger of the left hand, 5–8 for the index to little finger of the right hand). The sequence shows our 7 transition points of interest (i.e., points at which response times were evaluated with and without transcranial magnetic stimulation). There were two chunk boundary conditions (between- or within-chunk), each containing the same three hand-switch conditions (switch toward the right hand, switch away from the right hand, and stay on the right hand), plus the sequence initiation condition.
Using 16 different target sequences, rather than just one or a few target sequences, had several advantages. First, having different chunk orders allowed us to counterbalance the relative position of the transition points of interest. In a single target sequence, the same (within or between) chunk transition would always appear at the same sequence position and it would thus not be possible to dissociate between the effect of chunk boundary type and that of ordinal sequence position. Second, changing the order of the subsequence between each trial ensured that participants would not develop chunking patterns different from the intended 4 × 4 pattern or fuse two or more chunks together.
Statistical analysis.
Only correctly performed sequences were included in the analysis. For every sequence element, we calculated the interkey interval of the response (i.e., the time between the previous and the current response). The response time of the first key press was the time between stimulus onset and the first key press. We analyzed the effect of TMS in seven different conditions: at sequence initiation, at three different hand-switch conditions (switch toward right hand, stay on right, switch toward left) between chunks, and at the same three hand-switch conditions within chunks. For each condition, we aligned the responses to the transition point of interest (i.e., the first key press after the dTMS pulses), and extracted the response times from the element just before the dTMS up to six elements after the dTMS. However, because the ordinal position of elements of interest varied between the 2nd and the 14th sequence elements, not all trials contained six more button presses after the dTMS. From the trials without TMS, we extracted the response times at the corresponding transition points of interest for each condition. As is common for these kinds of tasks, response times in each condition were not normally distributed, as their distribution was skewed to the right and contained some severe outliers with very slow responses. We thus followed recommendations by Whelan (2008) and used an RT cutoff of 3 s to remove outliers. Furthermore, we applied a log-transform (natural logarithm) on the raw millisecond RTs to obtain a normal distribution. Finally, for each subject we calculated the mean of the log-transformed response times per condition. We also calculated the mean and SD over subjects' raw (nontransformed) median RTs to provide an indication of the RTs in a more intuitive unit.
For each transition point of interest (i.e., for the element following dTMS pulses or the corresponding element without TMS), these values were then submitted to a repeated-measures ANOVA (rmANOVA) with the within-subject factor stimulation condition (no TMS, M1, and preSMA). We used Greenhouse-Geisser corrected values to account for violations of the sphericity assumption. If the rmANOVA showed a significant difference between stimulation conditions, we performed two planned pairwise comparisons between the no-TMS and M1 conditions, and between the no-TMS and preSMA conditions to test which of the TMS conditions differed from baseline. Pairwise comparisons were Bonferroni-corrected for multiple comparisons. Additionally, to test whether the effect of TMS is dependent on the chunk position (between or within chunks) at which it is applied, we performed rmANOVA with the within-subject factors stimulation condition (no TMS, M1, and preSMA), chunk position (between chunks, within chunks), and the interaction term stimulation condition × chunk position. SPSS software (version 21.0 for Windows; IBM, Armonk, NY) was used to perform rmANOVAs.
RESULTS
All participants succeeded in learning the subsequences and tolerated the TMS procedure well. In two participants, TMS over M1 at 110% RMT elicited muscle twitches that interfered with sequence performance. For these participants stimulation intensity was lowered to 105% RMT for one and 100% RMT for the other participant to reduce muscle twitching. The mean RMT across participants was 46.7% (SD = 8.0) of the maximal stimulator output.
