Abstract
Evidence accumulating over the past 15 years soundly refutes the dogma that the Drosophila nervous system is hardwired. The preponderance of studies reveals activity-dependent neural circuit refinement driving optimization of behavioral outputs. We describe developmental, sensory input-dependent plasticity in the brain olfactory antennal lobe, which we term long-term central adaption (LTCA). LTCA is evoked by prolonged exposure to an odorant during the first week of posteclosion life, resulting in a persistently decreased response to aversive odors and an enhanced response to attractive odors. This limited window of early-use, experience-dependent plasticity represents a critical period of olfactory circuit refinement tuned by initial sensory input. Consequent behavioral adaptations have been associated with changes in the output of olfactory projection neurons to higher brain centers. Recent studies have indicated a central role for local interneuron signaling in LTCA presentation. Genetic and molecular analyses have implicated the mRNA-binding fragile X mental retardation protein and ataxin-2 regulators, Notch trans-synaptic signaling, and cAMP signal transduction as core regulatory steps driving LTCA. In this article, we discuss the structural, functional, and behavioral changes associated with LTCA and review our current understanding of the molecular pathways underlying these developmental, experience-dependent changes in the olfactory circuitry.
Keywords: critical period, fragile X mental retardation protein, ataxin, notch, cAMP
developmental refinement of brain circuitry is critical for tuning sensory perception to environmental stimuli. The prototypical system used to probe this developmental plasticity has been ocular dominance in the mammalian primary visual cortex (Espinosa and Stryker 2012). Hubel and Wiesel pioneered this field using monkeys and cats, with a focus on the anatomical and functional effects of monocular deprivation (Hubel and Wiesel 1970; LeVay et al. 1980; Wiesel et al. 1974). Since these classic experiments, much has been learned about the circuit changes that occur in response to sensory deprivation and other forms of biased developmental environments (Erzurumlu and Gaspar, 2012; Espinosa and Stryker 2012; Hensch 2005; Hübener and Bonhoeffer 2014). Although the use of rodent models has allowed for some limited genetic dissection, the molecular pathways that restrict developmental plasticity to the critical period of early-use development are still largely unknown. Recent work has established the powerhouse Drosophila genetic system as an exciting new model to study experience-dependent neural circuit refinement during early-use critical periods (Devaud et al. 2003a; Doll and Broadie 2014, 2015, 2016; Sachse et al. 2007).
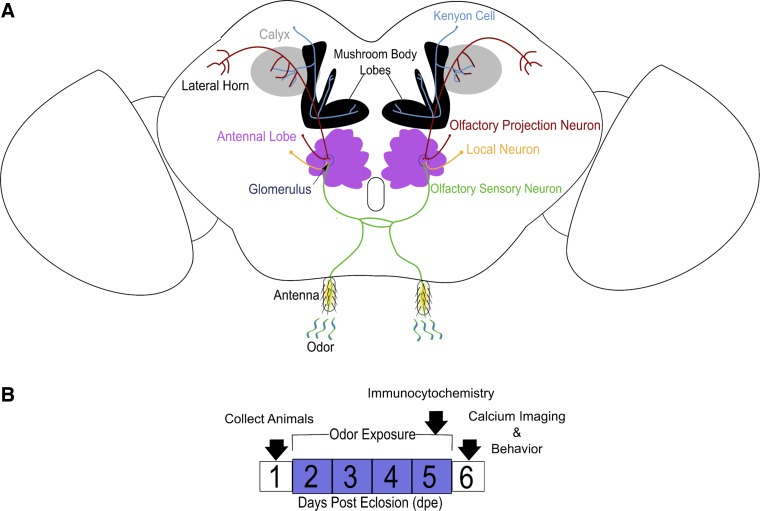
The Drosophila olfactory system has emerged as a particularly useful model for studying developmental plasticity, with a tremendous array of genetic and functional tools available to dissect connectivity and assess underlying molecular pathways (Hales et al. 2015; Wilson 2013). The olfactory circuitry is extremely well characterized, with information processing in four neuropils: antenna, antennal lobe (AL), mushroom body (MB), and lateral horn (LH; Fig. 1). First, odors are transduced into electrochemical signals within the antenna by an array of ∼50 olfactory receptors (ORs) expressed by olfactory sensory neurons (OSNs; Fig. 1A; Couto et al. 2005). OSN dendrites are housed in pairs in stalklike projections (sensilla) on the antenna and maxillary palps (Couto et al. 2005; Wilson 2013). Next, information from numerous OSNs expressing a single OR class converges on AL synaptic subcompartments called glomeruli (Fig. 1A; Couto et al. 2005; Fishilevich and Vosshall 2005; Vosshall et al. 2000; Vosshall and Stocker 2007; Wilson 2013). The pooling of OSN activation serves to average input within each AL glomerulus, thus facilitating more accurate odor discrimination by higher order brain centers.
Fig. 1.
Diagram of the Drosophila olfactory circuitry and the odor exposure paradigm used to drive critical period plasticity in the antennal lobe. A: the olfactory system. Odors are transduced by the dendrites of olfactory sensory neurons on the antenna. The axons of these sensory neurons project bilaterally into the same antennal lobe glomerulus of each brain hemisphere. Within a glomerulus, olfactory sensory neurons synapse onto local interneurons and olfactory projection neurons. The axons of these projections neurons synapse in two major central brain neuropils: the mushroom body calyx and the lateral horn. B: the odor exposure procedure used in experiments throughout this article (unless noted otherwise). Newly eclosed Drosophila are collected over the course of 1 day. Next, juvenile animals are transferred into vials with a specific odor for 4 days. At the end of the 5th day, flies are processed for immunocytochemistry. To avoid short-term plasticity due to odor exposure, animals are kept in odor-free conditions for 1 day before behavioral or functional analyses are carried out.
