Abstract
Introduction
Spontaneous lead dislodgement into the pulmonary circulation is a rare complication of permanent pacing with unproven harmfulness and an indication of controversial class for transvenous lead extraction (TLE).
Aim
To assess TLE safety in patients with leads dislodged into the pulmonary artery.
Material and methods
A retrospective analysis of a 9-year-old database of transvenous lead extraction procedures comprising 1767 TLEs was carried out, including a group of 19 (1.1%) patients with leads dislodged into the pulmonary artery (LDPA).
Results
Under univariate analysis the factors that increased the likelihood of the presence of an electrode in the pulmonary artery were mean lead dwelling time (increase of risk by 9% per year), total number of leads in the heart before TLE (increase of risk by 66% for one lead) and the number of abandoned leads (increase of risk by 119%). The presence of LDPA was associated with frequent occurrence of intracardiac lead abrasion (increase by 316%) and isolated lead-related infective endocarditis (LRIE) (increase by 500%). There were no statistically significant differences in clinical (p = 0.3), procedural (p = 0.94) or radiological (p = 0.31) success rates in compared (LDPA and non-LDPA) groups. Long-term mortality after TLE was comparable in both groups.
Conclusions
As the effectiveness and safety of TLE in patients with LDPA are comparable to those in standard TLE procedures, in our opinion, such patients should be considered TLE candidates.
Keywords: lead dislodgement, transvenous lead extraction, intracardiac lead abrasion
Introduction
A growing number of pacemaker (PM) and defibrillator (ICD) implantations is associated with the occurrence of infective and non-infective complications and the need for revision or lead extraction procedures. All situations of lead dysfunction and upgrades increase the likelihood of lead abandonment. Improper lead fixation/stabilization in its venous entry may favour lead fracture or failure of ligature following lead shift into the vascular bed (especially not sufficiently fixed, short cut, non-functional, abandoned leads). Moreover, excess length of the lead might result in the creation of a loop in the right atrium or ventricle [1–4]. The consequences of this phenomenon such as lead-dependent tricuspid dysfunction have been described in the literature [3, 4]. Migration of a lead proximal ending or even a lead loop via the tricuspid valve and pulmonary valve into the pulmonary bed has been inadequately described; several case reports have been published to date [2, 5–9], but there is no general consensus that will provide clear guidelines for managing such patients. According to the current transvenous lead extraction (TLE) guidelines (Heart Rhythm Society), indications for TLE in these cases would be: presence of leads in a place where they may pose an immediate threat (class I) or potential future threat (class IIb) to the patients [10]. Meanwhile, the threat level in patients with leads dislodged into the pulmonary artery (LDPA) is unknown, because this is the first study evaluating the significance of LDPA to the development of further complications and subsequent necessity for early transvenous lead extraction.
Aim
The objectives of this study were to estimate the risk factors of leads dislodged into the pulmonary artery, analyze indications for TLE procedures and compare TLE safety and effectiveness in patients with a lead dislodged into the pulmonary artery as opposed to standard procedures (LDPA and non-LDPA groups).
Material and methods
We analyzed data from 1767 patients undergoing transvenous lead extraction for infectious and non-infectious indications in the single TLE Reference Centre in Poland in 2006–2015. Based on medical data the patients were divided into two groups: group I consisted of 19 (1.1%) subjects with LDPA, and group II consisted of the remaining 1748 (98.9%) patients serving as the controls. Patients were assigned to group I if they had LDPA documented by echocardiography and/or fluoroscopy. In order to identify the effect of LDPA on a patient’s condition, we carried out a comparative analysis of indications for TLE, number, type and dwell time of the leads, as well as number of abandoned leads, number of procedures before lead extractions and presence of intracardiac lead abrasion (ILA). A comparative assessment of the effectiveness and safety of TLE procedures and long-term mortality was also conducted in examined LDPA and non-LDPA groups.
Definitions
The term “loop of the lead” means an excessive extension of the lead located in the right atrium or ventricle, protruding into the tricuspid ostium or pulmonary artery and resulting in persistent mechanical collision or dynamic contact with itself or with other electrodes [11, 12].
Intracardiac lead abrasion was defined according to previous descriptions as macroscopically visible damage of the external insulation of the lead, located only in its intracardiac part, usually in the first 15–20 cm from the tip. The lesion of the external lead tube results in exposure of the metal wire with its colour change and possible serum or purulent effusion from inside the lead [11, 12].
