Using immersive virtual reality we compared the behavioral and physiological reactivity of participants who observed pain and pleasure stimuli delivered to the body of an avatar that was embodied when seen from an egocentric perspective. This novel paradigm allowed us to investigate the reactivity to vicarious pain and pleasure without actually delivering any real stimuli and to explore the influence of different physical perspectives on basic empathic reactivity to pain and pleasure.
Keywords: empathy, pleasant touch, skin conductance and heart rate, body ownership
Abstract
Studies have explored behavioral and neural responses to the observation of pain in others. However, much less is known about how taking a physical perspective influences reactivity to the observation of others' pain and pleasure. To explore this issue we devised a novel paradigm in which 24 healthy participants immersed in a virtual reality scenario observed a virtual: needle penetrating (pain), caress (pleasure), or ball touching (neutral) the hand of an avatar seen from a first (1PP)- or a third (3PP)-person perspective. Subjective ratings and physiological responses [skin conductance responses (SCR) and heart rate (HR)] were collected in each trial. All participants reported strong feelings of ownership of the virtual hand only in 1PP. Subjective measures also showed that pain and pleasure were experienced as more salient than neutral. SCR analysis demonstrated higher reactivity in 1PP than in 3PP. Importantly, vicarious pain induced stronger responses with respect to the other conditions in both perspectives. HR analysis revealed equally lower activity during pain and pleasure with respect to neutral. SCR may reflect egocentric perspective, and HR may merely index general arousal. The results suggest that behavioral and physiological indexes of reactivity to seeing others' pain and pleasure were qualitatively similar in 1PP and 3PP. Our paradigm indicates that virtual reality can be used to study vicarious sensation of pain and pleasure without actually delivering any stimulus to participants' real body and to explore behavioral and physiological reactivity when they observe pain and pleasure from ego- and allocentric perspectives.
NEW & NOTEWORTHY
Using immersive virtual reality we compared the behavioral and physiological reactivity of participants who observed pain and pleasure stimuli delivered to the body of an avatar that was embodied when seen from an egocentric perspective. This novel paradigm allowed us to investigate the reactivity to vicarious pain and pleasure without actually delivering any real stimuli and to explore the influence of different physical perspectives on basic empathic reactivity to pain and pleasure.
empathy, i.e., the social ability that allows one to share the emotions, feelings, and beliefs of other individuals, consists of a variety of components ranging from the self-centered reactivity at the basis of the process of mapping on the self what we see in others (e.g., sensorimotor contagion) to the other-oriented stance that allows us to understand others through cognition (i.e., perspective taking) or emotion (i.e., empathic concern) (Decety and Jackson 2004). Studies indicate that people who see or imagine others in pain tend to empathically share what others feel at both behavioral and neural levels (Lamm et al. 2011). Although studies originally suggested that empathy for pain involves only the anterior cingulate cortex and the anterior insula, i.e., the two main affective nodes of the pain matrix (Singer et al. 2004), subsequent evidence demonstrated that also the regions that are part of the sensory node of the pain matrix, such as the primary somatosensory and motor cortexes, play an important role in the basic form of empathy for pain called sensorimotor contagion (Betti and Aglioti 2016). Interest in the study of empathy for positive states (e.g., the conditions in which imagining, recalling, and observing joy in others trigger positive states in the empathizer) is rapidly increasing (Van der Gaag et al. 2007; Morelli et al. 2015a, 2015b; Chiesa et al. 2015; Wang et al. 2016). A recent study, for example, reported shared activation during first-hand and vicarious experience in the orbitofrontal cortex for pleasant touch and the right frontoinsular cortex for unpleasant touch (Lamm et al. 2015). It is worth noting that pleasant touch represents an important element in interpersonal connections and leads to positive feelings that forge attachment and social bonds (Suvilehto et al. 2015). Expanding on classical studies that investigated the induction of illusory feelings of ownership (FO) on artificial physical body parts (i.e., like a rubber hand; Botvinick and Cohen 1998), immersive virtual reality (IVR) studies have demonstrated that FO over virtual body parts can be easily induced, particularly when the virtual character is seen from a first-person perspective (1PP) (Tieri et al. 2015a; Pavone et al. 2016). Autonomic responses [e.g., skin conductance responses (SCR) or heart rate (HR)] indicate that there is a link between physiological correlates of self-experienced emotions and vicarious physiological responses to others' pain (Avenanti et al. 2010). However, because the study of positive empathy is still in its infancy, much less is known about autonomic activation contingent upon viewing pleasant stimuli in others.