Each OSN axon synapses onto both olfactory projection neurons (PNs) and local interneurons (LNs) in a single glomerulus (Fig. 1A; Couto et al. 2005; Das et al. 2013; Fishilevich and Vosshall 2005; Vosshall et al. 2000; Vosshall and Stocker 2007; Wilson 2013). PNs send axons to higher olfactory centers via the inner, middle, or outer antennocerebral tracts (iACT, mACT, and oACT, respectively). The mACT and oACT project directly to the LH, forming connections with output neurons (LHONs) and LNs (Liang et al. 2013; Strutz et al. 2014), whereas the iACT axons synapse first in the MB calyx and then in the LH (Fig. 1A). In the MB calyx, incoming information from PNs is sparsely coded by synapsing on a relatively larger number of intrinsic MB neurons called Kenyon cells (KCs). The MB acts as an associative learning center by pooling sensory information from the KCs with a valence signal from dopaminergic neurons (DANs) onto MB output neurons (MBONs; Barnstedt et al. 2016; Hige et al. 2015a, 2015b). Although the exact output circuitry has not been fully described, both the LHONs and MBONs likely converge on downstream neurons to activate appropriate behavioral motor programs (Barnstedt et al. 2016; Hige et al. 2015a, 2015b; Parnas et al. 2013).
The well-mapped connectivity of the Drosophila olfactory circuitry provides an excellent system to investigate developmental plasticity. Immature animals exposed to odorants during the initial phase of olfactory experience exhibit developmental changes in this circuitry and resultant persistent alterations in odorant response behaviors (Fig. 1B; Devaud et al. 2001). This olfactory experience-dependent modulation is restricted to a short critical period of less than 1 wk immediately following eclosion (Fig. 1B). In this article, we focus on developmental plasticity in the AL, which we term long-term central adaption (LTCA). A growing body of LTCA literature indicates that circuit refinement in the AL is driven by activity-dependent modulations during the early-use critical period (Das et al. 2011; Devaud et al. 2001; Kidd et al. 2015). In this article, we discuss three LTCA topics. First, we start by discussing general aspects of LTCA defining the discovery of behavioral plasticity within the critical period. Second, we explore subsequent identification of the olfactory circuit rewiring that occurs during LTCA, and the molecular pathways underlying activity-dependent synaptic refinement. Finally, we consider important unanswered questions to highlight key future directions in LTCA research.
Behavioral Plasticity in LTCA
The phenomenon of LTCA was discovered in the laboratory of Alberto Ferrús at the Cajal Institute in Spain and described in a seminal paper showing that odor exposure during juvenile development leads to changes in both AL glomeruli architecture and odor discrimination behavior (Devaud et al. 2001). The odor exposure protocol used in this classic work has become the standard for the field (Fig. 1B). Drosophila collected during the first day posteclosion (0–1 dpe) are moved to new food vials with a specific odorant from 2 to 5 dpe (4-day odorant exposure). At 5 dpe, the olfactory circuitry is interrogated with immunocytochemical labeling (Fig. 1B). To avoid short-term plasticity from odorant exposure, animals are maintained odorant free overnight and then the following day (6 dpe) are tested in functional and behavioral assays. Devaud et al. (2001) found that preexposure to specific odorants leads to a significant reduction in odor avoidance during testing. The same odor exposure leads to a significant reduction in the volume of peripheral AL glomeruli, measured using a synaptic marker (green fluorescent protein-tagged n-synaptobrevin). Although this study measured several glomeruli, the ones chosen were selected for convenience and not based on matching to exposed odorants (Devaud et al. 2001). Finally, this study provided key evidence ruling out peripheral adaption as a mechanism, using electroantennogram recordings to measure local field potentials. This ground-breaking work revealed LTCA for the first time, but subsequent studies have been imperative in defining the temporal and spatial specificity of LTCA.
In two follow-up articles, Devaud and colleagues showed that LTCA is developmentally restricted to a short period immediately following eclosion and affects odorant responses in a stimulus-specific manner (Devaud et al. 2003a, 2003b). These studies used two time points, comparing early odorant exposure (2–5 dpe) in juveniles with late exposure (8–11 dpe) in mature adults (Devaud et al. 2003a). Immature flies exposed at the earlier time point showed strong LTCA, whereas mature animals did not show any significant changes after odor exposure. Furthermore, the LTCA that was induced during 2–5 dpe did not show any reversal by 12 dpe, indicating a persistent modification (Devaud et al. 2003a). To test the odor specificity of LTCA, the authors drove expression of tetanus toxin [which blocks synaptic vesicle (SV) fusion by acting as an n-synaptobrevin protease (Sweeney et al. 1995)] into two restricted sets of two to three AL glomeruli and then assayed for changes in olfactory behavior (Devaud et al. 2003b). Although the two sets of glomeruli both affected odor perception when synaptically silenced, each had a characteristic pattern that differed between the odors tested (Devaud et al. 2003b). These studies established the temporal and odor specificity of LTCA and laid the foundations for subsequent studies investigating the functional and molecular underpinnings of LTCA.