Procedural success and complication definitions were based on current TLE guidelines [10]:
complete procedural success: all targeted leads removed without permanently disabling complications or procedure-related death;
clinical success: all targeted leads removed, or residue of small parts (< 4 cm) of the lead without increasing the risk of derivative complication or persistence of infection;
radiological success: all targeted leads removed, with the absence of any permanently disabling complications. We differentiated complete radiological success and partial radiological success when less than 4 cm lead fragments remained.
Major complication was defined as death, significant disability, or any event that required significant surgical intervention. Minor complication was defined as adverse event that required medical intervention or minor procedural intervention and did not limit the patient’s function.
Extraction technique
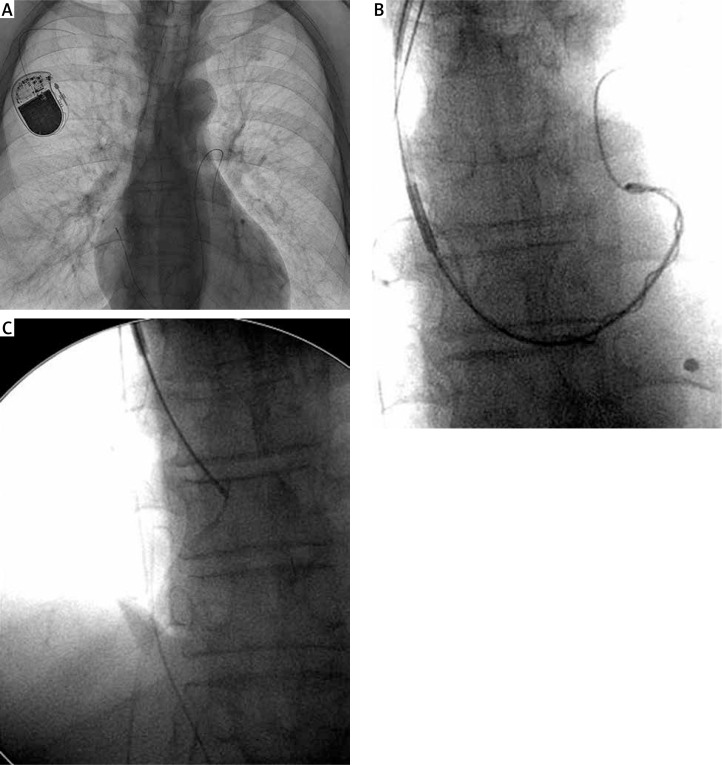
The extraction technique depended on the type of dislocation. If the proximal end of the lead was available in the pocket and there was only a loop in the pulmonary artery, the procedure was similar to standard TLE using the lead venous entry approach. After stylet introduction (locking or standard, according to technical conditions), a Byrd dilator sheath (Cook Medical, USA) was introduced over the lead, and after gentle traction the loop was pulled down into the right ventricle. If a lead was broken and the proximal end was dislocated into the cardiovascular system – even into the pulmonary artery – a pigtail catheter was introduced via a coronary sinus cannulation catheter (Medtronic Attain Command, USA) into the pulmonary artery. The catheter was positioned parallel to the lead, and after rolling around the lead (the lead was wound over the catheter) both were retracted into the superior vena cava. Then, using a lasso catheter (introduced via the same CS-dedicated sheath) the lead was recaptured and removed with the Byrd dilator sheath positioned over the CS catheter (Figure 1). We previously described in detail the technique of grasping the free end of a lead in the vena cava and the subsequent extraction of the grasped lead (broken lead or lead fragment) using conventional tools [13–15]. The procedures were performed in a cardiovascular operating room with on-site cardiac surgery backup.
Figure 1.
Broken lead dislocated into pulmonary artery (A), using a pigtail catheter the lead was retracted into the superior vena cava (B) and recaptured with a lasso (C)
Statistical analysis
Normality of the data was tested by the Shapiro-Wilk test. Because of the lack of a normal distribution of some variables, continuous data are presented both as means with standard deviation (SD) and medians with interquartile range (IQR). Categorical data are presented as absolute numbers and percentages.