In the present study we expanded our previous research (Avenanti et al. 2005; Bufalari et al. 2007; Costantini et al. 2008; Valentini et al. 2012) by combining IVR with recording of SCR and HR in healthy participants who observed painful or pleasurable stimuli delivered to the hand of a virtual avatar seen from a 1PP or a third-person perspective (3PP). In particular, we capitalized on the power of IVR to make a specific empathogenic scenario realistic and looked for differences in reactivity to pain or pleasure depending on whether the virtual body was perceived as belonging to the self (when seeing it from 1PP) or others (3PP).
METHODS
Participants
Twenty-four healthy volunteers took part in the study (12 females; mean age ± SD, 26.1 ± 5.2). All participants were right-handed (Handedness Inventory; Briggs and Nebes 1975) with normal visual acuity and were naïve as to the purposes of the experiment. The experimental protocol was approved by the ethics committee of the Fondazione Santa Lucia and was carried out in accordance with the ethical standards of the 2013 Declaration of Helsinki. All participants gave their written informed consent to take part in the study.
Experimental Stimuli and Setup
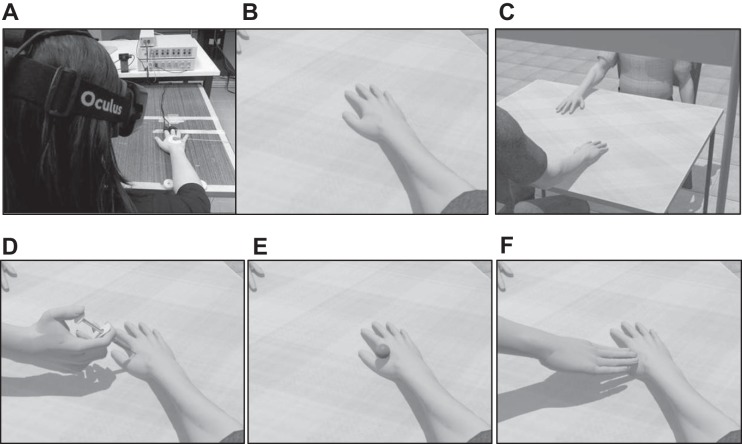
The virtual scenario was designed using 3DS Max 2015 (Autodesk) and implemented in XVR 2.0 (Tecchia et al. 2010; http://www.vrmedia.it/en.html). The virtual avatars were created using Poser Pro 2010 library (Smith Micro) and implemented in XVR. The scenario was presented by means of the Oculus Rift DK 2.0 head-mounted display (HMD; www.oculus.com) (Fig. 1, A and B). The scene consisted in a real-size room, with two virtual avatars sitting on the two opposite sides of a table and a virtual gray panel placed between the avatars that occluded the view of their faces (Fig. 1C). Three different clip stimuli were designed and implemented in the virtual scenario, namely: 1) a virtual arm holding a needle on the avatar's right hand and penetrating it (pain; Fig. 1D), 2) a virtual hand approaching the avatar's right hand and caressing it (3 cm/s; Loken et al. 2009; Fig. 1E) (pleasure), and 3) a virtual ball gently touching it (neutral; Fig. 1F).
Fig. 1.
A–C: virtual reality scenario. A: schematic representation of a participant wearing a head-mounted display (HMD) and positioning his/her real hand on a table in the same location and posture as the virtual body; appearance of the virtual limb on which the stimuli were delivered seen from a 1-person perspective (1PP). B: appearance of the virtual scenario during stimulations to the limb seen from a 3-person perspective (3PP). C: on the opposite side of the table and the gray screen covering the other's face. D–F: stimuli [pain (D), neutral (E), and pleasure (F)].
We used AD Instruments PowerLab 8/35 and ML116 Galvanic Skin Response (GSR) Amplifier [providing a 75-Hz AC excitation with low constant voltage of 22 millivolt root mean square (mVrms)] devices to amplify the signals and specific GSR sensors consisting of two bipolar finger electrodes placed on the right hand. The signal was sampled at 1 kHz, recorded, and analyzed using LabChart 7 (AD Instruments) software. The same devices and software have been used to record participant's electrocardiogram activity. More specifically, two electrodes (DORMO pregelled electrodes 50 mm) were placed on the back of each hand, and the reference was placed on the left ankle. Signals were sampled at 1 kHz and filtered using a 30-Hz low-pass filter.
