Abstract
A temporary and reversible inhibition of the hypothalamo-pituitary-gonadal axis is adaptive when energy reserves are diminished, allowing individual survival and energy accumulation for eventual reproduction. The AMP-activated protein kinase (AMPK) works as a cellular sensor of the AMP to ATP ratio and ultimately of energy availability. Activation of AMPK suppresses ATP-consuming processes and stimulates ATP-producing pathways. The AMPK α2 catalytic subunit is expressed in multiple hypothalamic nuclei including those associated with reproductive control, ie, the anteroventral periventricular nucleus and the arcuate nucleus. Subsets of kisspeptin neurons in the anteroventral periventricular nucleus (20% in females) and arcuate nucleus (45% in males and 65% in females) coexpress AMPKα2 mRNA. Using the Cre-loxP approach, we assessed whether AMPKα2 in Kiss1 cells is required for body weight and reproductive function. The AMPKα2-deleted mice show no difference in body weight and time for sexual maturation compared with controls. Males and females are fertile and have normal litter size. The AMPKα2-deleted and control females have similar estradiol feedback responses and show no difference in Kiss1 mRNA expression after ovariectomy or ovariectomy plus estradiol replacement. In males, acute fasting decreased Kiss1 mRNA expression in both groups, but no effect was observed in females. However, after an acute fasting, control mice displayed prolonged diestrous phase, but AMPKα2-deleted females showed no disruption of estrous cycles. Our findings demonstrate that the AMPKα2 catalytic subunit in Kiss1 cells is dispensable for body weight and reproductive function in mice but is necessary for the reproductive adaptations to conditions of acute metabolic distress.
The AMP-activated protein kinase (AMPK) has a fundamental role in the regulation of whole-body energy homeostasis. AMPK works as a cellular sensor of the AMP to ATP ratio and, ultimately, energy availability (1–3). In conditions of decreased energy requirements (eg, the transition from fasted to fed state) or elevated levels of anorexigenic hormones (eg, leptin and insulin), AMPK is inhibited resulting in decreased ATP production (4, 5). During negative energy balance, AMPK activity increases and energy becomes available through the inhibition of anabolic pathways and stimulation of ATP production (2, 6).
The role of AMPK in metabolic control is well established (1–3). In addition, studies have postulated that AMPK works as a key molecular pathway that translates energy insufficiency to the reproductive neuroendocrine axis (7). In many species, when energy reserves are diminished, a temporary and reversible inhibition of the hypothalamo-pituitary-gonadal (HPG) axis is adaptive, allowing individual survival and energy accumulation for eventual reproduction (8–10). The negative impact of the metabolic dysfunction in the reproductive physiology has been demonstrated by different laboratories (8–13). However, the neural network and the molecular basis for this physiological regulation are not completely understood. One potential mechanism is the regulation of AMPK activity in kisspeptin neurons in response to energy demands (7).
Kisspeptin is a potent secretagogue of GnRH. Upon binding to the G protein-coupled receptor-54 (also known as Kiss1 receptor), kisspeptin activates the HPG axis and controls the estrous cycles and fertility (14, 15). Short periods of fasting decrease Kiss1 mRNA in the arcuate nucleus (Arc) and suppress estradiol-induced Kiss1 expression in the anteroventral periventricular nucleus (AVPV) of rats, concomitant with a decrease in the reproductive capacity (16, 17). Thus, we hypothesize that AMPK acts as an energy sensor in Kiss1 neurons to modulate the activity of the HPG axis.
AMPK is a heterotrimeric complex comprised of catalytic α-subunits (α1 or α2) and regulatory β- (β1 and β2) and γ (γ1, γ2 and γ3)-subunits. The α-subunits confer kinase activity, whereas the β and γ-subunits have structural and regulatory functions (1, 18). Because previous studies have shown that AMPKα2 is regulated by metabolic cues and is highly expressed in hypothalamic neurons (5, 19, 20), we focused our studies on the conditional deletion of α2-subunits in Kiss1 cells using the Cre-loxP system. We assessed whether AMPKα2 expression in Kiss1 cells is required for the regulation of body weight, reproductive function, and responses to acute metabolic challenges in adult mice.