Statistics
Patients were divided into two groups based on LDPA presence: 1 – with LDPA and 2 – others. The Mann-Whitney U test was used for the comparison of continuous variables. For categorical data Yates’ χ2 test was used.
Regression analysis
Univariate regression analysis was applied to identify the parameters associated with LDPA occurrence.
Because there was a relatively small number of LDPA cases initially, we decided to build a three-variable model of multivariate regression analysis. All unrelated parameters reaching a significance level of p < 0.1 in the univariate analysis were added individually to this model in various combinations. Results of the regression analysis are presented as the hazard ratio (HR) with a 95% confidence interval (95% CI).
Survival curves and the log rank test
Survival analysis based on Kaplan-Meier curves and log-rank tests was used to assess the survival rates between examined groups.
Differences between groups were regarded as significant if the p-value was < 0.05 or when the 95% confidence interval did not include the value of one. If the 95% confidence interval was between 0.5 and 0.1, its value was written with two digits after the decimal point.
Statistical calculations were performed using Statistica 10.0 (StatSoft Inc., Minneapolis, USA).
Results
Patients and procedures
From January 2006 to 31 March 2015, 2991 leads (PM leads dwell time > 12 months, ICD leads > 6 months), from 1767 patients (mean age: 64.6 years; 60.5% male) were transvenously extracted. In 19 (1.1%) patients, a lead in the pulmonary vascular bed was observed. Eighteen (94.7%) cases of LDPA were PM leads; there was only 1 (5.3%) ICD lead; 13 leads (68.4%) were bipolar (BP) and 6 (31.6%) were unipolar (UP). The most frequently dislocated part of a lead present in the pulmonary artery was its proximal ending – 9 (47.4%); its loop was observed in 6 (31.6%) patients and its distal ending in 4 (21.1%). The lead was broken in 10 (52.6%) cases; there were 6 (31.6%) ligature failures and 3 (15.8%) lead dislodgements. The leads were most often located in the right pulmonary artery (8 cases, 42.1%), in the pulmonary trunk in 7 (36.8%) patients and in the left pulmonary artery in 4 (21.1%) subjects. Most of the dislocated leads were initially implanted in the RV – 13 (68.4%) (Table I).
Table I.
LDPA patients’ characteristics
| Lead in pulmonary artery (LDPA) | Number | Percentage | |
|---|---|---|---|
| Lead part in PA | Proximal ending | 9 | 47.4 |
| Lead loops | 6 | 31.6 | |
| Distal ending (tip) | 4 | 21.1 | |
| Mechanism | Lead break/fracture | 10 | 52.6 |
| Lead ligature failure | 6 | 31.6 | |
| Lead dislodgement | 3 | 15.8 | |
| Location in PA | Pulmonary trunk | 7 | 36.8 |
| Right pulmonary artery | 8 | 42.1 | |
| Left pulmonary artery | 4 | 21.1 | |
| Lead destination | Right atrium | 4 | 21.1 |
| Right ventricle | 13 | 68.4 | |
| Cardiac vein | 2 | 10.5 | |
| Unit chest side | Left | 16 | 84.2 |
| Right | 3 | 15.8 | |
| Lead type | PM lead | 18 | 94.7 |
| ICD lead | 1 | 5.3 | |
| Lead polarity | BP | 13 | 68.4 |
| UP | 6 | 31.6 | |
| Extraction approach | Subclavian (femoral, auxiliary) | 18 | 94.7 |
| Femoral (only) | 1 | 5.3 | |
Comparative analysis of patients with leads in pulmonary artery and without LDPA
Demographic data for the number of procedures before TLE did not differ significantly between examined groups. Presence of a lead in the pulmonary artery was associated with a longer dwelling time of implanted leads and with the number of leads in the patient. This resulted from a higher number of abandoned leads in the LDPA group. The number of functional leads in both groups was comparable. In the LDPA group parallel lead abrasions were detected more often. Except for the statistically significant difference in the number of cases of isolated LRIE, the groups did not differ in the incidence of other infectious complications (Table II).
Table II.
