We measured cortical responses to abrupt shifts in interaural time difference, changes typically associated with change in lateral position, and identified distinct age effects for listeners with clinically normal hearing. Reduced magnitudes and slower latencies in the responses relative to younger listeners were interpreted in the context of an opponent-channel model of spatial hearing as a decline in neural synchrony. Results have consequences for age-related models of spatial hearing, speech perception, and auditory stream segregation.
Keywords: aging, auditory temporal processing, binaural hearing, hemifield code, electroencephalography, auditory event-related potentials
Abstract
Previous electrophysiological studies of interaural time difference (ITD) processing have demonstrated that ITDs are represented by a nontopographic population rate code. Rather than narrow tuning to ITDs, neural channels have broad tuning to ITDs in either the left or right auditory hemifield, and the relative activity between the channels determines the perceived lateralization of the sound. With advancing age, spatial perception weakens and poor temporal processing contributes to declining spatial acuity. At present, it is unclear whether age-related temporal processing deficits are due to poor inhibitory controls in the auditory system or degraded neural synchrony at the periphery. Cortical processing of spatial cues based on a hemifield code are susceptible to potential age-related physiological changes. We consider two distinct predictions of age-related changes to ITD sensitivity: declines in inhibitory mechanisms would lead to increased excitation and medial shifts to rate-azimuth functions, whereas a general reduction in neural synchrony would lead to reduced excitation and shallower slopes in the rate-azimuth function. The current study tested these possibilities by measuring an evoked response to ITD shifts in a narrow-band noise. Results were more in line with the latter outcome, both from measured latencies and amplitudes of the global field potentials and source-localized waveforms in the left and right auditory cortices. The measured responses for older listeners also tended to have reduced asymmetric distribution of activity in response to ITD shifts, which is consistent with other sensory and cognitive processing models of aging.
NEW & NOTEWORTHY
We measured cortical responses to abrupt shifts in interaural time difference, changes typically associated with change in lateral position, and identified distinct age effects for listeners with clinically normal hearing. Reduced magnitudes and slower latencies in the responses relative to younger listeners were interpreted in the context of an opponent-channel model of spatial hearing as a decline in neural synchrony. Results have consequences for age-related models of spatial hearing, speech perception, and auditory stream segregation.
information-rich acoustic settings, such as a crowded restaurant, are commonly described by older adults as the most difficult listening environments they encounter. To parse relevant from irrelevant stimuli in these settings accurately, proper coding of binaural cues are needed, and, perhaps not surprisingly, converging evidence indicates older listeners are less able to take advantage of these cues (for a review, see Eddins and Hall 2010). The two primary azimuthal spatial cues, interaural intensity differences (IIDs) and interaural time differences (ITDs), result from differences in signal intensity and differences in signal arrival times at the two ears, respectively. The frequency content of a stimulus determines which cue is dominant: IID cues dominate for high-frequency signals that are susceptible to acoustic head shadow, and ITD cues prevail for low-frequency signals in which phase differences at the two ears are unambiguous. The bulk of the stimulus power in human communication sounds occurs at low frequencies (i.e., ≤1.5 kHz; Byrne et al. 1994), and, correspondingly, the bulk of relevant spatial cues are ITD cues (Wightman and Kistler 1992). At the neural level, there is general consensus that ITDs are processed at the earliest binaural structures through coincidence detection (Goldberg and Brown 1969; Yin and Chan 1990), yet models of cortical representation of ITDs have been debated (cf. Joris and Yin 2007; Salminen et al. 2012). Neurophysiological data from barn owls have supported topographic representation of ITDs (place code) in cortex as originally suggested by Jeffress (1948; for a review, see Konishi 2003); however, in mammals, research has pointed toward a population rate code in which sound-source location is deduced from the relative activity from opposing channels broadly tuned to the two spatial hemifields (hemifield code; Grothe et al. 2010). That is, as sounds move toward the left hemifield, they produce greater rates of cortical activity in neurons tuned to the left hemifield and reduced rates of activity in those tuned to the right hemifield, with the relative balance of activity indicating the source location.
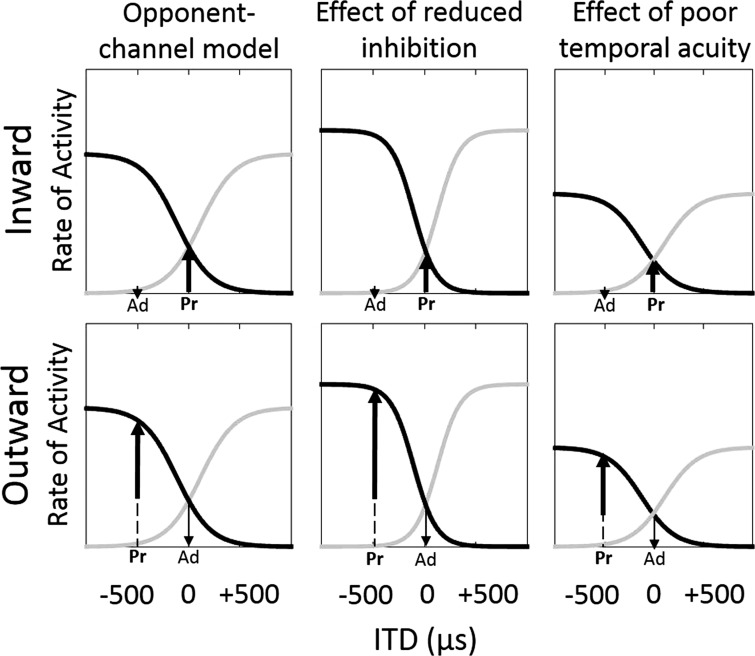
To evaluate the possibility that the human auditory system codes ITDs by an opponent-channel, hemifield coding process, Magezi and Krumbholz (2010) used electroencephalography (EEG) to record cortical responses to a binaural adapter signal of one ITD followed by a probe signal having another ITD. The adapter and probe combine in a manner typical of stimuli used to measure the acoustic change complex (Luck 2014). Based on the hemifield model, a shift in ITD (at the transition from adapter to probe) elicits a response that is dependent on the size of the shift and the relative laterality of the shift between the adapter and probe (Magezi and Krumbholz 2010). For a given hemifield, if the probe ITD is larger than the adapter ITD (i.e., lateralized further away from midline; referred to here as an outward shift), the transition to the probe should elicit a larger neural response than when the probe ITD is smaller than the adapter ITD (i.e., lateralized nearer to midline; referred to here as an inward shift). As summarized by others (Briley et al. 2013; Magezi and Krumbholz 2010; Salminen et al. 2010), Fig. 1 depicts the normal activity levels of two opposing channels as a function of ITDs, ranging from left-leading (−) to right-leading (+) ITDs. The left and right channels are assumed to cross at an ITD of 0, where neither hemifield dominates (i.e., lateralization at midline). In the examples presented in Fig. 1, the length of the upward-pointing arrow is associated with the difference in activity between the adapter and probe alone. A key assumption with the hemifield model is that changes in ITD are observed in the neural channel that is increasing in activity. Therefore, for inward ITD shifts toward the midline (top row), the right channel increases with the shift, and so the measured electrophysiological activity reflects the change in activity in the right channel. Conversely, outward ITD shifts away from midline (bottom row) result in an increase in activity in the left channel. Another assumption of the hemifield model is that the stimulus-response curves are symmetric with the maximal slope off-center and nearer the favored hemifield; however, recent work also has explored the potential for asymmetric curves and even a third, midline-specific channel (Briley et al. 2016). Nevertheless, the expected responses to the present conditions would be supported by both a two-channel and a three-channel model. A comparison of resulting activity (depicted here by the length of the upward-pointing arrow) between inward and outward shifts indicates that outward shifts will result in greater responses than inward shifts for the same adapter-probe pairs. The present study tested the potential effects of aging on the hemifield model for both inward and outward ITD shifts.