Materials and Methods
Animals
Male and female Kiss1-Cre (21) (JAX mice, stock number 023426), AMPKα2-floxed (JAX mice, stock number 014142) (22), and R26 enhanced green fluorescent protein (eGFP) (JAX mice, stock number 004077) (23) mice were kept in the University of Michigan animal facility in a light- (12 h on/off) and temperature (21–23°C)-controlled environment with free access to water and food, unless otherwise stated. Mice were fed a phytoestrogen-reduced Envigo diet 2016 (16% protein/4% fat) except during breeding when they received higher protein and fat, phytoestrogen-reduced Envigo diet 2019 (19% protein/8% fat). All the procedures and experiments were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Committee on Use and Care of Animals (animal protocol number 04380).
Kiss1-Cre is a transgenic mouse line with Cre-recombinase driven by Kiss1 regulatory elements (21). The AMPKα2-floxed mouse is a knock-in targeted mutation with loxP sites flanking exon 2 of the Prkaa2 gene coding for the AMPKα2 catalytic subunit (22). The ROSA26 (R26)-eGFP mice carry targeted mutations of the R26 locus with a loxP-flanked transcription-blocking cassette, preventing the expression of the CAG promoter-driven eGFP reporter (23). Cre-mediated excision of the loxP-flanked transcription-blocking cassette allows the expression of the eGFP.
The Kiss1-Cre reporter mice were generated by crossing the Kiss1-Cre with the R26-eGFP mice. These mice (Kiss1-Cre/GFP) were used to reliably detect Kiss1-expressing neurons (21, 23). Mice with the deletion of AMPKα2 catalytic subunit in Kiss1 cells were generated by crossing the Kiss1-Cre/GFP and the AMPKα2-floxed homozygous mice. All mice were tail genotyped before defining the groups and after euthanasia using the primers described in Table 1. Tail DNA was extracted using Sigma RED Extract-N-Amp tissue PCR kit (catalog number XNAT). Cre activity and DNA recombination were assessed in the hypothalamus, cerebellum, liver, and tail tips using forward 5′-TAGTGATGTTAAATAGAAGATGAA-3′ and reverse 5′-CCCTGGTCCCCGAGTGA-3′ primers and the genotyping protocol described on the JAX mice web site. Tissues were processed with the Sigma kit and DNA recombination was defined by the presence of an approximately 366-bp band using a 100-bp DNA ladder (New England Biolabs). Groups were divided into wild-type controls (mice with Kiss1-Cre and no AMPKα2-loxP alleles or homozygous for AMPKα2-loxP and no Kiss1-Cre allele) and experimental (mice expressing at least one copy of Kiss1-Cre allele and homozygous for AMPKα2-loxP, named Kiss1-Cre AMPKα2loxP).
Table 1.
List of Primers Used for Genotyping
| Mice | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Kiss1-Cre | GCTCTGGTGAAGTACGAACTCTGA | TGCGAACCTCATCACTCGTTGCAT |
| AMPKα2-floxed | GCAGGCGAATTTCTGAGTTC | TCCCCTTGAACAAGCATACC |
| R26 eGFP | AAGTTCATTCGCACCACCG | TCCTTGAAGAAGAATGGTGCG |
Perfusion and histology
Mice were deeply anesthetized with isoflurane (Fluriso; Vet One) and perfused intracardially with 10% buffered formalin (Sigma). Brains were dissected and cryoprotected overnight at 4°C in diethylpyrocarbonate (DEPC)-treated 0.1 M PBS (pH 7.4), containing 20% sucrose. The brains were cut (30 μm sections) in the frontal plane in a freezing microtome. Five series were collected and stored at −20°C in cryoprotectant until processing for in situ hybridization and immunohistochemistry.
Reproductive phenotyping
Females were monitored for sexual maturation (first estrus) and estrous cyclicity, defined by vaginal cytology. Males and females were tested for fertility by mating within genotypes and with control breeders of proven fertility. Because metabolic dysregulation can affect the reproductive function, potential changes in body weight were monitored throughout the experiment.
Ovariectomy and estradiol replacement
Bilateral ovariectomy (OVX) was performed in mice anesthetized with isoflurane. A capsule, prepared as previously described (23), containing either 1 μg of estradiol (E2; 17β-estradiol 3-benzoate; Sigma) suspended in sesame oil (OVX+E2) or oil alone (OVX) was implanted under the skin at the time of the OVX. Mice were perfused 3 days (OVX+E2) or 10 days (OVX) later, and brains were processed for histology. Blood was collected from the right atrium before perfusion for hormonal assessment.