Demographic, cardiac implantable electronic device (CIED)-related parameters, the reason for TLE in groups depending on LDPA presence
| Parameter | Group 1 With lead in pulmonary artery (N = 19) | Group 2 (control) Without lead in pulmonary artery (N = 1748) | P-value Mann-Whitney U/χ2 test |
|---|---|---|---|
| Patient’s age (first implantation) Mean ± SD, median, IQR |
51.6 ±18.2, 56.17, 28.75 | 57.3 ±17.4, 60.08, 19.17 | NS |
| Patient’s age (TLE) Mean ± SD, median, IQR |
62.8 ±17.5, 68.0, 28.0 | 64.7 ±15.9, 68.00, 15.86 | NS |
| Gender (female) | 6 (31.6%) | 691 (39.5%) | NS |
| LRIE (isolated) | 8 (42.1%) | 189 (10.8%) | < 0.001 |
| LRIE with or without pocket infection | 9 (47.4%) | 488 (27.9%) | NS |
| Infective indications (all) | 10 (52.6%) | 709 (40.6%) | NS |
| Non-infective indications | 9 (47.4%) | 1039 (59.4%) | NS |
| Number of leads in heart before TLE Number, mean ± SD, median, IQR |
47, 2.47 ±0.90, 2.0, 1.0 | 3507, 2.01 ±0.83, 2.0, 1.0 | < 0.05 |
| Number of leads in the system Mean ± SD, n, % of all leads |
1.79 ±0.54, 34/47, 72.3% | 1.80 ±0.64, 3142/3507, 8.01% | NS |
| Number of abandoned leads Mean ± SD, n, % of all leads |
0.68 ±0.82, 13/47, 72.3% | 0.21 ±0.55, 365/3507, 10.4% | < 0.001 |
| Intracardiac lead abrasion | 11 (57.9%) | 289 (16.5%) | < 0.001 |
| Number of procedures before lead extraction Number, mean ± SD, median, IQR |
44, 2.32 ±0.20, 2.0, 2.0 | 3289, 1.88 ±1.16, 2.0, 1.0 | NS |
| ICD lead extraction | 1 (5.3%) | 450 (25.7%) | NS |
| Mean lead body dwelling time [years] Mean ± SD, median, IQR |
9.46 ±5.65, 9.92, 8.50 | 6.91 ±4.99, 5.73, 6.10 | < 0.05 |
Uni- and multivariate regression analysis
Under univariate analysis the factors that increased the likelihood of the presence of an electrode in the pulmonary artery were mean lead dwelling time (increase of risk by 9% per year), total number of leads in the heart before TLE (increase of risk by 66% for one lead) and the number of abandoned leads (increase of risk by 119%). The presence of LDPA was associated with frequent occurrence of intracardiac lead abrasion (increase by 316%) and isolated LRIE (increase by 500%). Risk of all LRIE cases increased by 132% with p = 0.068. The ICD lead presence was consistent with a lower risk of LDPA, but there was borderline statistical significance as well.
Multivariate analysis showed that the strongest predictive factor of LDPA presence was the presence of abandoned leads (increase of risk by 85%). It may result in intracardiac lead abrasions (increased risk by 190%) and also in lead-related infective endocarditis (increased risk by 314%) (Table III).
Table III.
Relationship between demographic, CIED-related parameters, the reason for TLE and LDPA presence under uni- and multivariable regression analysis
| Parameter | Univariable regression | Three-variable regression model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Patient’s age (first implantation) | 0.98 | 0.96–1.01 | NS | |||
| Patient’s age (TLE) | 0.99 | 0.97–1.02 | NS | |||
| Gender (female) | 1.42 | 0.54–3.75 | NS | |||
| Non-infective indications | 0.61 | 0.25–1.51 | NS | |||
| Infective indications (all) | 1.64 | 0.66–4.05 | NS | |||
| LRIE (all) | 2.32 | 0.94–5.75 | 0.068 | |||
| LRIE + pocket infection | 0.28 | 0.04–2.02 | NS | |||
| LRIE (isolated)* | 6.00 | 2.38–15.1 | < 0.001 | 4.14 | 1.57–10.91 | < 0.001 |
| Pocket infection (isolated) | 0.37 | 0.05–2.85 | NS | |||
| Number of leads in heart before TLE | 1.66 | 1.11–2.49 | 0.015 | |||
| Number of leads in the system | 0.98 | 0.55–1.76 | NS | |||
| Number of abandoned leads* | 2.19 | 1.39–3.46 | 0.001 | 1.85 | 1.14–3.00 | < 0.05 |
| Intracardiac lead abrasion* | 4.80 | 1.92–12.0 | 0.001 | 2.90 | 1.09–7.73 | < 0.001 |
| Number of procedures before lead extraction | 1.28 | 0.95–1.73 | NS | |||
| ICD lead presence** | 0.15 | 0.02–1.12 | 0.064 | 0.21 | 0.03–1.66 | NS |
| Mean lead body dwelling time** | 1.09 | 1.01–1.17 | 0.024 | 1.04 | 0.97–1.12 | NS |
Variables included in three-variable regression model
Variables added individually to three-variable regression model, LRIE all – lead-related infective endocarditis all.