On average, participants made 35.7 (SD = 23.6; range, 10–100) mistakes throughout the experiment. There were more errors in the first block (mean = 11.8; SD = 14.1; range, 1–53) than in the remaining blocks 2–8 (range of means across blocks, 2.7 to 4.2; SD = 1.7 to 3.1; range, 0–14). Errors did not seem to be primarily due to dTMS, because on average, only 2.2 (SD = 1.9) mistakes were committed right after M1 stimulation and 1.9 (SD = 2.0) mistakes were committed right after preSMA stimulation. Thus, only 6.2% and 5.3% of all errors were preceded by dTMS to M1 and preSMA, respectively. Note that because incorrect trials were repeated until they were correct, the number of correct trials (168) was equal for all participants. From the correct trials, 21 responses were removed due to excessively slow RTs (>3 s). All of these responses corresponded to sequence initiation elements (8 after no TMS, 6 after M1 stimulation, and 7 after preSMA stimulation).
The means and standard deviations of the subjects' median raw (non-log transformed) RTs are shown in Table 2. Note, however, that because of the skewed RT distributions, all further calculations and statistical tests were carried out on the log-transformed RTs and that these values are presented only to provide an indication of the RTs in raw units.
Table 2.
Mean and SD values of subjects' raw median response times in milliseconds
| No TMS | TMS Over Left M1 | TMS Over Left preSMA | |
|---|---|---|---|
| Sequence initiation | 1,061.7 (192.8) | 932.1 (260.9) | 879.2 (198.9) |
| Between chunks, switch toward | 221.8 (94.6) | 223.4 (98.1) | 231.6 (91.5) |
| Between chunks, stay on right | 209.8 (98.2) | 215.1 (123.7) | 211.2 (113.1) |
| Between chunks, switch away | 226.3 (127.8) | 234.3 (124.4) | 233.3 (111.1) |
| Within chunks, switch toward | 202.2 (102.9) | 201.4 (105.4) | 185.3 (97.3) |
| Within chunks, stay on right | 228.9 (75.1) | 232.0 (90.4) | 223.5 (70.5) |
| Within chunks, switch away | 183.5 (81.2) | 176.0 (80.4) | 174.3 (77.6) |
Values are presented as mean (SD).
TMS, transcranial magnetic stimulation; M1, primary motor cortex; preSMA, presupplementary motor area.
Chunking pattern and effects of TMS after cue onset.
Participants' performances showed a clear chunking structure with longer RTs for the first element of a new subsequence (i.e., for the 1st, 5th, 9th, and 13th sequence elements). This chunking pattern was evident in trials both with and without TMS (see Fig. 3). A rmANOVA of the first sequence element revealed a significant effect of stimulation condition [F(1.7,28.4) = 14.78, P < 0.001], and post hoc analysis showed that both M1 stimulation and preSMA stimulation led to significantly faster first responses than the no-TMS condition [M1 vs. no TMS: t(17) = 3.9, P = 0.002; preSMA vs. no TMS: t(17) = 6.8, P < 0.001, Bonferroni corrected] (Fig. 3).
Fig. 3.

Transcranial magnetic stimulation (TMS) at sequence initiation. TMS over both left primary motor cortex (M1) and left presupplementary motor area (preSMA) (flash symbol indicates TMS) led to faster RTs than at baseline (no TMS). Note also the long RTs for the 5th, 9th, and 13th elements, which correspond to the start of each subsequence. Error bars show standard errors of measurement. Repeated-measures ANOVA for the difference between stimulation sites was performed on the first sequence element. **M1 vs. no TMS and preSMA vs. no TMS were both significant at P < 0.05, Bonferroni corrected.
TMS between chunks.
We hypothesized that preSMA stimulation between chunks would interfere with the preparation of the next motor chunk and would thus delay the next response, irrespective of whether the next chunk required a hand switch. Alternatively, if the preSMA is more involved in hand switching rather than chunking per se, we would expect a delay in between-chunk conditions that involved a hand switch, but not in those that continued with the same hand. For stimulation over M1, we expected a delay of any subsequent movement that is performed with the contralateral (right) hand.
In the between chunks switch toward right condition, the RTs for the element just after the TMS pulse were significantly different between stimulation sites [F(1.5,25.9) = 5.65, P < 0.014]. Post hoc analyses revealed that both M1 and preSMA stimulation delayed the following response significantly compared with no TMS [M1 vs. no TMS: t(17) = 4.0, P = 0.002; preSMA vs. no TMS: t(17) = 2.8, P < 0.022, Bonferroni corrected]. Figure 4A shows increased RTs for the element immediately following TMS in the M1 and preSMA stimulation conditions.