Procedure
Participants were seated on a chair in front of a table wearing the HMD (Fig. 1A) and carefully fixated the right virtual hand (in 1PP or 3PP) (Fig. 1, B or C). Moreover, they were informed that a virtual needle, caress, or ball could be delivered to the virtual hand but that no stimulation of their real hand could occur. The experiment was composed of two separate blocks (one for each perspective) presented in a counterbalanced way across subjects. Each block consisted of 21 trials (7 pain, 7 pleasure, and 7 neutral). Each trial started with the observation of the right virtual hand (in 1PP or 3PP). After 6,000 ± 500 ms, the stimulus was delivered to the virtual hand on a region overlying the first dorsal interosseous. The stimulus remained on for ∼1 s. Participants continued to observe the virtual hand for 6,000 ± 500 ms after the stimulus and, at the end of the trial (and of the observation), the visual analog scale (VAS) appeared, and they provided three ratings about the stimuli: 1) illusory ownership: how strong was the sensation that the virtual hand was part of your body (0 = no ownership to 100 = maximal ownership); 2) intensity: how intense was the stimulus for you (0 = very weak, 100 = very strong) referring to the sensory quality of the observed stimuli (Bufalari et al. 2007; Valeriani et al. 2008); and 3) (un)pleasantness: was the stimulus pleasant or unpleasant for you (0 = strongly unpleasant, 50 = neutral, 100 = strongly pleasant)? The order of the three questions was counterbalanced across blocks and participants. It is worth noting that all of the questions referred to the feelings experienced by the onlooker and thus may have triggered self-oriented reactivity.
SCR and HR were continuously recorded for the entire duration of each block. At the end of the experimental session, participants were asked to fill out two questionnaires aimed at measuring trait-empathy [i.e., the interpersonal reactivity index (IRI); Davis et al. 1980] and state-empathy [empathy for pain scale (EPS Scale); Giummarra et al. 2015].
RESULTS
VAS Reports in the Virtual Pain and Pleasure Observation Task
VAS responses were normally distributed according to the Kolmogorov-Smirnov test. Thus, three (one for each question) different two-way repeated-measures ANOVAs with perspective (1PP vs. 3PP) and condition (pain-pleasure-neutral) as main factors were run. Multiple-comparison post hoc tests were performed using the Newman-Keuls test.
Analysis of the illusory ownership responses revealed a significant main effect of perspective [F(1,23) = 171.46, P < 0.001], accounted for by higher ownership in 1PP than in 3PP (mean ± SD: 1PP = 68.47 ± 14.05 vs. 3PP = 14.76 ± 14.71) (Fig. 2A) and a significant main effect of condition [F(2,46) = 7.70, P = 0.001]. Post hoc comparisons showed that ownership was higher during pain compared with pleasure (pain = 44.19 ± 31.56 vs. pleasure = 41.43 ± 30.13; P = 0.034) and the neutral condition (39.29 ± 30.13; P < 0.001). Only a trend toward a difference between pleasure and neutral (P = 0.088) was found. The nonsignificant perspective × condition [F(2,46) = 0.20, P = 0.818] interaction indicates that the modulating effect of the observed painful stimuli was not distinguishable for the two perspectives. Analysis of subjective reports concerning intensity revealed a significant main effect of perspective [F(1,23) = 15.688, P < 0.001] that was accounted for by higher values in 1PP than 3PP (mean ± SD: 54.67 ± 18.64 vs. 43.12 ± 22.30; P < 0.001), and a significant main effect of condition [F(2,46) = 16.50, P < 0.001] that was accounted for by higher-intensity ratings for pain (57.47 ± 19.21) than for pleasure (49.73 ± 18.70; P = 0.017) and neutral (39.48 ± 22.22; P < 0.001) stimuli. Moreover a significant difference was found between pleasure and neutral stimuli (P = 0.002) (Fig. 2B). No significant perspective × condition interaction was found [F(2,46) = 0.307; P = 0.736].
Fig. 2.
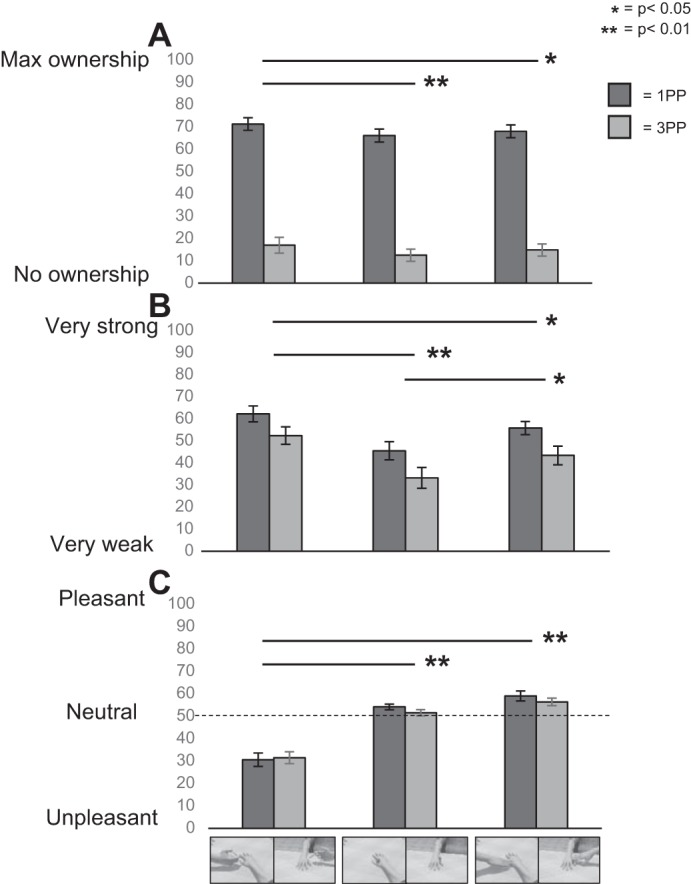
Visual analog scale (VAS) ratings (means and SE) of ownership (A), intensity (B), and (un)pleasantness (C) elicited by the different stimuli in 1PP and 3PP.