TLE procedural analysis
The duration of the whole procedure in patients with LDPA was significantly longer (p = 0.0001) and more technical problems were observed during TLE (p = 0.1). There were no statistically significant differences in clinical (p = 0.3), procedural (p = 0.94) or radiological (p = 0.31) success rates among compared groups. Major complications occurred in 1 (5.3%) vs. 27 (1.5%) patients (p = 0.19); minor complications were observed only in the second group of patients, 26 (1.5%) (p = 0.59) (Table IV).
Table IV.
Assessment of TLE effects in patients with LDPA
| Patient/procedure information | With lead in pulmonary artery | Without lead in pulmonary artery | P-value χ2 with Yates correction/Mann-Whitney U test |
|---|---|---|---|
| Full radiological success | 19 (100.0%) | 1664 (95.2%) | 0.31 |
| Clinical success | 18 (94.7%) | 1713 (98.0%) | 0.30 |
| Procedural success | 18 (94.7%) | 1663 (95.1%) | 0.94 |
| Technical problems during TLE | 6 (31.6%) | 268 (15.3%) | 0.100 |
| Major complications | 1 (5.3%) | 27 (1.5%) | 0.19 |
| Minor complications | 0 (0.0%) | 26 (1.5%) | 0.595 |
| Operating room stay-in time (whole procedure duration) [min], mean ± SD | 159.2 ±69.2 | 108.0 ±44.4 | < 0.001 |
Survival analysis
Long-term mortality after TLE was comparable in both groups of patients.
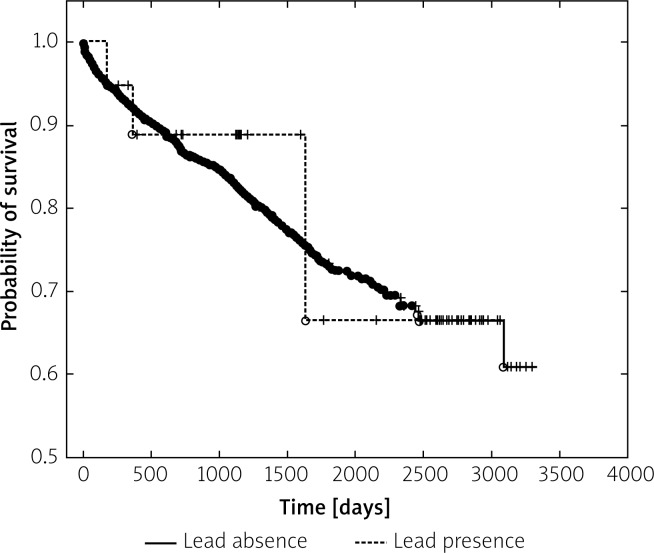
During the follow-up (0–3303 days, 3.043 ±2.051 years, median: 2.86; IQR: 3.21) of 341 (19.5%) deaths, respectively 3 (15.8%) occurred in LDPA and 338 (19.3%) occurred in the control group. The survival curves are presented in Figure 2.
Figure 2.