Fig. 4.
TMS between chunks. All trials were aligned to the element preceding the TMS pulse (flash symbol). Repeated-measures ANOVA was performed on the sequence element after the TMS pulses (i.e., element 2). In the switch toward right condition, TMS over M1 and over preSMA led to delayed RTs compared with no TMS trials (A). In the stay on right (B) and switch away from right (C) conditions TMS over M1 but not over preSMA led to increased RTs. Error bars show standard errors of measurement. *M1 vs. no TMS is significant at P < 0.05, **M1 vs. no TMS and preSMA vs. no TMS are both significant at P < 0.05, all values are Bonferroni corrected.
Also in the between chunks stay on right condition there was a significant difference between stimulation sites [F(1.6,27.5) = 8.6, P < 0.02]. Post hoc analyses showed a significant delay in response times only after M1 stimulation [M1 vs. no TMS: t(17) = 3.7, P = 0.004, Bonferroni corrected], but not after preSMA stimulation [preSMA vs. no TMS: t(17) = 0.7, P = 0.89, Bonferroni corrected]. An increase in RT for the post-TMS element after M1, but not preSMA stimulation, can be seen in Fig. 4B.
In the between chunks switch away from right condition, there was a strong trend toward a difference between stimulation sites [F(1.8,30.4) = 3.4, P = 0.051]. Post hoc comparisons indicated that responses were delayed after M1 stimulation [M1 vs. no TMS: t(17) = 2.5, P = 0.042, Bonferroni corrected], but not after preSMA stimulation [preSMA vs. no TMS: t(17) = 0.9, P = 0.72, Bonferroni corrected]. A strong trend for a response slowing after M1 stimulation can be seen in Fig. 4C.
TMS within chunks.
For the conditions in which TMS was delivered within chunks, we expected that preSMA stimulation would not have an effect because the next response was presumably already prepared before the current chunk was initiated. Similar to the between-chunks condition, we expected M1 stimulation within chunks to interfere with the execution of the next response of the right hand.
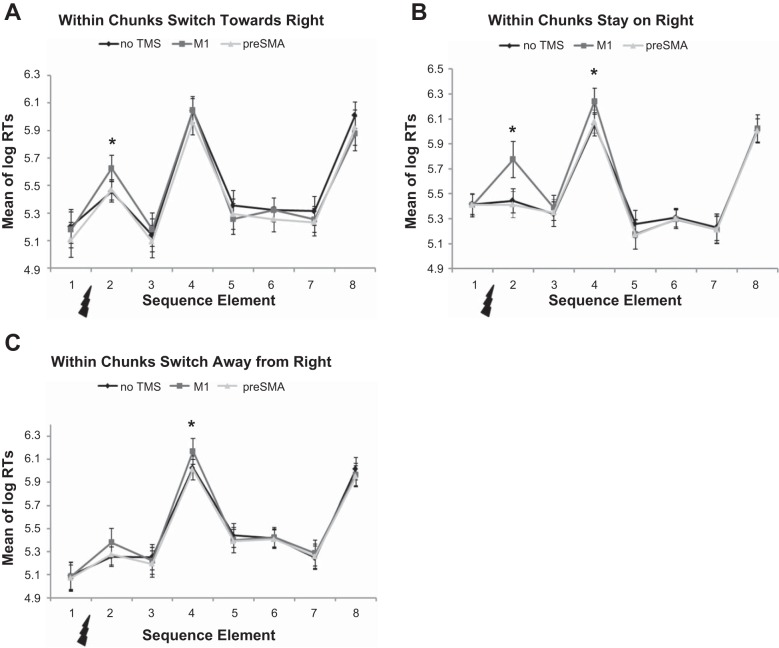
In the within chunks switch toward right condition there was a significant difference between stimulation sites [F(1.4,23.2) = 9.73, P < 0.002], and post hoc analyses revealed that this was due to a slower response following M1 stimulation [M1 vs. no TMS: t(17) = 3.3, P = 0.008, Bonferroni corrected], but not following preSMA stimulation [preSMA vs. no TMS: t(17) = 0.5, P = 1, Bonferroni corrected]. A slowing of the post-TMS response after M1 stimulation site can be seen in Fig. 5A.