Circuit Plasticity in LTCA
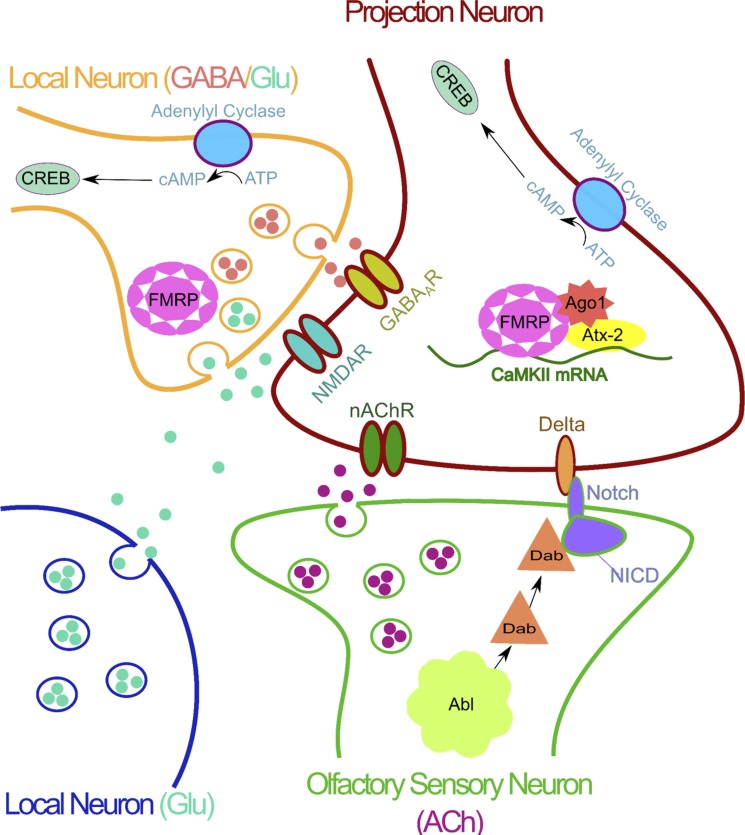
Taking advantage of new tools and a growing knowledge of the Drosophila olfactory circuit, Sachse et al. (2007) described a critical period for activity-dependent plasticity in the AL. GAL4 driver lines that specifically labeled either OSN (axon) or PN (dendrites) in the CO2-responsive V glomerulus were used to assess volume changes caused by odor exposure. High-level CO2 increased V glomerulus volume while not affecting surrounding glomeruli (Sachse et al. 2007). A glomerulus volume increase was evident with both axonal and dendritic markers, extending earlier findings (Devaud et al. 2001, 2003a, 2003b). Interestingly, when the FLP-out technique (Ng et al. 2002) was used to analyze individually labeled OSNs, no significant differences in morphology were found between ambient and high levels of CO2 exposure. This finding suggests that changes in glomerular volume reflect a broadening of the space that an OSN axon can occupy, but not a growth in axon size. Sachse et al. (2007) reported that CO2 exposure must persist for at least 2 days to cause LTCA and is reversible if high CO2 is removed at least 2 days before the end of the first week posteclosion. This finding conflicts with earlier studies showing LTCA is irreversible (Devaud et al. 2003a) but has since been further supported for CO2 and geranyl acetate exposure (Kidd et al. 2015; Kidd and Lieber 2016). The ability to specifically label different classes of neurons in the AL circuit helped define presynaptic and postsynaptic contributions to glomerular changes (Fig. 2) and also paved the way to detect functional changes underlying LTCA.
Fig. 2.
The antennal lobe circuitry underlying long-term central adaption (LTCA) presentation. Olfactory sensory neuron (OSN): the OSN provides the odor signal to the antennal lobe via synaptic release of acetylcholine (ACh). LTCA volume changes are mediated by noncanonical Notch signaling with the Abl target Disabled (Dab) acting on the Notch receptor. Volume changes are controlled by a retrograde Delta signal activating a canonical Notch signaling pathway. Projection neuron (PN): the PN is the output of the antennal lobe. Changes in PN function require 3 different receptor classes: nicotinic acetylcholine receptors (nAChRs), N-methyl-d-aspartate receptors (NMDARs), and ionotropic γ-aminobutyric acid receptors (GABAARs). Functional presentation of LTCA requires Delta expression on the PN. RNA-binding proteins fragile X mental retardation protein (FMRP) and ataxin-2 (Atx-2) interact with the RNA-induced silencing complex (RISC) protein Argonaut1 (Ago1) to mediate translation of activity-dependent transcripts. One target transcript bound by both FMRP and Atx-2 encodes calcium/calmodulin-dependent kinase II (CaMKII). Local neurons (LNs): local neurons modify PN output via the release of GABA, glutamate (Glu), and other neuromodulators. A variety of LNs are important for LTCA presentation. The release of both GABA and Glu from LNs is required for LTCA. Two sites of Glu release are 1) synaptic corelease with GABA and 2) volume release from distinct glutamatergic neurons. FMRP and cAMP-response element binding protein (CREB) are both required in LNs for LTCA.
To begin to tease apart functional changes underlying LTCA, Sachse et al. (2007) used the genetically encoded calcium indicator GCaMP to pair the first functional investigations to AL glomerulus structural changes. The authors started by assaying single OSN clones using the FLP-out technique (Ng et al. 2002), showing that OSN termini within the AL showed no significant difference when compared at high (5%) and ambient (0.4%) CO2 exposure. From this observation, the authors concluded that the changes in the AL must be due to downstream circuit changes in the PNs and/or LNs (Fig. 2). To test this new hypothesis, the authors examined the PN output in the LH brain center (Fig. 1A). Although structural analysis proved unfeasible for these authors because of the dense synaptic projections, GCaMP calcium imaging revealed a significant reduction in PN synaptic functional output, consistent with a lower behavioral response to CO2 (Sachse et al. 2007). The decreased PN function was subsequently linked to an enhancement in the activity a specific group of LNs, as measured with a GCaMP reporter driven by the LN2-GAL4 driver. On the basis of this finding, Sachse et al. (2007) concluded that LTCA is the result of potentiation of odor-selective LN2 class inhibitory synapses onto specific olfactory PNs (Fig. 2). Subsequent studies in honeybees support the idea that critical period odor exposure can have long-term effects on the AL (Arenas and Farina 2008; Arenas et al. 2009). In this work, scented sugar sources were used to provide critical period learning, which created a strong memory and a long-lasting increase in AL function. In general, this proposed circuit model has stood up to further investigation (Acebes et al. 2011; Lieber et al. 2011), although some of the details and predictions of this mechanisms have been challenged in subsequent studies (Das et al. 2011; Sudhakaran et al. 2014).
There are two major complications with LTCA circuitry studies using specific odorants: 1) the complexity of LN innervation patterns (Chou et al. 2010) and 2) the dearth of specific tools for studying LNs (Wilson 2013). These limitations have led to three similar, but separable, hypotheses about LN roles in LTCA. The first hypothesis is that GABAergic LNs labeled by LN2-GAL4 are important for LTCA, at least for CO2 and perhaps for other odorants (Fig. 2; Sachse et al. 2007). The second hypothesis posits that LNs labeled instead by LN1-GAL4 are sufficient for LTCA, in response to both CO2 and ethyl butyrate (EB) (Das et al. 2011). Confoundingly, both LN1-GAL4 and LN2-GAL4 populations, which are groups of largely nonoverlapping GABAergic interneurons (Fig. 2), have received support from different laboratories as being central to LTCA (Das et al. 2011; Lieber et al. 2011; Sachse et al. 2007). The third hypothesis, from the laboratory of Alberto Ferrús, suggests that olfactory perception is modified by the balance of excitatory and inhibitory input of LNs within the olfactory circuit, with no direct differentiation between OSNs and PNs (Acebes et al. 2011). Because of the broad projections of LNs, as well as lateral excitation between PNs, it is difficult to rule out any of these competing theories. Likely the LTCA mechanism involves the interplay of several LN populations, together with PN processing and output (Fig. 2). Electrophysiology as well as more targeted functional imaging studies are needed to elucidate the exact nature of LN changes underpinning LTCA.