Fig. 1.
Schematic representation of the rates of neural activity in response to ITDs as modeled by a hemifield code. Black lines represent the response pattern of a neural population favoring left-leading ITDs, and gray lines represent the activity pattern for an opposing neural population that favors right-leading ITDs. In the present study, a probe (Pr) ITD immediately follows an adapter (Ad) ITD, and the relative direction of ITD shift, either inward (top row) or outward (bottom row), determines a distinct response (upward-pointing arrow). The left column presents the normally occurring output, whereas the middle column represents the hypothesized effect of reduced inhibition, and the right column represents the hypothesized effect of poor temporal acuity.
Many older listeners exhibit stereotypical, high-frequency peripheral hearing loss (i.e., presbycusis; Cruickshanks et al. 1998; Gates et al. 1990); however, a number of studies have also reported age-related declines in ITD sensitivity independent of hearing status (Babkoff et al. 2002; Herman et al. 1977; Ross et al. 2007; Strouse et al. 1998). Poor sensitivity to ITDs in older listeners could be attributed to underlying temporal processing declines (Grose and Mamo 2010; Lister and Roberts 2005; Ozmeral et al. 2016; Ross et al. 2007; Snell 1997), although not all find a correlation between behavioral measures of ITD sensitivity and temporal resolution (e.g., gap detection; Strouse et al. 1998). Nonetheless, precise ITD coding has been linked to auditory spatial coding (Grothe et al. 2010), and older listeners tend to have poorer spatial acuity (Freigang et al. 2014). In a recent study, Briley and Summerfield (2014) demonstrated age-related broadening of a spatial filter measured by event-related potentials to noise bursts shifting between −60 and +60° in the free field. As further evidence for a hemifield code of auditory space, listeners' behavioral measures (minimum audible angle; Mills 1958) were predicted well by an opponent-channel model fitted to the electrophysiological data. Although acoustic stimuli were not restricted only to ITD cues, the authors could comfortably assume that ITDs were the dominant cue. The underlying cause of the age-related decline in ITD coding, however, remains elusive. Two possibilities are considered below.
Neurophysiological evidence from rodents suggests that the aging sensory system undergoes a progressive deafferentation, which causes a compensatory downregulation of central inhibition. The effects of reduced inhibition, including changes to temporal response properties, have been shown at multiple levels of the central auditory pathway (for a review, see Caspary et al. 2008). At the cortical level, inhibition plays a key role in suppressing task-irrelevant information, and poor inhibitory control has been attributed to larger early-latency components (e.g., P1 and N1) for auditory processing (Alain and Woods 1999; Chao and Knight 1997). Alternatively, a possible explanation for age-related decline in spatial coding lies in the precise relaying of temporal information along the auditory pathway. Neurophysiological evidence has shown age-related declines in temporal precision, or neural synchrony (for a review, see Frisina 2001), and human electrophysiological measures in brain stem have shown poorer temporal coding of periodic stimuli (Anderson et al. 2012; Mamo et al. 2016). Considering the extraordinary ITD sensitivity of normal-hearing young adults (on the order of 10 μs), any loss in neural synchrony would have compounding effects upstream. Whereas these age-related changes may predict continued sensitivity across physiologically relevant ITDs, greater changes in ITDs may be necessary to evoke the same level of cortical responses seen in younger listeners.
The purpose of the present study was to evaluate the effect of aging on an ITD-specific opponent-channel process. Age-related downregulation of neural inhibition would predict that cortical responses increase and saturate more quickly as ITDs increase due to poorer regulation of activity, and sensitivity would be limited to a narrow range around zero ITD (Fig. 1, middle column). Alternatively, reduced neural synchrony and temporal precision predicts an overall shallower (less sensitive) opponent-channel function and smaller responses across the physiological range of ITDs (Fig. 1, right column). These alternative outcomes were assessed in younger and older listeners with normal hearing.
MATERIALS AND METHODS
Participants.
Ten younger listeners (mean age ± SD, 24.9 ± 2.5 yr; nine women) and nine older listeners (70.0 ± 2.7 yr; five women) completed the study. All listeners had clinically normal hearing at octave frequencies from 0.25 to 2 kHz with pure-tone thresholds ≤25-dB hearing loss (HL) and thresholds ≤60-dB HL for frequencies ≤8 kHz. Exclusion criteria included middle ear dysfunction, history of retrocochlear disorder, ear surgery, head injury, allergy to study materials, and/or cognitive impairment. All listeners were administered the Montreal Cognitive Assessment (MoCA; Nasreddine et al. 2005) to screen for cognitive impairment, and all achieved a score >26. Participants provided written, informed consent and received compensation for their participation, as approved by the University of South Florida Institutional Review Board. One older participant withdrew from the study before its completion and, therefore, was not included in the analysis.
Stimuli and procedure.