Fig. 5.
TMS within chunks. All trials were aligned to the element preceding the TMS pulse (flash symbol). Repeated-measures ANOVA was performed on the sequence element after the TMS pulses (i.e., element 2). Compared with no stimulation, TMS over M1 led to increased RTs in the switch toward (A) and stay on right (B) conditions, but not in the switch away from right (C) condition. Stimulation over preSMA did not delay RTs in any of the conditions. Note also the significant RT increases for the first element of the following chunk (i.e., 3rd element after TMS), for stimulation over M1 in the stay on right (B) and switch away from right (C) conditions. Error bars show SE. *M1 vs. no TMS is significant at P < 0.05.
Similarly, the within chunks stay on right condition showed a significant difference between stimulation sites [F(1.3,21.5) = 18.5, P < 0.001], with post hoc analyses revealing a significant delay in RTs only after M1 stimulation [M1 vs. no TMS: t(17) = 4.4, P = 0.001, Bonferroni corrected], but not after preSMA stimulation [preSMA vs. no TMS: t(17) = 1.0, P = 0.66, Bonferroni corrected]. Figure 5B shows a clear increase in RT for the element following M1 stimulation.
Finally, in the within chunks switch away from right condition RTs showed no significant difference between stimulation sites [F(1.4,23.8) = 3.4, P = 0.065]. RTs for all three stimulation sites are shown in Fig. 5C.
Effects of TMS within chunks on the onset of the next chunk.
Although it was not part of our original hypotheses, we noted that in the within chunks stay on right and the within chunks switch away from right conditions there seemed to be a delay in the onset of the next chunk after M1 stimulation (see element 4 in Fig. 5, B and C). To test whether this effect of stimulation condition on the onset of the next chunk (i.e., on the third element after the TMS pulse) was significant, we performed the same rmANOVA as for the first element after the TMS pulse. For both conditions there was a significant difference between stimulation conditions [within chunks stay on right: F(1.9,31.5) = 5.5, P = 0.01; within chunks switch away from right: F(1.6,27.8) = 7.4, P = 0.004]. Post hoc pairwise comparisons showed that in both conditions M1, but not preSMA stimulation, significantly delayed the response to the first element of the next chunk [within chunks stay on right, M1 vs. no TMS: t(17) = 2.7, P = 0.03, preSMA vs. no TMS: t(17) = 0.4, P = 1; within chunks switch away from right, M1 vs. no TMS: t(17) = 3.4, P = 0.006, preSMA vs. no TMS: t(17) = 0.4, P = 1].
Dependency of the TMS effect on chunk position.
Because in the switch toward condition preSMA stimulation delayed RTs when dTMS was given between chunks but not within a chunk, whereas M1 stimulation delayed RTs both between and within chunks, we directly tested whether there was an interaction between stimulation site (preSMA, M1, no TMS) and chunking position (between or within chunks). The rmANOVA suggested a trend for an interaction [F(1.92,32.64) = 3.16, P = 0.058], with the RT delay at chunk boundaries compared with within chunks being more pronounced after preSMA stimulation. Figure 6 shows that within-chunks RTs are delayed only after M1 stimulation, whereas between-chunks RTs are delayed after both M1 and preSMA stimulation. Significant main effects further showed that overall, responses were slower for between-chunk than for within-chunk elements [F(1,17) = 29.28, P < 0.001] and that RTs differed between stimulation sites [F(1.46,24.81) = 8.75, P = 0.003], with responses after M1 and preSMA stimulation being slower than after no stimulation [P < 0.01 and P = 0.039, respectively, Bonferroni corrected] (Fig. 6).