Unlike the uncertainty of LNs, targeted genetic manipulations in olfactory PNs support the idea that activation of these long-distance neurons facilitates many of the changes attributed to odorant exposure. Changes in olfactory PN output due to developmental odorant exposure have been generalized to a variety of odorants and activity paradigms. Unlike what was seen with CO2 in response to developmental activity during the critical period, PN dendrites decrease in volume, at least with direct optogenetic stimulation of a VL1 PN (Doll and Broadie 2015). Moreover, targeted critical period silencing of a VL1 PN, as well as broader silencing of many PNs for longer periods, leads to an increase in dendritic volume in the AL glomeruli and decreased axonal output synapses in the MB calyx (Doll and Broadie 2015; Kremer et al. 2010). Single-cell functional changes caused by 0–1 dpe optogenetic stimulation of a VL1 PN during the critical period include a strong increase in GCaMP calcium signaling (Doll and Broadie 2016). Longer periods of developmental exposure with fruit leads to decreased PN calcium signaling (Pech et al. 2015), similar to changes with CO2 exposure (Sachse et al. 2007). Therefore, direct stimulation/inhibition of PNs in the absence of OSN activation leads to opposite changes compared with odorant sensory stimulation. However, because these studies examine different glomeruli, the results may rather reflect glomerulus-specific effects. Direct side-by-side comparison of optogenetic and sensory stimulation during the LTCA critical period will be needed to fully elucidate activity-dependent changes. In disagreement with most of the studies presented thus far, some work has suggested a more important role for peripheral OSN changes (Chakraborty et al. 2009; Gupta and Stopfer 2011; Iyengar et al. 2010).
Siddiqi and colleagues have presented the idea of “imaginal conditioning,” which argues that peripheral changes in OSNs can account for the behavioral changes seen in response to developmental odor exposure (Chakraborty et al. 2009; Iyengar et al. 2010). This work takes advantage of an odor-free medium, which may serve as a better control condition compared with typical cornmeal food sources. Odor-dependent changes from single sensillum recordings have been reported to correlate with both an increased attraction at low odorant concentrations and decreased avoidance at higher odorant concentrations (Chakraborty et al. 2009; Iyengar et al. 2010). At present, it is not clear how peripheral effects that underlie imaginal conditioning relate to LTCA. It is quite possible that these two types of plasticity simply represent consequences of critical period odor exposure at different levels of the olfactory circuitry. Within all neuronal components of the olfactory circuit analyzed, the source and/or nature of developmental activity modulations seem to suggest different levels of activity-dependent changes, likely mediated by trans-synaptic signaling (Fig. 2). The molecular mechanisms behind the AL synaptic connectivity changes should help to explain the results.
Molecular Underpinnings of LTCA
The earliest experiments exploring the molecular basis for LTCA derived from classic MB-dependent learning and memory studies (Dudai et al. 1976; Keene and Waddell 2007; Livingstone et al. 1984; Waddell and Quinn 2001). Specifically, cAMP pathway learning/memory mutants dunce (phosphodiesterase) and rutabaga (adenylyl cyclase) were reported to lack LTCA (Fig. 2; Devaud et al. 2001, 2003a). These findings were further substantiated by use of a heat-shock-inducible form of cAMP-dependent response-element binding protein 2 (CREB2), an inhibitory form of CREB, which was shown to suppress LTCA when expressed in either PNs or LNs (Das et al. 2011). In addition to the requirement for cAMP signal transduction, several neurotransmitter receptors and transporters have been shown to be required for LTCA. Genetic knockdown experiments provide evidence for acetylcholine (ACh), γ-aminobutyric acid (GABA), and glutamate (Glu) signaling in the behavioral presentation of LTCA (Fig. 2; Das et al. 2011). However, recent studies have shown increases in AL glomerular volume occur in the absence of cholinergic PN activation (Kidd and Lieber 2016). ACh acts as the primary Drosophila brain excitatory neurotransmitter, acting both via nicotinic ACh receptors (nAChRs; Campusano et al. 2007; Gu and O'Dowd 2006; Lee and O'Dowd 1999; Su and O'Dowd 2003) and as a neuromodulator through less studied muscarinic AChRs (Collin et al. 2013; Silva et al. 2015). In contrast, GABA is considered the major Drosophila brain inhibitory neurotransmitter, acting via GABAA receptors (e.g., rdl) and GABAB receptors (Mezler et al. 2001).
The question of neurotransmitter LTCA requirements in the AL circuit has led to a targeted genetic dissection using the UAS/GAL4 system to drive small interfering RNA (siRNA) against different neurotransmitter system components in both PNs and LNs (Das et al. 2011). These studies demonstrated that GABAA receptors and glutamatergic N-methyl-d-aspartate receptors (NMDARs) in PNs, as well as vesicular Glu transporter (VGluT) and GABA synthesis enzyme glutamate decarboxylase (GAD) in LN1 neurons, are all required for LTCA (Fig. 2; Das et al. 2011). Interestingly, glutamate has been suggested to act as an AL inhibitory neurotransmitter, signaling via volume transmission to GluCl receptors and acting mainly on GABAergic LNs (Liu and Wilson 2013). However, the role of NMDARs in LTCA suggests that glutamate may also play additional roles within the AL (Fig. 2; Das et al. 2011). This work has led to a proposed model with five sequential steps: 1) Odorants bind to OSNs, leading to the release of the ACh onto both PNs and LNs. 2) The activation of LNs leads to the corelease of GABA and Glu onto PNs. 3) The simultaneous depolarization by ACh and binding of Glu to NMDARs causes an influx of calcium. 4) The influx of calcium stimulates the production of cAMP and the activation of CREB-dependent transcription. 5) The result of this transcription is to potentiate GABAergic synapses, increasing inhibition onto PNs (Fig. 2; Das et al. 2011). This model has appeal because it links functional and behavioral studies, but it still lacks a downstream mechanism that can regulate synaptic plasticity in response to neurotransmitter activity.