ANOVA of (un)pleasantness ratings revealed only a highly significant effect of condition [F(2,46) = 59.50, P < 0.001] (Fig. 2C), which was accounted for by stronger unpleasantness (lower ratings) elicited by the observation of pain (mean ± SD: 31.10 ± 13.81) compared with pleasure (57.80 ± 9.49; P < 0.001) and neutral (52.92 ± 6.57; P < 0.001) stimuli. The comparison of pleasure and neutral stimuli was marginally significant (57.80 ± 9.49 vs. 52.92 ± 6.57; P = 0.066). No significant effects of the perspective [F(1,23) = 1.423, P = 0.24] and the perspective × condition [F(2,46) = 0.890, P = 0.42] interaction were found. It is worth noting that we performed the one-sample t-test (using a Bonferroni correction for 6 comparisons, the resulting P level was 0.008) between (un)pleasantness reports (0 = unpleasant; 100 = pleasant) and the value 50 (which represented the neutral score) to verify if pleasure and pain were different from neutral stimuli. In 1PP, pain was perceived as significantly unpleasant (mean ± SD: 30.66 ± 14.81 vs. 50; P < 0.001), whereas pleasure was perceived as more pleasant than the neutral value of 50 (59.14 ± 10.79; P < 0.001). It is worth noting that ratings for neutral stimuli were more pleasant with respect to the value of 50 (54.22 ± 6.13; P = 0.003). In 3PP, pain was perceived as significantly more unpleasant than 50 (31.52 ± 13.04; P < 0.001), and pleasant stimuli were perceived as more pleasant than the neutral value of 50 (56.44 ± 7.98; P = 0.001). Neutral stimuli did not differ from 50 (51.61 ± 6.85; P = 0.260). These results indicate that the neutral stimulus was actually neutral only when seen from a 3PP and thus less embodied with respect to the 1PP.
Skin Conductance Responses
Data preprocessing and analysis were carried out using Matlab 7.9.0 (The MathWorks, Natick, MA) and the Matlab-based toolbox Ledalab V3.4.2 (Leipzig, Germany) (www.ledalab.de). Continuous decomposition analysis (Benedek and Kaernbach 2010a) was performed to separate phasic components from tonic activity based on standard deconvolution. SCR was analyzed using the mean amplitude of the phasic SCR within a window of 6 s (Madden et al. 2016) after visual stimulus onset with a minimum response of 0.01 μS.
To correct for nonnormally distributed responses the following transformation was computed: log(SCR + 1) (Armel and Ramachandran 2003). Figure 3 shows the temporal dynamics of the SC signal from 6 s before the event to 6 s after the event.
Fig. 3.
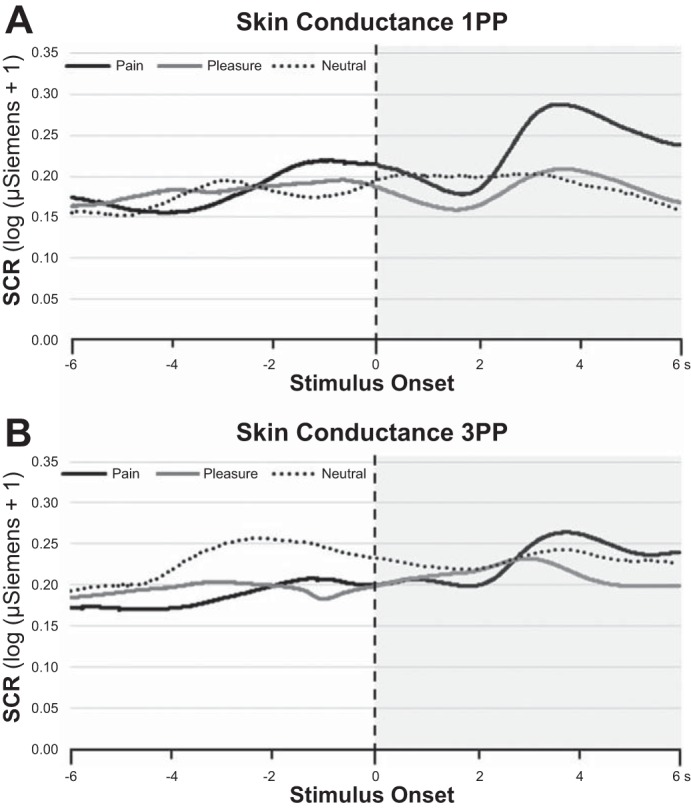
Grand average of the SC signals [data were preprocessed by separating the phasic components from tonic activity and then transformed in log(SCR + 1)] over 12 s. 1PP and 3PP conditions are represented in A and B, respectively. Zero refers to the time at which the needle (black line), caress (gray line), and ball (broken line) touched the hand of the avatar. The time window from −6 to 0 and from 0 to 6 s refers to observation of the virtual hand before the stimulus or after it impinged on the virtual hand, respectively. The gray transparent box indicates the data used for the quantitative analysis reported in Fig. 4, top.