Fig. 6.

Interaction between TMS site and chunking position. The direct comparison of within-chunk TMS and between-chunk TMS for the three stimulation sites shows a strong trend toward a significant interaction (P = 0.058) between TMS site (i.e., no TMS, M1, and preSMA) and chunk position (i.e., within chunks, between chunks) when switching toward the right hand. Note that TMS over M1 delayed RTs at both chunk positions, whereas TMS over preSMA only delayed RTs when applied between chunks. Error bars show standard errors of measurement.
DISCUSSION
TMS effects on sequence initiation.
Contrary to previous findings by Kennerley et al. (2004; experiment 4) and Ruitenberg et al. (2014), TMS over preSMA at sequence initiation did not delay the first response, but instead it decreased RTs for the first-sequence element. A similar RT improvement was present after M1 stimulation. One possible explanation might be that the RT decrease was due to intersensory facilitation from the clicking sound or somatosensory stimulation of the scalp (Nickerson 1973; Terao et al. 1997) rather than to any specific TMS effects on motor planning or execution. However, a sham or nonmotor cortex TMS condition would have been needed to support this interpretation. Because our sequence cue and task were rather complex (a row of four differently colored boxes each representing a subsequence), TMS pulses at 120 and 160 ms after cue onset might have been too early to interfere with motor planning or chunk preparation, but they might have facilitated responses by altering excitability thresholds. Different effects for early and late suprathreshold TMS have been previously reported for simpler choice reaction time tasks, with early TMS (i.e., stimulation soon after an imperative movement cue) leading to faster RTs, and late TMS (i.e., stimulation close to the response onset) causing delayed RTs (Leocani et al. 2000; Pascual-Leone et al. 1992; Ziemann et al. 1997).
TMS effects on motor chunking.
When TMS was applied between chunks, stimulation over left preSMA only delayed the next key press if it required a switch toward the contralateral (right) hand, but not if the switch was away from the contralateral hand or if no switch was necessary. Note that in this condition switching toward the right hand and staying on the right hand required the next response to be made with the same (middle) finger. Thus, the difference between these conditions cannot be due to different effectors being involved. In contrast, control stimulation over left M1 delayed the following response independent of hand switches. As expected, the effect of preSMA stimulation was specific for chunk boundaries, because TMS within chunks did not interfere with performance in any of the hand-switch conditions. Stimulation over left M1, however, delayed RTs also when applied within chunks, given that the next key press was with the right hand (i.e., in the switch toward and stay on right conditions). Thus, the left preSMA seems to be particularly important for tasks that require both initiation of a new motor chunk and a switch toward the contralateral (right) hand. These results are in line with previous findings by Kennerley et al. (2004) in showing that preSMA stimulation only delayed responses when applied between chunks but not within chunks. However, the current study extends these findings by differentiating between different types of chunk boundaries and demonstrating that the effect of preSMA stimulation at chunk boundaries depends on more task parameters such as the presence and directionality of a hand switch during chunking. Thus, the preSMA seems to be most vulnerable to TMS interference under more complex task conditions that require chunking and hand switching simultaneously. This view is in agreement with previous findings that the preSMA enhances M1 excitability during action reprogramming, but not when the same action represented a standard response (Mars et al. 2009). Similarly, a role for preSMA in switching between response selection rules has been demonstrated by Rushworth et al. (2002).
In line with the role of M1 in motor execution rather than in motor planning, stimulation over the left M1 delayed contralateral responses independent of chunk boundaries or hand-switch conditions. Interestingly, however, left M1 stimulation between chunks also delayed the next key press with the left (ipsilateral) hand in the switch-away condition. This points toward a role for left M1 also in ipsilateral motor sequences, either directly or more indirectly via interhemispheric inhibition (Perez and Cohen 2009). This would be in line with previous reports showing that left M1 is coactivated during left-hand movements (Kawashima et al. 1993; Kim et al. 1993) and that it tends to be dominant during complex sequential motor tasks (Serrien et al. 2006). Alternatively, right-hand muscle twitches or sensations arising from left M1 stimulation may have diverted attention away from the left hand, thereby delaying the upcoming response.