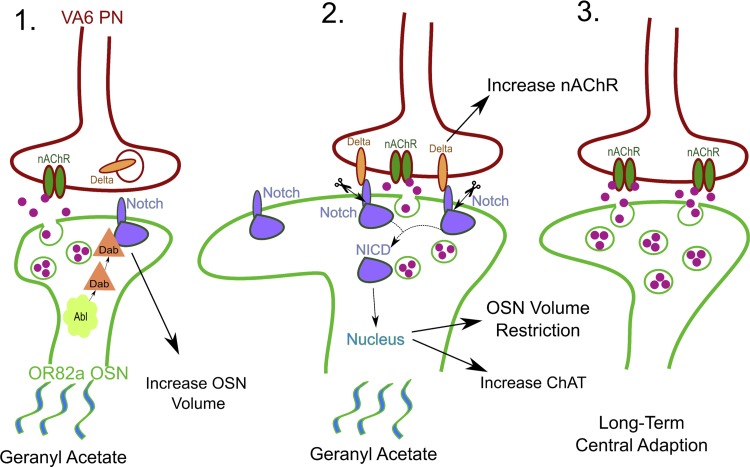
One important mechanism for LTCA generation and stability is Notch trans-synaptic signaling. Classically, Notch signaling has been shown to drive developmental cell fate and proliferation decisions; however, Notch has recently emerged as a player in experience-dependent plasticity (Zhang et al. 2013). Working through both canonical and noncanonical pathways, Notch plays a critical role in dictating AL glomerular volume and function (Fig. 3; Kidd et al. 2015; Kidd and Lieber 2016; Lieber et al. 2011). In the context of LTCA, Notch canonical signaling begins with membrane-bound Delta binding to the Notch receptor and the internalization of the Delta/Notch complex (Fig. 3). The Notch intercellular domain (NICD) is then proteolytically cleaved and translocates to the nucleus, where it causes changes in gene expression as part of a transcriptional regulator complex containing Suppressor of Hairless (Fig. 3; Guruharsha et al. 2012). The Notch noncanonical pathway is defined simply as a response requiring only Notch, but not the other canonical pathway components (Kidd and Lieber 2016; see description below). In a series of papers, Lieber and colleagues present a central role for Notch in both anatomical and functional changes associated with developmental odor exposure. In their model, odor activates noncanonical Notch signaling in OSNs, leading to increased glomerular volume, but interestingly no corresponding developmental change in PN function (Fig. 3; Kidd et al. 2015; Kidd and Lieber 2016; Lieber et al. 2011). This Notch signaling pathway interacts with the Abl pathway member Disabled but does not involve the canonical mechanism.
Fig. 3.
Odorant-driven LTCA requires Notch trans-synaptic signaling. 1) The odorant geranyl acetate activates the Or82a class of olfactory sensory neurons (OSNs), which synapse onto projections neurons (PNs) of the VA6 glomerulus. Activation of the OSNs leads to a noncanonical Notch signaling pathway involving interaction between Notch and the Abl-activated Disabled (Dab) that increases OSN volume. 2) Delta displayed on the PN membrane binds to Notch receptors on the OSN to activate a canonical Notch signaling pathway, with consequent intracellular Notch cleavage and translocation of the Notch intracellular domain (NICD) to the nucleus. Delta-Notch signaling increases the number of nicotinic acetylcholine receptors (nAChRs) on the PN, increases the amount of acetylcholine synthesis in the OSN, and restricts the increase in VA6 glomerulus volume. 3) Overall, the result of developmental exposure to chronic geranyl acetate odorant during the early-use critical period is to enhance the olfactory PN output and to increase the VA6 glomerulus volume. These changes cause persistent modification of olfactory behavior.
A role for the canonical pathway in activity-dependent regulation of glomerular volume was ruled out by showing that Notch knockdown (Notch-RNAi) in OSNs prevents structural changes but that reducing Delta signaling from PNs (Delta-RNAi) or reducing NICD transport to the nucleus (Aph1-RNAi) augments the volume increase (Fig. 3; Kidd et al. 2015; Kidd and Lieber 2016). This mechanism of Notch signaling is cell autonomous in OSNs, because silencing output with tetanus toxin does not affect the volume increase (Kidd and Lieber 2016). Canonical signaling occurs when OSN odor stimuli activate downstream PNs, increasing PN expression of Delta. Notch binding restricts OSN volume and increase PN calcium transients, at least for geranyl acetate (Fig. 3; Kidd and Lieber 2016). The enhanced activity is correlated with an increase in presynaptic choline acetyltransferase (rate-limiting enzyme in ACh production) and postsynaptic nAChR abundance, as measured by fluorescence microscopy. Since both pre- and postsynaptic markers increase, it is appealing to speculate that more synapses form (Kidd and Lieber 2016). This prediction is consistent with the original finding that developmental odor exposure changes synapse number (Devaud et al. 2001). In addition to functional plasticity, reversal of the OSN volume increase depends on canonical Notch signaling, because Delta knockdown (Delta-RNAi) or synapse silencing with a dominant negative nAChR Dα-7 subunit blocks reversal (Kidd and Lieber 2016). Because canonical Notch signaling relies on neural activity, the actions of LNs can modify glomerular output and therefore Notch cleavage (Lieber et al. 2011). It will be of interest to explore whether other forms of OSN inhibition can also modify downstream Notch signaling. For example, nonsynaptic inhibition of OSNs within the same sensillium could be another potential mechanism to modify Notch signaling (Su et al. 2012). Overall, Notch signaling has been shown to be a crucial player in LTCA and may be a useful starting point for investigating the developmental time window defining LTCA.