Data were analyzed using a two-way repeated-measures ANOVA with perspective (1PP vs. 3PP) and condition (pain-pleasure-neutral) as main factors. Statistical analysis revealed a significant main effect of condition [F(2,46) = 14.572; P < 0.001] (Fig. 4A), which was accounted for by higher amplitudes of SCR when participants observed pain compared with pleasure (mean ± SD: 0.176 ± 0.157 vs. 0.131 ± 0.117 μS; P < 0.001) and neutral (0.122 ± 0.116 μS; P < 0.001) conditions that in turn did not differ from one another (P = 0.378). A significant effect of perspective [F(1,23) = 6.571; P = 0.017] was found, with a higher amplitude for 1PP than 3PP (0.155 ± 0.13 vs. 0.131 ± 129 μS). Tellingly, the perspective × condition interaction was not significant [F(2,46) = 1.009; P = 0.372].
Fig. 4.

Skin conductance responses (SCR) {mean amplitude of the phasic SCR in [log(μS + 1)] and SE} and heart rate (HR) (means and SE) values for the different clips in 1PP and 3PP.
Heart Rate
The HR index was calculated by the number of heart beats in a 6-s poststimulus time window (as for the SCR; Slater et al. 2010). Data were analyzed using two-way repeated-measures ANOVA with perspective (1PP vs. 3PP) and condition (pain-pleasure-neutral).
No significant effect of perspective was found [F(1,23) = 0.640; P = 0.432]. By contrast, the effect of condition [F(2,46) = 4.876; P = 0.012] was significant. This effect was accounted for by lower HR during the pain (7.764 ± 1.051) than the neutral (mean ± SD vs. 7.891 ± 0.990; P = 0.01) condition but not compared with the pleasant condition (7.764 ± 1.051 vs. 7.803 ± 1.043; P = 0.358). Moreover, a significant difference was found between pleasure and the neutral conditions (P = 0.039) (Fig. 4B).
Questionnaires Evaluating Trait- and State-Empathic Reactivity
The mean values of the IRI subscales were as follows: 19.54 ± 3.91 for “perspective taking,” 17.25 ± 4.34 for “fantasy,” 18.12 ± 4.30 for “empathic concern,” and 11.5 ± 4.25 for “personal distress.” These values are in accord with previous reports in samples of comparable size (Betti et al. 2009). The mean values of the EPS subscales were 2.61 ± 0.85 for “affective distress,” 2.03 ± 0.95 for “vicarious pain,” and 3.02 ± 0.65 for empathic concern. These values are in keeping with Giummarra et al. (2015). No participants reported to have experienced pain or touch synesthesia.
Correlation Analyses
VAS reports in the virtual pain and pleasure observation tasks.
Indexes were calculated (separately for each perspective) by subtracting neutral scores from those of pain and pleasure. We obtained two indexes of the effect of seeing pain (1PP and 3PP) and two of the effect of seeing pleasure (1PP and 3PP) concerning ownership, (un)pleasantness, and intensity. Each of the indexes was correlated with indexes of SCR and HR reactivity (calculated as seeing pain minus neutral and seeing pleasure minus neutral). No correlations were found between explicit reports and physiological measures.
Trait and state empathic reactivity.
Correlation analyses were performed between IRI and EPS subscales and the previously described indexes of physiological measures. No correlations were found between questionnaire subscales and physiological measures. A significant negative correlation was found between the IRI perspective taking subscale and SCRs in the 1PP pain observation condition (r = −0.515; P = 0.010), suggesting that higher perspective-taking scores are linked to lower SCR responses.