TMS effects on later sequence elements.
A somewhat unexpected finding was that within-chunk stimulation over left M1 delayed the onset of the following chunk (i.e., the third sequence element after the TMS pulses) in the switch away and stay on right conditions. This cannot be a direct effect of the stimulation because, first, stimulation was very brief (40 ms) and was therefore terminated well before the key press, and second, the previous key press (i.e., the last element of the ongoing chunk) was not delayed. The effect thus seemed to be specific to the initiation of the following chunk. Because trials were sorted according to the hand switch condition at the TMS pulse and not at the following chunk boundary, the following chunk onset represented a mixture of hand switch conditions. Further sorting of the trials (according to hand switch condition at the following trial) would have left too few trials per condition to analyze. Interestingly, in both conditions that showed this delayed effect of left M1 stimulation, the corresponding right hand was active just prior to the stimulation, possibly suggesting a state-dependency of the effect.
Limitations.
Although TMS over preSMA was directed to stimulate the left preSMA, its close proximity to the midline also makes it possible that the right hemisphere was stimulated to some degree. Therefore, one should be careful in ascribing the observed effects exclusively to the left preSMA.
Compared with the studies by Kennerley et al. (2004) and Rushworth et al. (2002), which used short trains (5 and 4 pulses, respectively) of rTMS, the double-pulse stimulation in the present study was relatively short and mild. Therefore, the absence of stimulation effects in two of the between-chunk conditions (stay on right and switch away conditions) should not be regarded as evidence against preSMA involvement in these tasks. Rather, the role of the left preSMA might be more vulnerable to TMS interference during some (more complex) tasks, whereas it might be more robust or more easily compensated for during other tasks. In this study, we focused on the left preSMA and on hand switches with respect to the contralateral hand only. Because there may be laterality effects within preSMA, it may still be possible that the right preSMA plays a different role in motor chunking than the left. Different connectivity patterns for the left and right preSMA have been previously reported (Catani et al. 2012), suggesting at least partly different functional specializations.
Finally, one could argue that chunking consists of two processes, parsing of a long sequence into shorter chunks and concatenation of individual elements into one unit (Wymbs et al. 2012), and that the task in the present study required primarily concatenation. To balance hand switch conditions across within- and between-chunk transitions it was necessary to impose a specific chunking pattern onto our sequences. Therefore, it was not possible to let participants develop spontaneous segmentation points. However, it seems likely that many processes that take place at a chunk boundary, such as selecting and loading the next chunk, preparing the corresponding hand/fingers for movement, and suppressing competing motor plans, are similar for self-generated and externally imposed chunking structures. Although the preSMA has traditionally been considered to be more involved in internally than in externally guided movements (Deiber et al. 1991; Goldberg 1985; Jenkins et al. 2000), it has been suggested that this distinction is more likely to reflect differences in the complexity or sensorimotor context of internally and externally guided tasks (Mueller et al. 2007; Nachev et al. 2007, 2008). Given that our task required participants to select the correct motor chunk and its corresponding elements from memory, we expected the task to be sufficiently complex to recruit preSMA, even if the individual subsequences were externally cued during testing and had been practiced using an explicit sequence learning approach.
Conclusion.
Using a novel motor sequencing task and carefully designed sequences, we were able to dissociate between the role of the preSMA in motor chunking and hand switching. Proper functioning of the left preSMA is crucial when initiation of the next chunk also requires a switch toward the contralateral hand. The preSMA did not seem to be necessary for hand switching per se, because TMS before hand switches, in the absence of a chunk boundary, had no effect on performance.
GRANTS
Support for this study was provided by the National Institute of Neurological Disorders and Stroke Intramural Research Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M. and M.H. conceived and designed research; D.M., N.T., and H.S. performed experiments; D.M. analyzed data; D.M., N.T., and M.H. interpreted results of experiments; D.M. prepared figures; D.M. drafted manuscript; D.M., N.T., H.S., T.P., and M.H. edited and revised manuscript; D.M., N.T., H.S., T.P., and M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nguyet Dang for technical support with the experimental setup.