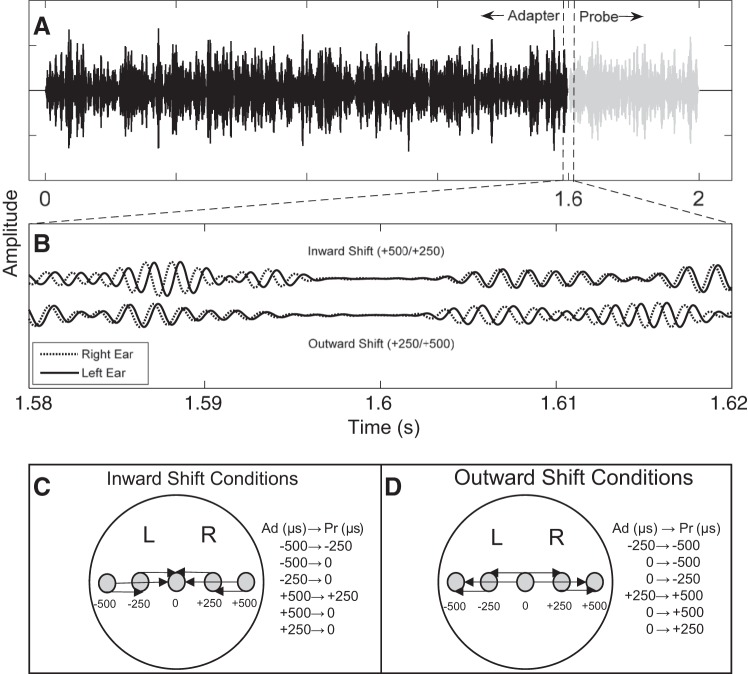
Stimuli were two sequential band-pass Gaussian noise bursts (adapter and probe) with lower and upper cutoffs of 500 and 750 Hz, respectively. Digital filtering was performed in the frequency domain using custom MATLAB (The MathWorks) software. New random noise samples were created each trial. The adapter and probe durations were 1,610 and 410 ms, respectively, including a 10-ms cosine-squared onset-offset window to avoid transients. Interaural time differences (ITDs) were applied to each stimulus with either the left or right channel temporally leading. Adapter and probe tokens were sequential with a 10-ms overlap (Fig. 2A). Figure 2B illustrates two such stimulus pairs, one in which the probe is perceived closer to midline than the adapter (top; inward shift) and one in which the probe is perceived farther from midline than the adapter (bottom; outward shift). Sampling rate was 24,414 Hz, corresponding to 41 μs per sample. Presentation level was fixed at 85-dB sound pressure level (SPL) and presented via TDT RZ6 real-time processor (Tucker-Davis Technology) and ER·2 Insert Earphones (Etymotic Research).
Fig. 2.
An example waveform of the stimuli presented to listeners. A shows the 1,600-ms-long adapter (black) immediately followed by the 400-ms-long probe (gray). B shows an example narrow view at the transition from adapter (Ad) to probe (Pr) for the binaural stimulus, either in the inward (top waveform) or outward (bottom waveform) direction. Example adapter-probe pair is +500/+250 μs. C and D depict the lateralization and shift in ITD per condition in the inward and outward directions, respectively. L, left; R, right.
Three different stimulus pairs were tested in each hemifield (i.e., left-leading or right-leading ITDs) and in both directions (i.e., inward or outward direction) for a total of 12 conditions. Stimulus pairs included: 0/±250, 0/±500, and ±250/±500 μs. Because of sampling, true ITDs were ±246 and ±492 μs for the ±250- and ±500-μs conditions, respectively. Figure 2, C and D, shows the inward and outward conditions, respectively, by indicating with an arrow the perceived lateral shift for each condition. Conditions were run in block format, and each block consisted of 150 trials with an interstimulus interval of 1,600 ms. Each block lasted ∼8 min, for a total of ∼96 min of listening per subject. The order of blocks was randomized across subjects. During listening blocks, participants were instructed to limit eye blinks and body movements while they watched a self-chosen video with captions. They were provided breaks during the recording sessions, and, on average, total time for sessions including applying EEG cap, listening blocks, and periodic breaks was 2.5 h.
EEG recording and processing.
Auditory-evoked potentials were recorded using an Advanced Neuro Technology (ANT) high-speed amplifier and an active shield, waveguard cap with sixty-four sintered Ag/AgCl electrodes (International 10-20 electrode system). Four additional electrodes were placed at the outer canthus of each eye and on the supra- and infraorbital ridges of the left eye to monitor eye movement and blink activity. Electrode impedance was <10 kΩ. Signals were referenced to the mean across channels, and the ground was located at the central forehead (AFz). The continuous EEG was recorded at a sampling rate of 512 Hz with 24-bit resolution using asalab acquisition software (ANT). Stimulus generation, presentation, and event triggering were controlled by custom MATLAB software. MATLAB and asalab were paired using ActiveX controllers in ASA Experiment Manager (ANT) via Excel (Microsoft).
The 64-channel, continuous EEG waveforms were processed using the software suite, Brainstorm (Tadel et al. 2011), and programmed within the MATLAB environment. All raw data files were preprocessed in the following manner: band-pass filtered (0.1–100 Hz), notch-filtered (60 Hz), artifact detection (eye blinks, physical movement, and other extraneous activity), artifact removal, epoched to stimulus onset (−200 to 1,500 ms) for adapter and probe signals separately, averaging, detrending, and baseline correction. Artifacts were automatically marked for removal if the eye electrode channels registered amplitudes >2 standard deviations over the entire block. This method led to ∼17% of the epochs rejected per subject.
Source estimation.
Underlying cortical sources were estimated in the Brainstorm software using sLORETA (Pascual-Marqui 2002). Default software parameters were selected, including: constrained source orientations, 3-dB signal-to-noise ratio, whitening via principal component analysis (PCA), full noise covariance, and depth weighting. Source files were created from the averaged trials per condition and for each subject individually. Sources were computed for a source space consisting of ∼15,000 cubic vertices constrained to the volume of the cortex. It should be noted that sources were mapped to the Montreal Neurological Institute (MNI) Colin27 brain template using a multilinear registration technique within Brainstorm and not to individually measured electrode positions.
Selection of ROI and data analysis.
Forward models were generated by the symmetric boundary element method from the open-source software OpenMEEG (Gramfort et al. 2010; Kybic et al. 2005). Regions of interest (ROI) were defined based on the MNI coordinates for the left and right primary auditory cortices [x, y, and z; −40, −28, and 6 mm (left); 48, −28, and 10 mm (right)]. A cluster of vertices was manually selected around each ROI until the total volume of the cluster was ∼5 cm2.
RESULTS
Morphology of cortical responses.
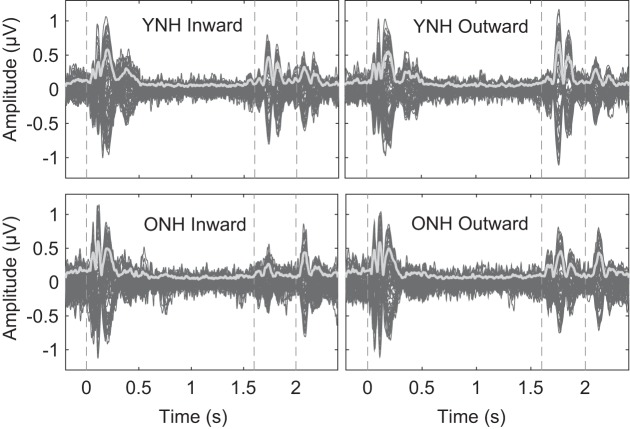
In Fig. 3, waveforms averaged across younger and older subjects are displayed across the full stimulus (adapter and probe) duration for each of the 64 recorded channels. The global field power (GFP; Skrandies 1990) is highlighted in light gray. The waveforms for inward-shift and outward-shift conditions for both groups exhibited a stereotypical auditory event-related potential (ERP), with the characteristic P1-N1-P2 complex (Luck 2014), associated with the onset of the adapter (t = 0 s), transition to the probe (t = 1.6 s), and the offset of the stimulus (t = 2 s). As seen elsewhere (Edmonds and Krumbholz 2014; Magezi and Krumbholz 2010), the offset of the probe ITD elicited prominent N1 and P2 components, whereas the P1 component was less evident.