DISCUSSION
We explored whether behavioral and physiological reactivity to the observation of painful and pleasurable stimuli delivered to the hand of an avatar was influenced by the visual perspective and the feeling of receiving the stimulation due to IVR-related induction of ownership over virtual body parts. In keeping with previous research (Maselli and Slater 2013; Tieri et al. 2015a, 2015b; Pavone et al. 2016), our behavioral data show that participants felt high illusory ownership of the virtual hand when they saw it in 1PP, suggesting that virtual embodiment can be induced even in the absence of visuotactile or visuomotor boosting. Our novel IVR scenario allowed us to expand previous research by showing that, although ratings of ownership over an avatar limb are much higher in 1PP than in 3PP, the effect of seeing different valence stimulation is qualitatively similar. Indeed, observation of painful stimuli induced a higher sense of ownership with respect to pleasure and neutral stimuli in both perspectives. Perceived intensity of the observed stimuli was maximal for pain, intermediate for pleasure, and minimal for the neutral touch. Importantly, however, this effect did not differ across the two perspectives.
While the observation of pain turned out to be significantly more unpleasant than all the other conditions, the observation of a pleasant touch was only marginally more pleasurable than the neutral stimulus. Although the pleasant touch stimuli used in our study might not have been maximally effective, it is worth mentioning that perception of touch on one's own body may not always be experienced as pleasant (Ellingsen et al. 2016). For example, the person who receives a caress before deciding whether interpersonal touch is positive or negative may need to perceive sensory cues (Taylor-Clarke et al. 2002) and the internal motivational state (Triscoli et al. 2014) of the toucher. In any case, future studies are needed in which virtual touch is more clearly pleasant. An important behavioral result of our study is that the valence of the stimuli was similarly perceived in 1PP and 3PP, indicating that reactivity to pain and pleasure in others cannot be explained by an egocentric perspective even when the specific questions raised by the task bias an egocentric stance. Thus, our study expands research based on using only 2D pain stimuli indicating that, although quantitatively different, the reactivity to stimuli seen on others or attributed to the self may be qualitatively similar.
The increase in SCR found during the observation of pain hints at the salience of this condition and is in keeping with studies showing a similar effect when a threatening event is applied over a full body (Petkova et al. 2008), a rubber hand (Armel and Ramachandran 2003), or a virtual hand (Tieri et al. 2015b). It is relevant that modulation of SCR has been reported during observation of others' pain (Avenanti et al. 2010; De Coster et al. 2013; Hein et al. 2011; Pfabigan et al. 2015). Interestingly, SCR variations are linked to a variation of activity in the anterior cingulate cortex (Purves et al. 2008), a region that is part of the pain matrix and is involved in empathy for pain (Singer et al. 2004). Importantly, our modulation of SCR during the observation of pain was found in both 1PP and 3PP, suggesting that the same qualitative reactivity was at play in the two observation perspectives. It is worth noting that the effect was higher in 1PP compared with 3PP. This difference could be due to somatosensory contagion: the effect is maximized when it is perceived on one's own body and reduced when it is perceived on others' bodies. Also, no correlation was found between ownership scores and SCR, thus ruling out that the reactivity to observed pain is due to embodiment. A negative correlation between SCR in the pain condition in 1PP and the perspective-taking subscale could suggest that the more the participants felt themselves separate from the avatar in 1PP the higher their skin response, as if they perceived the stimuli on the real body; conversely, if the avatar was fully embodied, responses were slower. Unlike pain, SCR did not differ for pleasant touch and neutral stimuli, a result what may be specific of this paradigm and needs to be confirmed in future studies.
A reduction of HR in a time window of 6 s after the stimulus was found for observation of both pain and pleasure with respect to a neutral stimulus. This effect was not different in 1PP vs. 3PP. Studies indicate that the application of painful stimuli induces a modulation of the sympathetic system when pain is felt (Moltner et al. 1990) or seen (Lamm et al. 2008). The heart modulation in the pain condition is consistent with a triphasic pattern of responses described by Bradley and Lang (2000) with an initial deceleration (in our case in the 6 s after the stimulus) followed by an acceleration and a second deceleration. This result is consistent with a study on the role of the perspective taken during empathy for pain (Lamm et al. 2008) where a reduction of HR was found in participants who watched videos of people in pain and tried to imagine that they were the people in pain. This initial deceleration of heart beats could be related to an orienting response (Porges 1992). We also found a deceleration of heart beats in the pleasure condition, which is in keeping with the notion that touch has a positive influence on parasympathetic activity. It is also interesting that the same pattern of deceleration in a time window of 10 s was found in 9-mo-old infants receiving a caress (Fairhurst et al. 2014). In any case, heart rate changes to the observation of pain and pleasure were similar in 1PP vs. 3PP, which suggests that this variable may simply track general arousal. Mirror-touch synesthesia (MTS) and mirror-pain synesthesia (MPS) refer to the conscious experience of tactile or pain sensations induced by seeing someone else being tactually or painfully stimulated (Ward and Banissy 2015). Despite none of the participants in this study reporting explicitly MTS or MPS, our virtual task may induce vicarious activation of touch or pain also on one's own body as in the MTS/MPS. A direct comparison of the behavioral and neural effects of real and virtual pain as well as the exploration of the specific links between first-hand and empathic pain remain important issues for future research. Our approach might also be relevant to develop a treatment of specific disorders related to pain (e.g., chronic pain or allodynia) or hedonic impulses (e.g., anhedonia).