Survival after TLE in mean 3-year follow-up. There were no significant differences in long-term mortality between compared groups (with LDPA and without LDPA)
Discussion
In the literature there are only a few case reports of accidentally found leads dislocated into the pulmonary artery [11, 16, 17]. As LDPA is a rare phenomenon, the factors favouring its development – as well as potential indications for TLE – have not been precisely analysed yet. In our study LDPA was caused mainly by lead proximal end dislocation into the pulmonary artery (47.4%) or by a loop crossing the pulmonary valve (31.6%). Both mechanisms were related to lead fracture and displacement, excessive lead elongation due to pocket ligature failure, or non-functional lead abandonment after cutting the proximal ending of the lead. Previous studies revealed that lead loops determine intracardiac lead abrasion, which is a risk factor for LRIE development [11, 12, 18–20]. Our research showed a similar sequence of events in patients with LDPA. The performed analysis confirmed that LDPA occurred frequently in patients with abandoned leads and contributed to the development of ILA, which increased the risk of LRIE. It is interesting to note that only isolated LRIE (without pocket infection) was correlated with LDPA presence. This coexistence probably confirms the different pathogenesis of isolated LRIE associated with the facilitated penetration of pathogens within the damaged insulation of the lead (ILA).
In our study, long-term mortality after TLE was comparable in both groups of patients, which proved that transvenous extraction in patients with LDPA is a proper strategy. However, in real life, management of such patients still remains problematic. Some of them underwent successful percutaneous extraction [4, 5, 7, 9, 21]. Despite a few cases of long-term asymptomatic course [8, 21], serious complications of prolonged observation such as lead-related infective endocarditis [22] or even lead-induced ventricular tachycardia resulting in cardiac arrest [5, 23] were described. Conservative treatment – long-term observation – of such patients presumably results from the anxiety of possible TLE complications in an asymptomatic patient. Therefore we decided to share our experience of extraction procedures in such patients. The results are optimistic. The difference in clinical success and complication rates between compared groups of patients undergoing TLE (with and without a dislocated lead) was statistically insignificant. We managed to achieve nearly 95% clinical success in such patients. Similarly, the number of major and minor complications was comparable to that of the control group of patients undergoing TLE due to other indications. Naturally, these procedures were technically more advanced and lasted longer, but this did not affect the final result.
We believe that leaving the patient with LDPA without extraction is a risky solution. However, to achieve a high rate of clinical success in such TLE procedures, the operator must be properly prepared and have experience with extra tools used mainly in interventional radiology.
Study limitations include the small number of LDPA patients and the absence of a control group of patients with LDPA who did not undergo TLE.
Conclusions
Displacement of a lead into the pulmonary artery is a rare but potentially dangerous phenomenon leading to serious complications. LDPA was related to the development of intracardiac lead abrasion, which increases the risk of LRIE. Current guidelines do not specify management in such cases, but our results indicate that patients with LDPA should be considered TLE candidates to prevent the development of life-threatening complications.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Smith MC, Love CJ. Extraction of transvenous pacing and ICD leads. Pacing Clin Electrophysiol. 2008;31:736–52. doi: 10.1111/j.1540-8159.2008.01079.x. [DOI] [PubMed] [Google Scholar]
- 2.Kutarski A, Małecka B, Ząbek A, et al. Broken leads with proximal endings in the cardiovascular system: serious consequences and extraction difficulties. Cardiol J. 2013;20:161–9. doi: 10.5603/CJ.2013.0029. [DOI] [PubMed] [Google Scholar]
- 3.Polewczyk A, Kutarski A, Tomaszewski A, et al. Lead dependent tricuspid dysfunction: analysis of the mechanism and management in patients referred for transvenous lead extraction. Cardiol J. 2013;20:402–10. doi: 10.5603/CJ.2013.0099. [DOI] [PubMed] [Google Scholar]