Fig. 3.
The average EEG responses to the inward (left column) and outward (right column) ITD shift conditions are displayed for the young, normal-hearing group (YNH; top row) and older, normal-hearing group (ONH; bottom row). The display consists of 64 scalp electrode recordings (dark gray lines) and the global field potential (light gray line) measured over the time window of −0.2 to 2.4 s relative to the adapter ITD onset. Vertical dotted lines indicated the onset of the adapter (t = 0 s), the transition to the probe ITD (t = 1.6 s), and the offset of the probe (t = 2 s).
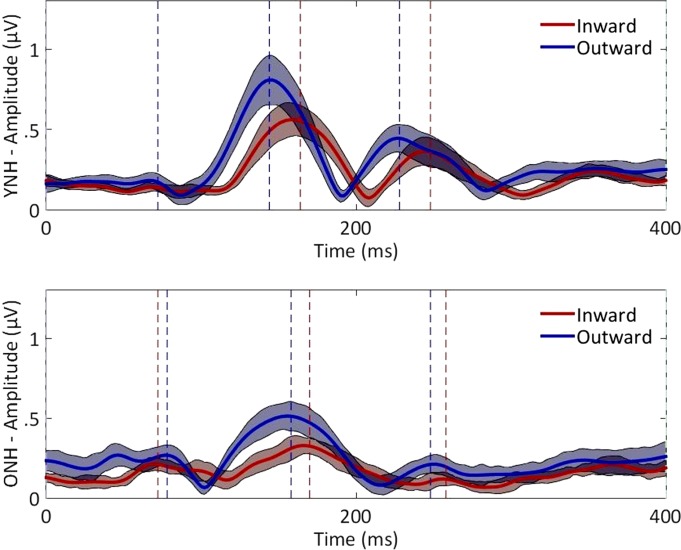
Between the age groups, the difference in ERP responses coinciding with the onset of the adapter or to the offset of the probe stimulus were minimal, whereas the response to the probe onset was visibly diminished in the older group. In Fig. 4, the average GFP as a function of time is shown relative to the onset of the probe for the younger and older groups with the peaks of the P1, N1, and P2 components for the inward and outward conditions. The responses from both age groups had similar morphology. To quantify individual peak magnitudes and latencies, peaks were first identified in the average waveforms, and then peaks in the individual subject waveforms were automatically selected via custom MATLAB script using a 40-ms window centered at the average latencies of the P1, N1, and P2 components. Based on these individual peaks, the statistics of the average peak magnitudes and corresponding latencies of each ERP component were computed and are reported in Table 1. The combined magnitudes of the N1 and P2 component serve as an aggregate response and were also included in the analysis. Data were submitted to separate two-way repeated-measures ANOVAs with age group (younger and older) and direction (inward and outward) as factors and the average pure-tone threshold at 500 Hz as a covariate, and results are summarized in Table 2. With respect to direction, latencies were significantly shorter at N1 and P2 for the outward direction relative to the inward direction. There also were significant age effects for N1 and P2 components, with older listeners having longer latencies on average, and at P2, there was an interaction with direction. The interaction could be explained by the more drastic latency difference between inward and outward conditions in the younger group than the older group. With respect to the component magnitudes, there also was a main effect of direction for the N1 and N1+P2 components, with outward conditions greater than inward conditions. No significant main effects of age or interactions were observed for magnitudes when pure-tone threshold at 500 Hz was used as a covariate.
Fig. 4.
The average GFP for inward (red) and outward (blue) ITD shift conditions are overlaid for the YNH (top) and ONH (bottom) groups. Color shaded regions indicate confidence intervals (1.96 × SD), and vertical dotted lines indicate latencies of the peaks for the P1, N1, and P2 components. Time is referenced to the onset of the probe ITD.
Table 1.
Average latencies and peak GFP magnitudes of the P1-N1-P2 complex
| Younger Listeners (n = 10) |
Older Listeners (n = 9) |
||||
|---|---|---|---|---|---|
| Direction of Shift | Component | Latency, ms | Magnitude, μV | Latency, ms | Magnitude, μV |
| Inward | P1 | 70.2 (7.0) | 0.3 (0.1) | 69.8 (6.6) | 0.3 (0.1) |
| N1 | 161.2 (5.5) | 0.7 (0.3) | 168.7 (6.8) | 0.4 (0.1) | |
| P2 | 247.2 (7.4) | 0.5 (0.3) | 255.8 (9.4) | 0.3 (0.1) | |
| N1+P2 | n/a | 1.1 (0.5) | n/a | 0.7 (0.2) | |
| Outward | P1 | 71.0 (6.7) | 0.4 (0.2) | 75.3 (7.7) | 0.4 (0.2) |
| N1 | 141.6 (4.3) | 0.9 (0.5) | 155.8 (6.2) | 0.7 (0.3) | |
| P2 | 228.2 (7.5) | 0.6 (0.3) | 247.6 (8.9) | 0.4 (0.2) | |
| N1+P2 | n/a | 1.5 (0.8) | n/a | 1.0 (0.3) | |
Mean values are presented with SD in parentheses.
n/a, Not applicable.
Table 2.
Results of 2-way repeated-measures ANOVA with factors direction and age group and the covariate average pure-tone threshold at 500 Hz
| Direction |
Age |
Direction × Age |
|||||
|---|---|---|---|---|---|---|---|
| Parameter | Component | F(1,16) | P | F(1,16) | P | F(1,16) | P |
| Latency | P1 | 0.96 | 0.341 | 0.18 | 0.677 | 0.01 | 0.928 |
| N1 | 7.19 | 0.016 | 24.55 | <0.001 | 3.76 | 0.070 | |
| P2 | 10.39 | 0.005 | 14.03 | 0.002 | 4.80 | 0.044 | |
| Magnitude | P1 | 0.48 | 0.499 | 1.20 | 0.290 | 0.13 | 0.719 |
| N1 | 11.02 | 0.004 | 1.45 | 0.247 | 0.26 | 0.616 | |
| P2 | 0.05 | 0.828 | 2.74 | 0.117 | 1.17 | 0.295 | |
| N1+P2 | 5.18 | 0.037 | 2.20 | 0.157 | 0.05 | 0.823 | |
P values in bold type are statistically significant.