The final molecular pathway known in LTCA involves two mRNA-binding proteins causally related to fragile X syndrome (Eberhart and Warren 1996) and cerebellar ataxia (Lastres-Becker et al. 2008), respectively, fragile X mental retardation protein (FMRP) and ataxin-2 (Atx-2). These proteins function together in PNs and LNs to mediate LTCA (Fig. 2; Sudhakaran et al. 2014). Genetic studies have shown that both proteins act via inhibitory regulation restricting target mRNA translation (McCann et al. 2011; Sudhakaran et al. 2014). In particular, CaMKII mRNA is bound and translationally regulated by both FMRP and Atx-2 (Fig. 2; Sudhakaran et al. 2014). In addition, knockdown of two proteins involved in miRNA-mediated translational repression, RNA-induced silencing complex (RISC) component ago1 and deadbox helicase me31B, abolishes LTCA from either EB or CO2 exposure (Sudhakaran et al., 2014). The role of FMRP in LTCA has been further supported by recent studies using optogenetics to developmentally modulate activity in single targeted PNs (Doll and Broadie 2015, 2016), demonstrating that stimulation reduces dendritic arbors and increases calcium signaling in VL1. These activity-dependent changes are restricted to the critical time period prior to 7 dpe. Importantly, both structural and functional changes are completely FMRP dependent, because they fail to occur in null mutants (Doll and Broadie 2015, 2016). We predict that sensory experience will likewise operate via this activity-dependent FMRP mechanism, working with partner mRNA-binding translational regulators on downstream targets mediating LTCA.
Conclusions and Future Directions
Over the past 15 years, research on LTCA has advanced enormously by capitalizing on new Drosophila transgenic technologies and improved maps of the olfactory circuit connectome. Emerging conceptual insights are better linking the initial behavioral observations with changes in circuit architecture, functional development, and recently defined molecular mechanisms. Although much ground has been gained toward a clearer understanding of LTCA, focused studies of both the functional and molecular underpinnings are needed to dissect the activity- and FMRP-dependent mechanism and to fully appreciate LTCA requirements in the developmental optimization of neural circuitry. It will be particularly important to unravel the temporal molecular requirements restricting circuit plasticity within the early-use critical period. This understanding will be central to the design of effective new disease treatment strategies, including fragile X syndrome (FXS) and related ataxia, intellectual disability (ID), and autism spectrum disorders (ASDs). In the above discussion, we have presented several areas for future study, including predicted outcomes based on recently published work. Below, three important open questions are examined in greater detail: 1) How do the architectural changes in glomerular morphology relate to functional circuit output? 2) How important are the changes at any single glomerulus for the overall behavioral response? 3) How may the implicated molecular pathways interact with each other in the context of critical period neural circuit refinement?
How do the architectural changes in glomerular morphology relate to functional circuit output?
A particularly robust anatomical correlate of LTCA is the increased AL glomerular volume in specific odor-responsive OSN classes. However, both functional and behavioral changes have been experimentally blocked without affecting this hallmark OSN structural change (Das et al. 2011; Kidd et al. 2015). The observation that developmental optogenetic stimulation of the PN without any input from the OSN still leads to functional plasticity provides additional support for the separation of OSN structural plasticity and PN functional plasticity (Doll and Broadie 2016). Notch signaling in the OSN might be an underlying pathway that links developmental structural and functional plasticity, because knocking down Notch can attenuate both the OSN volume increase and PN functional plasticity (Kidd et al. 2015). We propose three topics of particular interest poised for immediate future investigation in this arena. First, although excellent work has been done on LNs, very little is known about how LN connectivity relates to activity-dependent plasticity. Second, despite the advantages of transgenic calcium imaging for studying the activity of neuron populations, complementary electrophysiological studies are needed to define temporal shifts. Indeed, paired electrophysiology and calcium imaging can help to answer questions about OSN, PN, and, perhaps most importantly, LN plasticity. Third, it is not known how neural activity activates Notch signaling in the AL, and establishing a clear link between these two steps will be fundamental to understand the mechanisms controlling LTCA.
How important are the changes at any single glomerulus for overall behavioral response?
Activity-dependent changes in glomerular volume drive behavioral modifications. Evolutionary comparisons highlight the importance of changes within just a single glomerulus. For example, the feeding specialist subspecies D. erecta and D. sechellia eat screw pine and morinda fruits, which are either repulsive or toxic to D. melanogaster (Dekker et al. 2006; Linz et al. 2013). In both species, there is an increase in Or22a OSN and target DM2 glomerulus size that drives attraction to these host plant odors, demonstrating how a single change can alter behavioral adaptation. LTCA experiments to date have focused on single odor-glomerulus pairs. Although behavioral responses can be well correlated with changes in a single glomerulus, the output of the olfactory system depends on the combination of responses from all the combined glomeruli (Wilson 2013), as well as integration with multiple other sensory modalities. Therefore, perhaps a better approach to study LTCA would be to consider changes across the entire AL neuropil instead of isolated changes in single glomeruli. Several lines of evidence point to this being an important consideration. First, the LNs critical for LTCA presentation typically innervate many AL glomeruli (Chou et al. 2010). These different glomeruli receive not only differential excitation from a given odor but also show a different responsiveness to LN inhibition (Hong and Wilson 2015). Importantly, glomerular volume changes are not restricted only to the odor-sensitive glomeruli (Devaud et al. 2001). Second, Notch signaling for a given odor does not always align with the set of activated OSNs for that odor (Lieber et al. 2011). On the basis of these observations, it would be of interest to take advantage of functional imaging to consider population level changes in glomeruli function. This could be done using transgenic calcium imaging and defining regions of interest corresponding to distinct glomeruli, as has already been applied to link behavioral responses with distinct glomerular activation patterns (Badel et al. 2016; Bell and Wilson 2016).
How may the implicated molecular pathways interact with each other in the context of critical period neural circuit refinement?