Single- and dual-label in situ hybridization/immunohistochemistry
Single-label in situ hybridization histochemistry (ISHH) for AMPKα2 and Kiss1 mRNA was performed in series of hypothalamic sections, as previously described (24). Briefly, tissue sections from control males and diestrous females (n = 4/group for AMPKα2 and Kiss1 mRNA) from OVX+E2 and OVX mice (n = 4/group, for Kiss1 mRNA) were mounted onto SuperFrost plus slides (Fisher Scientific), air dried overnight, and fixed in 4% paraformaldehyde in DEPC-treated PBS for 20 minutes. Tissue was dehydrated in increasing concentrations of ethanol, cleared in xylenes, rehydrated in decreasing concentrations of ethanol, and placed in prewarmed sodium citrate buffer (pH 6.0). Slides were microwaved for 10 minutes followed by dehydration in graded ethanol. The Kiss1 probe has been described and validated before (21), and the AMPKα2 riboprobe was produced from hypothalamic cDNA using T3 (CAGAGATGCAATTAACCCTCACTAAAGGGAGAGTGGTGACCCTCAAGACCAG) and T7 (CCAAGCCTTCTAATACGACTCACTATAGGGAGAGCCAGTCAAAAGAGCCAGTGA) primers. Single-label ISHH was initially performed to evaluate the distribution of AMPKα2 mRNA in males and females and to validate the riboprobe. Single-label ISHH was also performed to evaluate changes in Kiss1 mRNA in OVX and OVX+E2 females after the AMPKα2 deletion.
The 35S-labeled AMPKα2 and Kiss1 riboprobes were diluted to 106 cpm/mL in a hybridization solution containing 50% formamide, 10 mM Tris-HCl (pH 8.0), 5 mg tRNA (Invitrogen), 10 mM dithiothreitol, 10% dextran sulfate, 0.3 M NaCl, 1 mM EDTA, and 1× Denhardt's solution. This hybridization solution with the riboprobe was applied to each slide and incubated overnight at 57°C. Slides were washed in 2× sodium chloride sodium citrate buffer (SSC) and treated with 0.02% ribonuclease A (Roche) for 30 minutes. Sections were then subjected to stringency washes in SSC. Tissue was dehydrated in increasing concentrations of ethanol, and slides were placed in X-ray film cassettes with BMR-2 film (Kodak) for 2 days. Slides were then dipped in NTB2 autoradiographic emulsion (Kodak), dried, and stored in light-protected boxes at 4°C for 2 weeks. Finally, slides were developed with D-19 developer (Kodak), dehydrated in graded ethanol, cleared in xylenes, and coverslipped with DPX (Electron Microscopy Sciences).
Dual-label ISHH and immunohistochemistry (IHC) was performed in series of brain sections of male and diestrous female mice, as previously described (21, 23). This procedure was used to determine the colocalization of AMPKα2 in Kiss1 neurons (Kiss1-Cre/GFP mice) and to validate the deletion of AMPKα2 from Kiss1 neurons (Kiss1-Cre AMPKα2loxP mice, n = 4). Briefly, free-floating sections from males (n = 4) and diestrous females (n = 7) were rinsed in DEPC-treated PBS followed by 0.1% sodium borohydride for 15 minutes. Sections were treated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) for 10 minutes and then washed in 2× SSC. Next, the sections were incubated overnight at 50°C in the above-described hybridization solution containing the 35S-labeled AMPKα2 riboprobe. Subsequently, sections were treated with ribonuclease A and submitted to stringency washes in SSC. Sections were then incubated in anti-GFP (made in chicken, 1:5000, catalog number GFP-1010; Aves Labs; please see Table 2) overnight at room temperature. The anti-GFP antibody has been validated before by our group and others (21, 23, 25–28). The next day, sections were incubated for 1–2 hours in secondary antibody (biotin conjugated goat antichicken, 1:1000; Jackson ImmunoResearch) and further incubated in avidin-biotin complex (1:500; Vector Labs) for 1 hour. The peroxidase reaction was performed using 3,3′-diaminobenzidine tetrahydrochloride (Sigma) as chromogen. Sections were mounted onto SuperFrost plus slides (Fisher Scientific) and processed for standard autoradiographic procedures, as described above.