Individual conditions also were examined because the size of ITD shift and the ITD hemifield may have had variable effects. The peak GFP magnitudes were extracted from the P1, N1, and P2 components for each adapter-probe pairing. The component magnitudes were submitted each to a 3-way repeated-measures ANOVA with factors of age group (younger and older), direction (inward and outward), stimulus-probe pairing (6 levels of ITD change between 0 and ±250, 0 and ±500, and ±250 and ±500 μs), and the average pure-tone threshold at 500 Hz as a covariate. For the N1 component, results showed significant main effects of direction [F(1,16) = 2.46, P = 0.04], but group and condition differences were not significantly different nor were there any significant interactions. Neither the P1 nor the P2 component yielded significant differences. The N1 response for each condition is presented in Fig. 5, and data were submitted to subsequent post hoc tests (1-way ANOVA). Results showed that younger listeners had significantly larger magnitudes than older listeners for 3 of the 6 inward conditions. Although these results are not conclusive, visual inspection of Fig. 5 indicates that the N1 component was smaller for older listeners than younger listeners at all 12 stimulus-probe pairings.
Fig. 5.
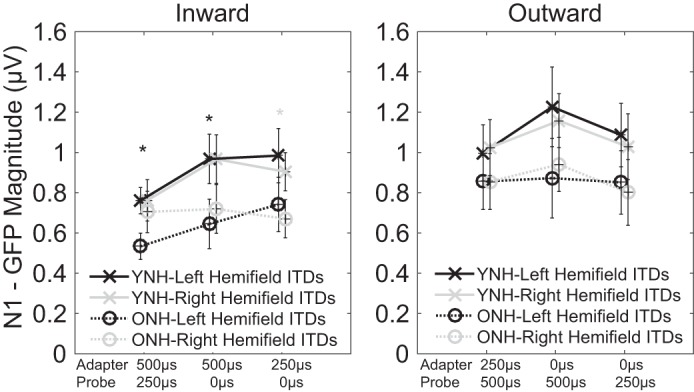
The magnitude of the GFP in the N1 component is plotted for each adapter-probe pairing. Younger, normal-hearing listeners (YNH; solid lines with × markers) generally had larger responses than older, normal-hearing listeners (ONH; dotted lines with ○ markers). Adapter-probe pairings could either be left-leading (black) or right-leading (gray). Shaded asterisks indicate significant between-group differences in the corresponding hemifield.
Source-localized responses.
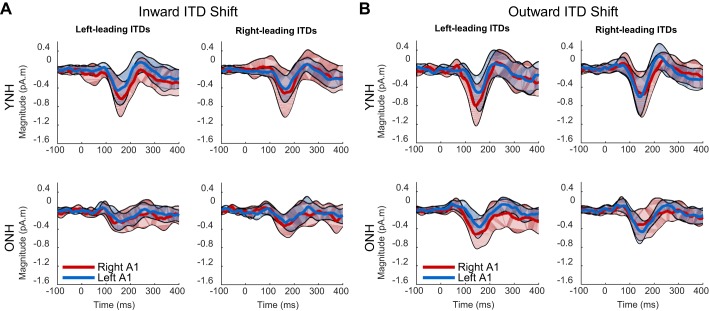
Distributions of raw EEG activity across the scalp can represent some of the hemispheric distribution of activity to ITD changes; however, interpretation of these data is limited by the fact that the response at a given electrode is influenced by volume conduction from multiple cortical sources. Using sLORETA analyses, the source of measured activity was estimated in an effort to quantify differential effects in the left and right primary auditory cortex (A1). In Fig. 6, source waveforms from the younger group (top row) and older group (bottom row) are displayed for both the inward (Fig. 6A) and outward (Fig. 6B) ITD shift conditions. Data are organized by spatial hemifield (columns) and cortical hemisphere (line color). As was the case with the GFP measures, source-localized waveforms appear to reveal larger responses to ITD shifts for younger listeners than older listeners (compare top and bottom rows). In addition, responses to outward-shifting ITDs tended to be larger than inward-shifting ITDs (compare right vs. left broad columns, respectively). Finally, there was no apparent magnitude difference between spatial hemifield of the stimuli, although it is possible that there was an interaction between the spatial hemifield of the stimulus and the cortical hemisphere that was more active (for example, compare red lines in left column with blue lines in right column of Fig. 6, A or B). To assess the strength of these observations, the magnitudes of each component in the P1-N1-P2 complex were extracted from the source waveforms in the same manner described above for the GFP waveforms and submitted to a separate four-way ANOVA with factors of age group (young vs. older), direction of shift (outward vs. inward), spatial hemifield (left- vs. right-leading ITDs), cortical area (left A1 vs. right A1), and the average pure-tone threshold at 500 Hz as a covariate. For the P1 component, there was a main effect of direction [F(1,16) = 8.3, P = 0.011], confirming a stronger response to outward shifts in ITD, although some caution should be taken due to a significant three-way interaction between direction, hemifield, and cortex [F(1,16) = 5.1, P = 0.038]. This interaction was explored further below. For the N1 component, there was a main effect of direction [F(1,16) = 9.6, P = 0.007]. No other effect or interaction reached significance for the N1 component. Finally, in the P2 component, no main effects or interactions were revealed.
Fig. 6.
The average source-localized waveforms for left-leading and right-leading adapter-probe pairs either in the inward (A) or outward (B) direction. The YNH (top) group showed generally larger responses relative to the ONH (bottom) group. In addition, the YNH group tended to have greater responses in the right auditory cortex (right A1; red lines) relative to the left auditory cortex (left A1; blue lines). Colored shaded regions show confidence intervals (1.96 × SD) for the mean waveforms.
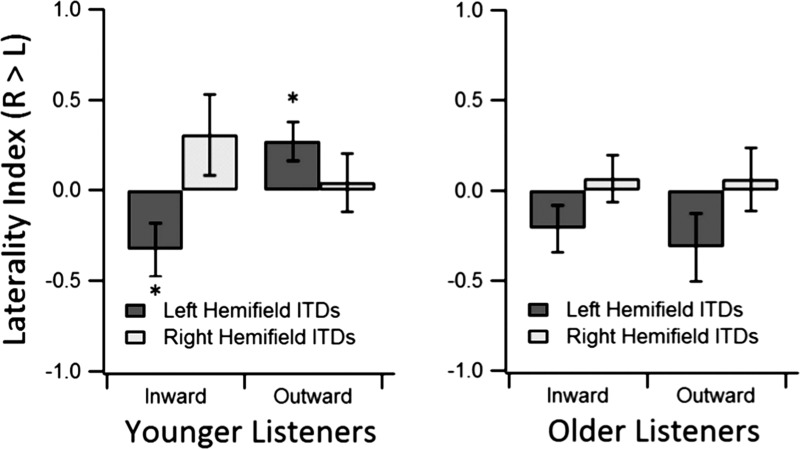
To explore further the three-way interaction between direction of ITD shift, spatial hemifield, and cortical area activity for the P1 component, a laterality index was calculated (Fig. 7). The laterality index was computed as the magnitude in the right minus the left cortex divided by the sum of the magnitudes in both cortices. Thus a positive value in the laterality index indicates asymmetric activity in favor of the right auditory cortex and a negative value indicates asymmetric activity in favor of the left auditory cortex. A null value indicates symmetric activity across the 2 cortices. Each measure of laterality was submitted to means testing (2-tailed t-test) to determine significant differences from 0 (i.e., asymmetry). In younger listeners, 2 of the 4 measures showed significant asymmetric activity; the response to an inward-shifting ITD in the left hemifield favored the left auditory cortex [t(9) = −2.23; P = 0.05], and the response to an outward-shifting ITD in the left hemifield favored the right auditory cortex [t(9) = 2.53; P = 0.03]. In older listeners, none of the measures indicated significant asymmetric activity.