- 4.Arapoglu M, Celiker A, Ozkan S. Severe tricuspid regurgitation secondary to dislodgement of the atrial loop into the right ventricle: an unusual complication of pacemaker implantation in a young adult. Acta Cardiol. 2012;67:235–8. doi: 10.1080/ac.67.2.2154215. [DOI] [PubMed] [Google Scholar]
- 5.Bõhm A, Pintér A, Préda I. Ventricular tachycardia induced by a pacemaker lead. Acta Cardiol. 2002;57:23–4. doi: 10.2143/AC.57.1.2005375. [DOI] [PubMed] [Google Scholar]
- 6.Lorsheyd A, DeBoeck BW, Guyomi SH, et al. A wandering defibrillator lead. Eur J Echocardiogr. 2009;10:156–9. doi: 10.1093/ejechocard/jen223. [DOI] [PubMed] [Google Scholar]
- 7.Michalak M, Kutarski A, Zawadzka-Byśko M, et al. Transvenous extraction of a broken atrial lead embolised into the pulmonary artery using a pigtail catheter. Kardiol Pol. 2015;73:464. doi: 10.5603/KP.2015.0107. [DOI] [PubMed] [Google Scholar]
- 8.Erkan H, Varol O, Karadeniz A, et al. Embolisation of permanent pacemaker lead to pulmonary artery: a 15-year follow up. Kardiol Pol. 2014;72:759. doi: 10.5603/KP.2014.0157. [DOI] [PubMed] [Google Scholar]
- 9.Oktay AA, Dibs SR, Silver JM, et al. Extreme externalisation of a Riata defibrillator lead conductor cable with prolapse into the left pulmonary artery. Heart Lung Circ. 2014;23:e276–8. doi: 10.1016/j.hlc.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 10.Wilkoff BL, Love CJ, Byrd CL, et al. Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA) Heart Rhythm. 2009;6:1085–104. doi: 10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Kutarski A, Małecka B, Kołodzinska A, et al. Mutual abrasion of endocardial leads: analysis of explanted leads. Pacing Clin Electrophysiol. 2013;36:1503–11. doi: 10.1111/pace.12216. [DOI] [PubMed] [Google Scholar]
- 12.Kołodzinska K, Kutarski A, Grabowski M, et al. Abrasions of the outer silicone insulation of endocardial leads in their intracardiac part: a new mechanism of lead-dependent endocarditis. Europace. 2012;14:903–10. doi: 10.1093/europace/eus003. [DOI] [PubMed] [Google Scholar]
- 13.Kutarski A, Pietura R, Czajkowski M. Breakage of extracted leads: another management option. Kardiol Pol. 2012;70:307–12. [PubMed] [Google Scholar]
- 14.Kutarski A, Chudzik M, Oszczygieł A, et al. Extraction of abandoned, potentially dangerous lead with uncovered proximal ending: a case report and method description. Cardiol J. 2012;19:192–6. doi: 10.5603/cj.2012.0033. [DOI] [PubMed] [Google Scholar]
- 15.Małecka B, Kutarski A, Zabek A, et al. Percutaneous removal of endocardial implantable cardioverter-defibrillator lead displaced to the right pulmonary artery. Cardiol J. 2010;17:293–8. [PubMed] [Google Scholar]
- 16.Stein A, Mazzitelli D, Kolb C. Very-late proarrhythmia of a migrant pacemaker lead. J Electrocardiol. 2011;44:232–4. doi: 10.1016/j.jelectrocard.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Ruparelia N, Newton J, Ormerod OJ, et al. Percutaneous retrieval of an embolized pacemaker lead from the pulmonary artery. Int J Cardiol. 2011;149:e106–7. doi: 10.1016/j.ijcard.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 18.Kołodzińska A, Kutarski A. Lead insulation failure, a serious complication: risk factors and management. Kardiol Pol. 2015;73:585–91. [PubMed] [Google Scholar]
- 19.Polewczyk A, Jacheć W, Janion M, et al. Lead-dependent infective endocarditis: the role of factors predisposing to its development in an analysis of 414 clinical cases. Pacing Clin Electrophysiol. 2015;38:846–56. doi: 10.1111/pace.12615. [DOI] [PubMed] [Google Scholar]
- 20.Polewczyk A, Janion M, Podlaski R, et al. Clinical manifestations of lead-dependent infective endocarditis: analysis of 414 cases. Eur J Clin Microbiol Infect Dis. 2014;33:1601–8. doi: 10.1007/s10096-014-2117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guler A, Karabay CY, Aung SM, et al. A successful percutaneous retrieval of a fractured pacemaker lead from a segmental pulmonary artery. Europace. 2012;14:605. doi: 10.1093/europace/eur317. [DOI] [PubMed] [Google Scholar]
- 22.Polewczyk M, Polewczyk AM, Kutarski A, et al. Proximal end of 15-year-old ventricular electrode penetrating pulmonary tissue – a source of infection and a challenge for transvenous lead extraction. Postep Kardiol Interw. 2015;11:248–9. doi: 10.5114/pwki.2015.54026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein A, Mazzitelli D, Kolb C. Very-late proarrhythmia of a migrant pacemaker lead. J Electrocardiol. 2011;44:232–4. doi: 10.1016/j.jelectrocard.2010.08.002. [DOI] [PubMed] [Google Scholar]