Three clusters of proteins have been implicated downstream of developmental changes important for LTCA: 1) mRNA-binding proteins FMRP and Atx-2 acting on CaMKII, 2) cAMP-signaling proteins including dunce and rutabaga, and 3) both canonical and noncanonical Notch signaling pathways. For downstream transcription, cAMP-responsive CREB might be the key target for all of these signaling pathways to converge upon, since all three signaling pathways act in the context of experience-dependent plasticity (Fig. 3; Hallaq et al. 2015; Sudhakaran et al. 2014; Zhang et al. 2013). Interestingly, a very recent study has shown that the age-dependent maintenance of the male-specific fruitless gene in OSNs requires posteclosion activation and downstream CaMK/CREB signaling (Hueston et al. 2016), which subsequently may alter odor-coding properties and OSN signaling to affect behavioral output. It is appealing to speculate that a similar mechanism could be important for driving Notch signaling in experience-dependent developmental plasticity. In addition to the potential convergence of these pathways on CREB signaling, it will be of interest to investigate the temporal mechanisms that limit plasticity only to the early posteclosion developmental window. One attractive theory is that the progressive reduction in FMRP expression during the course of this developmental interval limits plasticity to the critical period (Doll and Broadie 2014, 2015, 2016). Although studies have shown that loss of FMRP abolishes LTCA, examining the potential for reactivation of plasticity through ectopic FMRP expression after the critical period will be important in establishing that the FMRP mechanism is crucial for actually defining the critical period. Regardless of the outcome of such targeted genetic tests, Drosophila is amenable to unbiased genetic screens, which could produce a robust list of genes controlling LTCA.
Much has been learned about the cellular and molecular mechanisms that underlie LTCA. Early Drosophila behavioral work illustrated that developmental exposure to odors only during the initial early-use critical period drives plastic changes in the tuning of the olfactory system (Devaud et al. 2001). These behavioral observations were subsequently expanded by linking to corresponding architectural changes in the AL circuitry (Das et al. 2011; Devaud et al. 2001; Sachse et al. 2007). Although the exact nature of how changes in AL synaptic connectivity lead to alterations in local and overall circuit function remains unknown, the underlying circuit neural component requirements are beginning to emerge (Das et al. 2011; Sachse et al. 2007). The synaptic interplay between OSN, LN and PN appears complex (Fig. 2), and it is apparent that downstream changes in the central brain MB and LH centers will also participate in overall critical period plasticity. The molecular pathways identified to date are of the greatest interest, not only from the standpoint of critical period plasticity but also because they serve as models to study the function of two disease-relevant proteins (FMRP and Atx-2), as well as to study the largely unexplored role of Notch signaling in the postmitotic central nervous system (Doll and Broadie 2015, 2016; Kidd et al. 2015; Kidd and Lieber 2016; Lieber et al. 2011; Sudhakaran et al. 2014). The Drosophila AL is an excellent model to investigate critical period plasticity, providing foundational tenets for future work in mammalian systems.
GRANTS
This work was supported by National Institute of Mental Health Grant R01 MH084989 (to K. Broadie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.G. prepared figures; R.M.G. drafted manuscript; R.M.G. and K.B. edited and revised manuscript; R.M.G. and K.B. approved final version of manuscript.
REFERENCES
- Acebes A, Martín-Peña A, Chevalier V, Ferrús A. Synapse loss in olfactory local interneurons modifies perception. J Neurosci 31: 2734–2745, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas A, Farina WM. Age and rearing environment interact in the retention of early olfactory memories in honeybees. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 194: 629–640, 2008. [DOI] [PubMed] [Google Scholar]
- Arenas A, Giurfa M, Farina WM, Sandoz JC. Early olfactory experience modifies neural activity in the antennal lobe of a social insect at the adult stage. Eur J Neurosci 30: 1498–1508, 2009. [DOI] [PubMed] [Google Scholar]
- Badel L, Ohta K, Tsuchimoto Y, Kazama H. Decoding of context-dependent olfactory behavior in Drosophila. Neuron 91: 155–167, 2016. [DOI] [PubMed] [Google Scholar]
- Barnstedt O, Owald D, Felsenberg J, Brain R, Moszynski JPP, Talbot CB, Perrat PN, Waddell S. Memory-relevant mushroom body output synapses are cholinergic. Neuron 89: 1237–1247, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JS, Wilson RI. Behavior reveals selective summation and max pooling among olfactory processing channels. Neuron 91: 425–438, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campusano JM, Su H, Jiang SA, Sicaeros B, O'Dowd DK. nAChR-mediated calcium responses and plasticity in Drosophila Kenyon cells. Dev Neurobiol 67: 1520–1532, 2007. [DOI] [PubMed] [Google Scholar]
- Chakraborty TS, Goswami SP, Siddiqi O. Sensory correlates of imaginal conditioning in Drosophila melanogaster. J Neurogenet 23: 210–219, 2009. [DOI] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci 13: 439–449, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C, Hauser F, Gonzalez de Valdivia E, de Valdivia EG, Li S, Reisenberger J, Carlsen EMM, Khan Z, Hansen NO, Puhm F, Søndergaard L, Niemiec J, Heninger M, Ren GR, Grimmelikhuijzen CJ. Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods. Cell Mol Life Sci 70: 3231–3242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol 15: 1535–1547, 2005. [DOI] [PubMed] [Google Scholar]
- Das A, Gupta T, Davla S, Prieto-Godino LL, Diegelmann S, Reddy OV, Raghavan KV, Reichert H, Lovick J, Hartenstein V. Neuroblast lineage-specific origin of the neurons of the Drosophila larval olfactory system. Dev Biol 373: 322–337, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sadanandappa MK, Dervan A, Larkin A, Lee JA, Sudhakaran IP, Priya R, Heidari R, Holohan EE, Pimentel A, Gandhi A, Ito K, Sanyal S, Wang JW, Rodrigues V, Ramaswami M. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci USA 108: E646–E654, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr Biol 16: 101–109, 2006. [DOI] [PubMed] [Google Scholar]
- Devaud JM, Acebes A, Ferrús A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci 21: 6274–6282, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud JM, Acebes A, Ramaswami M, Ferrús A. Structural and functional changes in the olfactory pathway of adult Drosophila take place at a critical age. J Neurobiol 56: 13–23, 2003a. [DOI] [PubMed] [Google Scholar]
- Devaud JM, Keane J, Ferrús A. Blocking sensory inputs to identified antennal glomeruli selectively modifies odorant perception in Drosophila. J Neurobiol 56: 1–12, 2003b. [DOI] [PubMed] [Google Scholar]