Fig. 7.
The laterality index is shown for the P1 component. Data are separated by shift direction, age group, and hemifield of the stimulus percept. Asterisks indicate a significant difference from 0 (i.e., significant asymmetry; P < 0.05). Laterality index is computed by taking the difference in absolute magnitudes of the responses in the right (R) and left (L) auditory cortices and dividing by the sum. Error bars represent standard error of the mean.
DISCUSSION
The purpose of the present study was to explore the cortical representation of ITDs in younger and older listeners with clinically normal hearing within several octaves of the stimulus region of interest. Recent electrophysiological (Magezi and Krumbholz 2010; Salminen et al. 2010) data are consistent with a hemifield coding model for young, normal-hearing listeners, and although there have been a number of behavioral studies establishing an age-related decline in spatial hearing (for a review, see Dobreva et al. 2011), to date, only one other EEG study has addressed age-related effects on cortical processing of auditory spatial cues (Briley and Summerfield 2014). Changes to ITD processing in cortex may reflect disruptions to sensory-specific processing, such as reduced neural inhibition (Caspary et al. 2008), or they may reflect a more general reduction in temporal precision or neural synchrony (e.g., Marmel et al. 2013). In the context of the hemifield code, such physiological mechanisms lead to distinctly different predictions of age-related changes to ITD sensitivity: declines in inhibitory neurotransmission would lead to increased excitation and medial shifts to rate-azimuth functions (e.g., Fig. 1, middle column; Alain and Woods 1999; Chao and Knight 1997), whereas a general reduction in temporal precision would lead to reduced excitation and shallower slopes in the rate-azimuth function (e.g., Fig. 1, right column; Bertoli et al. 2002). Older listeners in the present study tended to have overall smaller N1 responses relative to younger listeners for ITD shifts but not to the initial onsets of the adapter, indicating an age effect specific to the change in ITD. Furthermore, the N1- and P2-response latencies in the older listening group were significantly longer than for younger listeners, which is consistent with previously observed auditory ERPs from older adults (Goodin et al. 1978; Picton et al. 1984; Ross et al. 2007).
Comparisons of ERP magnitudes.
The results for both listener groups showed a robust response (see Fig. 3) to the probe ITD. The resulting waveform was characterized by a positive deflection around 70 ms (P1) followed by a negative deflection between 140 and 170 ms (N1) and another positive deflection between 230 and 260 ms (P2) relative to the onset of the probe. Earlier studies have described this ITD change-response as the motion-onset response (Krumbholz et al. 2007), which has proven to be a robust response to spatial changes in general (Getzmann and Lewald 2012). Consistent with the results of Magezi and Krumbholz (2010), when ITD shifted inward, the P1-N1-P2 complex was smaller than when ITD shifted outward. Statistical analyses of the source-localized waveforms indicated that the P1 and N1 components drove the difference between inward and outward conditions, consistent with a modality-specific source (i.e., generated within auditory cortices; Liegeois-Chauvel et al. 1994; Näätänen and Picton 1987). The observed differences in the responses between outward or inward ITD changes add to the growing body of literature showing that ITDs are processed at the cortical level by a population rate code widely tuned to the left and right hemifields (for a review, see Salminen et al. 2012).
As described earlier (see Fig. 1), smaller observed magnitudes in older than younger listeners would suggest poorer neural synchrony in the older group. This would be consistent with simulations of reduced neural synchrony using temporal jitter, both behaviorally (Pichora-Fuller et al. 2007) and from the auditory brain-stem response (Mamo et al. 2016). The present data appear to show smaller magnitudes in the older than younger listeners (Fig. 5), although statistical significance was only present for some adapter-probe pairs. In fact, the prominent differences in the N1 component only appear for inward conditions. Large individual differences may have prevented a clearer result. It is also important to note that any observed reduction in amplitude for older listeners was specific to the probe onset and associated processing of the ITD shift, as there was no clear difference between groups in response to the onset of the adapter or offset of the full stimulus.
Unlike previous studies, there was a lack of an age effect in the P1 component, which is often significantly larger in older listeners and attributed to downregulation of inhibition (Alain et al. 2004; Ross et al. 2007). This may strengthen the conclusion that reduced inhibition was not responsible for the present results; however, it is also necessary to distinguish between the role inhibition has in cortical processing and the role it may still have played at the earliest binaural structures. To encode temporal information at the submillisecond level, it is believed that bilateral and temporally precise inhibitory inputs to medial superior olive (MSO) neurons are needed (Brand et al. 2002; Pecka et al. 2008). Although the present data suggest that the age effect is due to poor neural synchrony in the older listeners, it does not rule out the distinct possibility that downregulation of these inhibitory MSO inputs would cause poor neural synchrony. This potential confound may be inescapable, although recent work from Joris and colleagues has disputed the functional role of these inhibitory inputs for spatial coding (Franken et al. 2015; Joris and Yin 2007).
Together, these data provide support to a theory of reduced neural synchrony in older adults as others have found. In the free field with additional spatial cues, loudspeaker location shifts also evoke smaller responses in older listeners than younger listeners (Briley and Summerfield 2014), and in our own laboratory, a recent study on the binaural masking level difference has also shown a robust age-related decline in coding of interaural phase of stimulus fine structure (Eddins and Eddins 2013). To our knowledge, however, the present study is the only one that directly assesses age-related effects for changes in ITD-only processing at the cortical level.
Comparisons of ERP latencies.
Strong differences in peak ERP-component latencies were observed between the younger and older groups. Both N1 and P2 latencies were significantly longer in older adults. Previous studies of temporal processing have also reported prolonged latencies in the cortical evoked responses of older listeners relative to younger listeners and have attributed them to a general slowing of binaural processing in older adults (Goodin et al. 1978; Picton et al. 1984; Ross et al. 2007). Delayed ERPs also have been shown in older adults for early-latency responses like the auditory brain-stem response (Anderson et al. 2012) as well as for the middle-latency response (Kelly-Ballweber and Dobie 1984). Thus it is possible that delayed cortical responses are at least partially due to delays at lower levels. Potential causes of delayed responses include poorer brain connectivity (Forstmann et al. 2011), slower neural recovery (Walton et al. 1998), altered neural network pathways, reduced white matter myelination (Lu et al. 2011), and breakdowns in neural synchronization (Pichora-Fuller et al. 2007).