REFERENCES
- Arai N, Lu MK, Ugawa Y, Ziemann U. Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp Brain Res 220: 79–87, 2012. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex 48: 273–291, 2012. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage 44: 537–545, 2009. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402, 1991. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci 8: 567–588, 1985. [Google Scholar]
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B. Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 76: 617–621, 1996. [DOI] [PubMed] [Google Scholar]
- Janssen AM, Oostendorp TF, Stegeman DF. The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J Neuroeng Rehabil 12: 47, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123: 1216–1228, 2000. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, Fukuda H. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res 623: 33–40, 1993. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Sakai K, Rushworth MF. Organization of action sequences and the role of the pre-SMA. J Neurophysiol 91: 978–993, 2004. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science 261: 615–617, 1993. [DOI] [PubMed] [Google Scholar]
- Koch I, Hoffmann J. Patterns, chunks, and hierarchies in serial reaction-time tasks. Psychol Res 63: 22–35, 2000. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123: 1161–1173, 2000. [DOI] [PubMed] [Google Scholar]
- Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, Rushworth MF. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci 29: 6926–6931, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage 31: 1453–1474, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita A, Mushiake H, Shima K, Matsuzaka Y, Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat Neurosci 12: 502–507, 2009. [DOI] [PubMed] [Google Scholar]
- Mueller VA, Brass M, Waszak F, Prinz W. The role of the preSMA and the rostral cingulate zone in internally selected actions. Neuroimage 37: 1354–1361, 2007. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856–869, 2008. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage 36: T155–T163, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol 80: 2671–2687, 1998. [DOI] [PubMed] [Google Scholar]
- Nickerson RS. Intersensory facilitation of reaction time: energy summation or preparation enhancement? Psychol Rev 80: 489–509, 1973. [DOI] [PubMed] [Google Scholar]
- Obeso I, Robles N, Marron EM, Redolar-Ripoll D. Dissociating the role of the pre-SMA in response inhibition and switching: a combined online and offline TMS approach. Front Hum Neurosci 7: 150, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain 115: 1045–1059, 1992. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol 587: 725–726, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353, 1996. [DOI] [PubMed] [Google Scholar]
- Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, Koch G. Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol 24: 2940–2945, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D. Facial expression recognition takes longer in the posterior superior temporal sulcus than in the occipital face area. J Neurosci 34: 9173–9177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Curr Biol 17: 1568–1573, 2007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Kenny SB, Derr MA. Hierarchical control of rapid movement sequences. J Exp Psychol Hum Percept Perform 9: 86–102, 1983. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NM, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MF, Verwey WB, Schutter DJ, Abrahamse EL. Cognitive and neural foundations of discrete sequence skill: a TMS study. Neuropsychologia 56: 229–238, 2014. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592, 2002. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Pütz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci 19: RC1, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Putz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci 18: 1827–1840, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Kitaguchi K, Hikosaka O. Chunking during human visuomotor sequence learning. Exp Brain Res 152: 229–242, 2003. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci 7: 160–166, 2006. [DOI] [PubMed] [Google Scholar]
- Stadler MA. Implicit serial learning: questions inspired by Hebb (1961). Mem Cognit 21: 819–827, 1993. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, Kanazawa I. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res 115: 541–545, 1997. [DOI] [PubMed] [Google Scholar]
- Whelan R. Effective analysis of reaction time data. Psychol Rec 58: 475, 2008. [Google Scholar]
- Wymbs NF, Bassett DS, Mucha PJ, Porter MA, Grafton ST. Differential recruitment of the sensorimotor putamen and frontoparietal cortex during motor chunking in humans. Neuron 74: 936–946, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Netz J, Hömberg V. Delay in simple reaction time after focal transcranial magnetic stimulation of the human brain occurs at the final motor output stage. Brain Res 744: 32–40, 1997. [DOI] [PubMed] [Google Scholar]