In conclusion, the behavioral and physiological effects found in our study were qualitatively comparable in 1PP and 3PP. Moreover, none of these effects correlated with ratings of ownership, which indicates that they cannot be explained by embodiment. To the best of our knowledge, our experimental paradigm is the first IVR attempt to compare behavioral and physiological reactivity with stimuli that participants can perceive as delivered on their own or an avatar's body depending on the perspective. This paradigm may open new avenues for studying vicarious activation of pain and pleasure without actually delivering any stimuli and for studying whether taking a different physical perspective influences basic reactivity to pain and pleasure.
GRANTS
This work received financial support from the Italian Ministry of Health (RF-2010-2312912) and from H2020-SESAR-2015-1 (The Embodied Remote Tower, Project No. 699379).
DISCLOSURES
BrainTrends did not have any financial or scientific influence on the present study. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F., G.T., and S.M.A. conception and design of research; M.F. and G.T. performed experiments; M.F. and G.T. analyzed data; M.F., G.T., and S.M.A. interpreted results of experiments; M.F. and G.T. prepared figures; M.F., G.T., and S.M.A. drafted manuscript; M.F., G.T., and S.M.A. edited and revised manuscript; M.F., G.T., and S.M.A. approved final version of manuscript.
REFERENCES
- Armel KC, Ramachandran VS. Projecting sensations to external objects: evidence from skin conductance response. Proc R Soc B Biol Sci 270: 1499–1506, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci 8: 955–960, 2005. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Curr Biol 20: 1018–1022, 2010. [DOI] [PubMed] [Google Scholar]
- Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods 190: 80–91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Aglioti SM. Dynamic construction of the neural networks underpinning empathy for pain. Neurosci Biobehav Rev 63: 191–206, 2016. [DOI] [PubMed] [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F. Synchronous with your feelings: sensorimotor band and empathy for Pain. J Neurosci 29: 12384–12392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands “feel” touch that eyes see. Nature 391: 756, 1998. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, physiology. Cog Neurosci Emotion 25: 49–59, 2000. [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex 11.3: 230–238, 1975. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex 17: 2553–2561, 2007. [DOI] [PubMed] [Google Scholar]
- Chiesa PA, Liuzza MT, Acciarino A, Aglioti SM. Subliminal perception of others′ physical pain and pleasure. Exp Brain Res 233: 2373–2382, 2015. [DOI] [PubMed] [Google Scholar]
- Costantini M, Galati G, Romani GL, Aglioti SM. Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects. Eur J Neurosci 28: 1222–1230, 2008. [DOI] [PubMed] [Google Scholar]
- Davis MH. A Multidimensional Approach to Individual Differences in Empathy. Austin, TX: Univ of Texas, 1980. [Google Scholar]
- De Coster L, Verschuere B, Goubert L, Tsakiris M, Brass M. I suffer more from your pain when you act like me: being imitated enhances affective responses to seeing someone else in pain. Cog Affect Behav Neurosci 13: 519–532, 2013. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev 3: 71–100, 2004. [DOI] [PubMed] [Google Scholar]
- Ellingsen DM, Leknes S, Løseth G, Wessberg J, Olausson H. The neurobiology shaping affective touch: expectation, motivation, and meaning in the multisensory context. Front Psychol 6: 1–16, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst MT, Löken L, Grossmann T. Physiological and behavioral responses reveal 9-month-old infants' sensitivity to pleasant touch. Psychol Sci 25: 1124–1131, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giummarra MJ, Fitzgibbon BM, Georgiou-Karistianis N, Beukelman M, Verdejo-Garcia A, Blumberg Z, Chou M, Gibson SJ. Affective, sensory and empathic sharing of another's pain: The Empathy for Pain Scale. Eur J Pain 19: 807–816, 2015. [DOI] [PubMed] [Google Scholar]
- Hein G, Lamm C, Brodbeck C, Singer T. Skin conductance response to the pain of others predicts later costly helping. PloS One 6: e22759, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54: 2492–2502, 2011. [DOI] [PubMed] [Google Scholar]
- Lamm C, Porges EC, Cacioppo JT, Decety J. Perspective taking is associated with specific facial responses during empathy for pain. Brain Res 1227: 153–161, 2008. [DOI] [PubMed] [Google Scholar]