Comparisons across the conditions included in the current study also showed a main effect of direction (inward vs. outward) for latency in both the N1 and P2 components. In both cases, outward conditions evoked earlier responses relative to inward conditions. The significant interaction for the P2 component between age and direction, however, indicates that this difference between inward and outward conditions is somewhat subdued for older listeners relative to younger listeners. Shorter latencies for outward conditions were in contrast to the results of Magezi and Krumbholz (2010) who found shorter latencies for the outward condition only in the P1 component. One possible explanation for this is the potential interference of the offset in their stimulus. Whereas our stimuli had a duration of 400 ms after the probe onset, theirs was only 250 ms, which roughly coincided with the P2-component latency. Furthermore, their analysis was focused on the vertex electrode, which may not have fully characterized the broader scalp response that the GFP aims to do. Nevertheless, both studies show a more rapid response when the probe ITD is in the outward direction relative to the adapter ITD, which may reflect facilitation of shifts within a presently dominant hemifield channel. Recall from Fig. 1 that the response to a shift is due to the activity in the channel that is rising in activity at the onset of the probe. For an inward shift as in the example in Fig. 1, the dominant channel during the adapter is the left-hemifield channel; however, the response to the probe is due to the activity in the right-hemifield channel, perhaps at a timing cost due to switching between relevant channels. In the case of the outward shift example, there is no dominant channel during the adapter, so there is no potential cost of switching channels. Although further measures would need to confirm this, such switching may also explain the more robust magnitude differences seen between groups in the inward conditions.
Hemispheric distribution of activity.
Results from several electrophysiological studies exploring the hemifield model for ITD coding indicated distinctly different patterns of hemisphere-specific neural activity in young, normal-hearing listeners. Briley et al. (2013) showed greater N1-P2 cortical responses in the left hemisphere for contralateral stimuli, whereas responses in the right hemisphere were equivalent for stimuli from both spatial hemifields. In response to ITD shifts, Magezi and Krumbholz (2010) showed that N1 was greater in the right hemisphere for outward shifts, whereas P2 was greater in the left hemisphere for inward shifts. Getzmann (2011) also observed distinct hemispheric differences marked by N1 and P2 components: the N1 response was greatest in the contralateral hemisphere to the hemifield of the motion onset, whereas the P2 response was greatest for inward motion independent of the hemifield. In comparison, the present results for younger listeners also indicate that there are differential effects for inward- and outward-shifting ITDs, although these were observed only in the P1 component. Lateralization for binaural stimuli has also been shown with fMRI (von Kriegstein et al. 2008). Such auditory-specific hemispheric asymmetry parallels the hemispheric asymmetry observed in younger subjects in the context of cognition (Reuter-Lorenz et al. 2000) and visual perception (Reuter-Lorenz et al. 1999). Furthermore, signs of age-related reorganization and a reduction in hemispheric asymmetry led Cabeza (2002) to propose the hemispheric asymmetry reduction in older adults (HAROLD) model of cognitive aging. We speculated that such reorganization may generalize to acoustic processing, leading us to hypothesize that any asymmetries observed in younger listeners would be weaker or absent in older listeners. Indeed, the present study indicates asymmetric hemispheric activity in the P1 component of the auditory ERP for younger listeners (Fig. 7). Based on a laterality index in young listeners, we show that both inward and outward left-leading ITD changes are lateralized to the left and right hemispheres, respectively. In contrast, the mean laterality indices for older adults indicates a more balanced distribution of ITD processing. To our knowledge, age-related cortical reorganization has not been reported previously in the context of binaural processing. Much like explanations for reduced asymmetry in cognitive tasks (for a review, see Cabeza 2002), older listeners may undergo reorganization in binaural coding for one or both of the following reasons: it may be that the brain must compensate for a reduction in resources by recruiting different brain areas, or through greater experience, connectivity may become less compartmentalized with age and more efficient through broad distribution of processing.
Conclusion.
Based on the observed age effects and a simple formulation of the opponent-channel model supporting the hemifield code, the current results suggest that ITD shifts are detected but not as effectively relayed from the periphery in older listeners compared with younger listeners, consistent with a theory of age-related reduction in neural synchrony. Neural signatures of ITD processing in older adults included generally smaller and delayed responses for ITD shifts as well as broader and more symmetric cortical distribution of activity compared with younger listeners. Reduced hemispheric asymmetry with increasing age, demonstrated here in the context of spatial hearing, mirrors data previously observed for higher-level tasks designed to index cognitive rather than sensory function, suggesting a more general adaptive aging process related to hemispheric distribution of activity. The consequences of poorer neural synchrony are not limited to poorer spatial hearing but also degraded speech perception and auditory grouping, which are known to manifest with age. Greater understanding of the restrictions to neural processing in older listeners is key to providing effective treatments. Although the presence of a hemifield code for binaural stimuli had previously been shown in the auditory cortex for younger listeners, the effects of aging on the hemifield code had only been previously observed in the free field. The present study provides confirmation of effects seen in younger listeners, and it extends this work to include older, normal-hearing listeners. Further work is needed to test whether ITDs are coded similarly to IIDs in older listeners and how other spatial cues would interact.
GRANTS
This work was supported by National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders (NIDCD) Award F32-DC-013724 and NIH National Institute on Aging (NIA) Award P01-AG-009524.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.J.O., D.A.E., and A.C.E. conception and design of research; E.J.O. performed experiments; E.J.O. analyzed data; E.J.O., D.A.E., and A.C.E. interpreted results of experiments; E.J.O. prepared figures; E.J.O. drafted manuscript; E.J.O., D.A.E., and A.C.E. edited and revised manuscript; E.J.O., D.A.E., and A.C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the comments and suggestions by four anonymous reviewers.