- Doll CA, Broadie K. Impaired activity-dependent neural circuit assembly and refinement in autism spectrum disorder genetic models. Front Cell Neurosci 8: 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll CA, Broadie K. Activity-dependent FMRP requirements in development of the neural circuitry of learning and memory. Development 142: 1346–1356, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll CA, Broadie K. Neuron class-specific requirements for fragile X mental retardation protein in critical period development of calcium signaling in learning and memory circuitry. Neurobiol Dis 89: 76–87, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA 73: 1684–8, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart DE, Warren ST. The molecular basis of fragile X syndrome. Cold Spring Harb Symp Quant Biol 61: 679–687, 1996. [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci 35: 1540–1553, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 75: 230–249, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol 15: 1548–1553, 2005. [DOI] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci 26: 265–272, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Stopfer M. Insect olfactory coding and memory at multiple timescales. Curr Opin Neurobiol 21: 768–773, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 13: 654–666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales KG, Korey CA, Larracuente AM, Roberts DM. Genetics on the fly: a primer on the Drosophila model system. Genetics 201: 815–842, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallaq R, Volpicelli F, Cuchillo-Ibanez I, Hooper C, Mizuno K, Uwanogho D, Causevic M, Asuni A, To A, Soriano S, Giese KP, Lovestone S, Killick R. The Notch intracellular domain represses CRE-dependent transcription. Cell Signal 27: 621–629, 2015. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888, 2005. [DOI] [PubMed] [Google Scholar]
- Hige T, Aso Y, Modi MN, Rubin GM, Turner GC. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron 88: 985–998, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hige T, Aso Y, Rubin GM, Turner GC. Plasticity-driven individualization of olfactory coding in mushroom body output neurons. Nature 526: 258–262, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, Wilson RI. Simultaneous encoding of odors by channels with diverse sensitivity to inhibition. Neuron 85: 573–589, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206: 419–436, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell 159: 727–737, 2014. [DOI] [PubMed] [Google Scholar]
- Hueston CE, Olsen D, Li Q, Okuwa S, Peng B, Wu J, Volkan PC. Chromatin modulatory proteins and olfactory receptor signaling in the refinement and maintenance of fruitless expression in olfactory receptor neurons. PLoS Biol 14: e1002443, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar A, Chakraborty TS, Goswami SP, Wu CF, Siddiqi O. Post-eclosion odor experience modifies olfactory receptor neuron coding in Drosophila. Proc Natl Acad Sci USA 107: 9855–9860, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci 8: 341–354, 2007. [DOI] [PubMed] [Google Scholar]
- Kidd S, Lieber T. Mechanism of notch pathway activation and its role in the regulation of olfactory plasticity in Drosophila melanogaster. PLoS One 11: e0151279, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Struhl G, Lieber T. Notch is required in adult Drosophila sensory neurons for morphological and functional plasticity of the olfactory circuit. PLoS Genet 11: e1005244, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer MC, Christiansen F, Leiss F, Paehler M, Knapek S, Andlauer TF, Förstner F, Kloppenburg P, Sigrist SJ, Tavosanis G. Structural long-term changes at mushroom body input synapses. Curr Biol 20: 1938–1944, 2010. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Rüb U, Auburger G. Spinocerebellar ataxia 2 (SCA2). Cerebellum 7: 115–124, 2008. [DOI] [PubMed] [Google Scholar]
- Lee D, O'Dowd DK. Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons. J Neurosci 19: 5311–5321, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol 191: 1–51, 1980. [DOI] [PubMed] [Google Scholar]
- Liang L, Li Y, Potter CJ, Yizhar O, Deisseroth K, Tsien RW, Luo L. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron 79: 917–931, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T, Kidd S, Struhl G. DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron 69: 468–481, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J, Baschwitz A, Strutz A, Dweck HK, Sachse S, Hansson BS, Stensmyr MC. Host plant-driven sensory specialization in Drosophila erecta. Proc Biol Sci 280: 20130626, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Wilson RI. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc Natl Acad Sci USA 110: 10294–10299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell 37: 205–215, 1984. [DOI] [PubMed] [Google Scholar]
- McCann C, Holohan EE, Das S, Dervan A, Larkin A, Lee JA, Rodrigues V, Parker R, Ramaswami M. The ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc Natl Acad Sci USA 108: E655–E662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezler M, Müller T, Raming K. Cloning and functional expression of GABAB receptors from Drosophila. Eur J Neurosci 13: 477–486, 2001. [DOI] [PubMed] [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36: 463–474, 2002. [DOI] [PubMed] [Google Scholar]
- Parnas M, Lin AC, Huetteroth W, Miesenböck G. Odor discrimination in Drosophila: from neural population codes to behavior. Neuron 79: 932–944, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech U, Revelo NH, Seitz KJ, Rizzoli SO, Fiala A. Optical dissection of experience-dependent pre- and postsynaptic plasticity in the Drosophila brain. Cell Rep 10: 2083–2095, 2015. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK. Activity-dependent plasticity in an olfactory circuit. Neuron 56: 838–850, 2007. [DOI] [PubMed] [Google Scholar]
- Silva B, Molina-Fernández C, Ugalde MBB, Tognarelli EI, Angel C, Campusano JM. Muscarinic ACh receptors contribute to aversive olfactory learning in Drosophila. Neural Plast 2015: 658918, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz A, Soelter J, Baschwitz A, Farhan A, Grabe V, Rybak J, Knaden M, Schmuker M, Hansson BS, Sachse S. Decoding odor quality and intensity in the Drosophila brain. Elife 3: e04147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492: 66–71, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, O'Dowd DK. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by α-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J Neurosci 23: 9246–9253, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakaran IP, Hillebrand J, Dervan A, Das S, Holohan EE, Hülsmeier J, Sarov M, Parker R, VijayRaghavan K, Ramaswami M. FMRP and ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc Natl Acad Sci USA 111: E99–E108, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14: 341–351, 1995. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30: 505–533, 2007. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell 102: 147–159, 2000. [DOI] [PubMed] [Google Scholar]
- Waddell S, Quinn WG. Flies, genes, and learning. Annu Rev Neurosci 24: 1283–1309, 2001. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, Lam DM. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res 79: 273–279, 1974. [DOI] [PubMed] [Google Scholar]
- Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci 36: 217–241, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yin JC, Wesley CS. From Drosophila development to adult: clues to Notch function in long-term memory. Front Cell Neurosci 7: 222, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]