- Lamm C, Silani G, Singer T. Distinct neural networks underlying empathy for pleasant and unpleasant touch. Cortex 70: 79–89, 2015. [DOI] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12: 547–548, 2009. [DOI] [PubMed] [Google Scholar]
- Madden VJ, Bellan V, Russek LN, Camfferman D, Vlaeyen JW, Moseley GL. Pain by association? Experimental modulation of human pain thresholds using classical conditioning. J Pain 17: 1105–1115, 2016. [DOI] [PubMed] [Google Scholar]
- Maselli A, Slater M. The building blocks of the full body ownership illusion. Front Hum Neurosci 7: 83, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möltner A, Hölzl R, Strian F. Heart rate changes as an autonomic component of the pain response. Pain 43.1: 81–89, 1990. [DOI] [PubMed] [Google Scholar]
- Morelli SA, Lieberman MD, Zaki J. The emerging study of positive empathy. Soc Personal Psychol Compass 9: 57–68, 2015a. [Google Scholar]
- Morelli SA, Sacchet MD, Zaki J. Common and distinct neural correlates of personal and vicarious reward: a quantitative meta-analysis. NeuroImage 112: 244–253, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone EF, Tieri G, Rizza G, Tidoni E, Grisoni L, Aglioti SM. Embodying others in immersive virtual reality: electro-cortical signatures of monitoring the errors in the actions of an avatar seen from a first-person perspective. J Neurosci 36: 268–279, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova VI, Ehrsson HH. If I were you: perceptual illusion of body swapping. PLoS One 3: e3832, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan DM, Seidel EM, Wucherer AM, Keckeis K, Derntl B, Lamm C. Affective empathy differs in male violent offenders with high-and low-trait psychopathy. J Personality Dis 29: 42, 2015. [DOI] [PubMed] [Google Scholar]
- Porges SW. Autonomic Regulation and Attention. Attention and Information Processing in Infants and Adults. 1992, p. 201–223. [Google Scholar]
- Purves D, Brannon EM, Cabeza R, Huettel SA, LaBar KS, Platt ML, Woldorff MG. Principles of Cognitive Neuroscience. Sunderland, MA: Sinauer Associates, 2008. [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Dolan RJ, Kaube H, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162, 2004. [DOI] [PubMed] [Google Scholar]
- Slater M, Spanlang B, Sanchez-Vives MV, Blanke O. First person experience of body transfer in virtual reality. PLoS One 5: e10564, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvilehto JT, Glerean E, Dunbar RIM, Hari R, Nummenmaa L. Topography of social touching depends on emotional bonds between humans. Proc Natl Acad Sci USA 112: 13811–13816, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clarke M, Kennett S, Haggard P. Vision modulates somatosensory cortical processing. Curr Biol 12: 233–236, 2002. [DOI] [PubMed] [Google Scholar]
- Tecchia F, Carrozzino M, Bacinelli S, Rossi F, Vercelli D, Marino G, Gasparello P, Bergamasco M. A flexible framework for wide-spectrum VR development. Presence Teleoperators Virtual Environ 19: 302–312, 2010. [Google Scholar]
- Tieri G, Tidoni E, Pavone EF, Aglioti SM. Body visual discontinuity affects feeling of ownership and skin conductance responses. Sci Rep 5: 17139, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieri G, Tidoni E, Pavone EF, Aglioti SM. Mere observation of body discontinuity affects perceived ownership and vicarious agency over a virtual hand. Exp Brain Res 233: 1247–1259, 2015a. [DOI] [PubMed] [Google Scholar]
- Triscoli C, Olausson H, Sailer U, Ignell H, Croy I. CT-optimized skin stroking delivered by hand or robot is comparable. Front Behav Neurosci 7: 208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini E, Liang M, Aglioti SM, Iannetti GD. Seeing touch and pain in a stranger modulates the cortical responses elicited by somatosensory but not auditory stimulation. Hum Brain Mapp 33: 2873–2884, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM. Seeing the pain of others while being in pain: a laser-evoked potentials study. Neuroimage 40: 1419–1428, 2008. [DOI] [PubMed] [Google Scholar]
- Van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc Neurosci 2: 179–222, 2007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Song J, Guo F, Zhang Z, Yuan S, Cacioppo S. Spatiotemporal brain dynamics of empathy for pain and happiness in friendship. Front Behav Neurosci 10: 45, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Banissy MJ.. Explaining mirror-touch synesthesia. Cogn Neurosci 6: 118–133, 2015. [DOI] [PubMed] [Google Scholar]