REFERENCES
- Alain C, McDonald KL, Ostroff JM, Schneider B. Aging: a switch from automatic to controlled processing of sounds? Psychol Aging 19: 125–133, 2004. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychol Aging 14: 507–519, 1999. [DOI] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci 32: 14156–14164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babkoff H, Muchnik C, Ben-David N, Furst M, Even-Zohar S, Hildesheimer M. Mapping lateralization of click trains in younger and older populations. Hear Res 165: 117–127, 2002. [DOI] [PubMed] [Google Scholar]
- Bertoli S, Smurzynski J, Probst R. Temporal resolution in young and elderly subjects as measured by mismatch negativity and a psychoacoustic gap detection task. Clin Neurophysiol 113: 396–406, 2002. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature 417: 543–547, 2002. [DOI] [PubMed] [Google Scholar]
- Briley PM, Goman AM, Summerfield AQ. Physiological evidence for a midline spatial channel in human auditory cortex. J Assoc Res Otolaryngol 17: 331–340, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley PM, Kitterick PT, Summerfield AQ. Evidence for opponent process analysis of sound source location in humans. J Assoc Res Otolaryngol 14: 83–101, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley PM, Summerfield AQ. Age-related deterioration of the representation of space in human auditory cortex. Neurobiol Aging 35: 633–644, 2014. [DOI] [PubMed] [Google Scholar]
- Byrne D, Dillon H, Tran K, Arlinger S, Wilbraham K, Cox R, Hagerman B, Hetu R, Kei J, Lui C, Kiessling J, Kotby MN, Nasser NH, Elkholy WA, Nakanishi Y, Oyer H, Powell R, Stephens D, Meredith R, Sirimanna T, Tavartkiladze G, Frolenkov GI, Westerman S, Ludvigsen C. An international comparison of long-term average speech spectra. J Acoust Soc Am 96: 2108–2120, 1994. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17: 85–100, 2002. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211: 1781–1791, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex 7: 63–69, 1997. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol 148: 879–886, 1998. [DOI] [PubMed] [Google Scholar]
- Dobreva MS, O'Neill WE, Paige GD. Influence of aging on human sound localization. J Neurophysiol 105: 2471–2486, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins AC, Eddins DA. Age-related changes in neural processing of binaural temporal cues in normal and hearing-impaired adults. In: Aging and Speech Communication: 5th Interdisciplinary International Research Conference. Bloomington, IN: 2013. [Google Scholar]
- Eddins DA, Hall JW 3rd.. Binaural processing and auditory asymmetries. In: Springer Handbook of Auditory Research, edited by Gordon-Salant S, Frisina RD, Popper AN, and Fay RR. New York: Springer, 2010, chapt. 6, p. 135–165. [Google Scholar]
- Edmonds BA, Krumbholz K. Are interaural time and level differences represented by independent or integrated codes in the human auditory cortex? J Assoc Res Otolaryngol 15: 103–114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Tittgemeyer M, Wagenmakers EJ, Derrfuss J, Imperati D, Brown S. The speed-accuracy tradeoff in the elderly brain: a structural model-based approach. J Neurosci 31: 17242–17249, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken TP, Roberts MT, Wei LT, Golding NL, Joris PX. In vivo coincidence detection in mammalian sound localization generates phase delays. Nat Neurosci 18: 444–452, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freigang C, Schmiedchen K, Nitsche I, Rubsamen R. Free-field study on auditory localization and discrimination performance in older adults. Exp Brain Res 232: 1157–1172, 2014. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res 158: 1–27, 2001. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cooper JC Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear 11: 247–256, 1990. [PubMed] [Google Scholar]
- Getzmann S. Auditory motion perception: onset position and motion direction are encoded in discrete processing stages. Eur J Neurosci 33: 1339–1350, 2011. [DOI] [PubMed] [Google Scholar]
- Getzmann S, Lewald J. Cortical processing of change in sound location: smooth motion versus discontinuous displacement. Brain Res 1466: 119–127, 2012. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Squires KC, Starr A. Long latency event-related components of the auditory evoked-potential in dementia. Brain 101: 635–648, 1978. [DOI] [PubMed] [Google Scholar]
- Gramfort A, Papadopoulo T, Olivi E, Clerc M. OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed Eng Online 9: 45, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear 31: 755–760, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 90: 983–1012, 2010. [DOI] [PubMed] [Google Scholar]
- Herman GE, Warren LR, Wagener JW. Auditory lateralization: age differences in sensitivity to dichotic time and amplitude cues. J Gerontol 32: 187–191, 1977. [Google Scholar]
- Jeffress LA. A place theory of sound localization. J Comp Physiol Psychol 41: 35–39, 1948. [DOI] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci 30: 70–78, 2007. [DOI] [PubMed] [Google Scholar]
- Kelly-Ballweber D, Dobie RA. Binaural interaction measured behaviorally and electrophysiologically in young and old adults. Audiology 23: 181–194, 1984. [DOI] [PubMed] [Google Scholar]
- Konishi M. Coding of auditory space. Annu Rev Neurosci 26: 31–55, 2003. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Hewson-Stoate N, Schonwiesner M. Cortical response to auditory motion suggests an asymmetry in the reliance on inter-hemispheric connections between the left and right auditory cortices. J Neurophysiol 97: 1649–1655, 2007. [DOI] [PubMed] [Google Scholar]
- Kybic J, Clerc M, Faugeras O, Keriven R, Papadopoulo T. Fast multipole acceleration of the MEG/EEG boundary element method. Phys Med Biol 50: 4695–4710, 2005. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol 92: 204–214, 1994. [DOI] [PubMed] [Google Scholar]
- Lister JJ, Roberts RA. Effects of age and hearing loss on gap detection and the precedence effect: narrow-band stimuli. J Speech Lang Hear Res 48: 482–493, 2005. [DOI] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, Bartzokis G. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol 33: 1059–1068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Introduction to the Event-Related Potential Technique (2nd ed). Cambridge, MA: MIT Press, 2014, p. 1–406. [Google Scholar]
- Magezi DA, Krumbholz K. Evidence for opponent-channel coding of interaural time differences in human auditory cortex. J Neurophysiol 104: 1997–2007, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo SK, Grose JH, Buss E. Speech-evoked ABR: effects of age and simulated neural temporal jitter. Hear Res 333: 201–209, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Linley D, Carlyon RP, Gockel HE, Hopkins K, Plack CJ. Subcortical neural synchrony and absolute thresholds predict frequency discrimination independently. J Assoc Res Otolaryngol 14: 757–766, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. J Acoust Soc Am 30: 237–246, 1958. [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology 24: 375–425, 1987. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005. [DOI] [PubMed] [Google Scholar]
- Ozmeral EJ, Eddins AC, Frisina DR, Eddins DA. Large cross-sectional study of presbycusis reveals rapid progressive decline in auditory temporal acuity. Neurobiol Aging 43: 72–78, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24: 5–12, 2002. [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci 28: 6914–6925, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, MacDonald E, Pass HE, Brown S. Temporal jitter disrupts speech intelligibility: a simulation of auditory aging. Hear Res 223: 114–121, 2007. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Champagne SC, Nelson RF. The effects of age on human event-related potentials. Psychophysiology 21: 312–325, 1984. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 12: 174–187, 2000. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller AC. Neural recruitment and cognitive aging: two hemispheres are better than one, especially as you age. Psychol Sci 10: 494–500, 1999. [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci 27: 11172–11178, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen NH, Tiitinen H, May PJ. Auditory spatial processing in the human cortex. Neuroscientist 18: 602–612, 2012. [DOI] [PubMed] [Google Scholar]
- Salminen NH, Tiitinen H, Miettinen I, Alku P, May PJ. Asymmetrical representation of auditory space in human cortex. Brain Res 1306: 93–99, 2010. [DOI] [PubMed] [Google Scholar]
- Skrandies W. Global field power and topographic similarity. Brain Topogr 3: 137–141, 1990. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. J Acoust Soc Am 101: 2214–2220, 1997. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am 104: 2385–2399, 1998. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kriegstein K, Griffiths TD, Thompson SK, McAlpine D. Responses to interaural time delay in human cortex. J Neurophysiol 100: 2712–2718, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O'Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci 18: 2764–2776, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. J Acoust Soc Am 91: 1648–1661, 1992. [DOI] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990. [DOI] [PubMed] [Google Scholar]