Table 2.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| GFP | Anti-GFP | Aves Labs, Catalog number GFP-1010 | Chicken, polyclonal | 1:5000 |
Hormone profile
The LH and FSH levels were assessed from blood samples taken from the heart before perfusion. After 40 minutes of incubation at room temperature, blood samples were centrifuged at 1000 × g for 20 minutes at 4°C. Serum was collected and stored at −20°C. The analyses of LH and FSH levels were performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, Virginia) using the EMD Millipore mouse/rat LH/FSH multiplex assay. The detection limits were 0.24 ng/mL for LH and 2.4 ng/mL for FSH.
Metabolic challenges
Female Kiss1-Cre AMPKα2loxP and control mice (4–5 mo of age) were monitored for estrous cyclicity for 3 weeks and were subjected to an acute metabolic stress. They were fasted for 24 hours on either the diestrus or estrus day, and the estrous cyclicity was monitored for another week. Males Kiss1-Cre AMPKα2loxP and controls (4–5 mo of age) and female controls (3 mo of age) were also fasted overnight and euthanized to evaluate changes in Kiss1, Tac2, and Dyn mRNA expression using quantitative PCR (qPCR).
Quantitative PCR
Kiss1-Cre AMPKα2loxP and control mice were deeply anesthetized with isoflurane and euthanized by decapitation. Blood was collected from the trunk, and the hypothalamus was rapidly dissected and frozen on dry ice. In females, hypothalamic blocks were divided in two, limited by an incision in the optic chiasm, another incision 1 mm anterior to the optic chiasm (to collect the preoptic area, containing the AVPV), and one incision across the mammillary bodies (to collect the mediobasal hypothalamus, containing the Arc). In males, only hypothalamic blocks containing the Arc were harvested. Lateral limits were defined by the optic tract, and superior limits were defined by the dorsal tip of the third ventricle. Tissues were stored at −80°C until qPCR was performed. The RNA was isolated using an RNA extraction kit and Qiazol reagent (miRNeasy; QIAGEN), followed by deoxyribonuclease treatment (DNA-Free; QIAGEN). The cDNA was synthesized using Superscript II and random primers (Invitrogen) according to the manufacturer's protocol. qPCR was performed on a CFX-384 Bio-Rad real-time PCR detection system (Bio-Rad Laboratories) using SYBR Green gene expression assays. Primers are described in Table 3. Fasting-induced changes in expression of Kiss1, Tac2, and Pdyn genes were evaluated. The increase in Npy gene expression was used as a positive control for the fasting protocol (29), and Gapdh was used as a housekeeping gene. Water instead of cDNA was used as a negative control. All samples (unknown and standard curves) were assessed in triplicate. The quantification of transcript for each gene was obtained by the delta-delta cycle threshold method (DDCt). The fold change of mRNA in the experimental group relative to the control group was determined by 2−DDCt. Data are shown as a percentage of the relative mRNA expression of the control group.
Table 3.
List of Primers Used for qPCR Studies
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Gapdh | GCTCATGACCACAGTCCATGC | GTTGGGGGGGGATAGGGCCTCTCTTG |
| Kiss1 | GGCAAAAGTGAAGCCTGGAT | GATTCCTTTTCCCAGGCAT |
| Npy | CAGAAAACGCCCCCAGAACAAGC | GGCAGACTGGTTTCAGGGGATGGAT |
| Pdyn | GTTGCTGTCAAGATCTGTTGC | ACCACGCCATTCTGACTCAC |
| Tac2 | AAGATGCCCGAGCTTTGAGGG | AACAGCATGGCGCTCCTCAT |
Quantification, data analyses, and production of digital images
All sections used for ISHH and ISHH/IHC were analyzed using an Axio Imager M2 microscope (Carl Zeiss). For single-label ISHH, the hybridization signal was estimated by the analysis of the integrated optical density (IOD) using the ImageJ software (http://rsb.info.nih.gov/ij), and comparisons among groups (diestrus, OVX, and OVX+E2) were carried out. Dark-field photomicrographs were acquired using the same illumination and exposure time for every section, and no image editing was performed. The IOD values for AMPKα2 and Kiss1 mRNA were calculated as the total IOD of a constant area subtracting the background. The background was obtained from adjacent nuclei that do not express Kiss1 mRNA. For dual-label ISHH/IHC assays, cells were considered dual labeled if the density of silver grains overlying the cytoplasm (GFP-ir) was at least 3 × greater than the background level. Only one representative section and one side of the brain were counted per mouse per group, and therefore, no correction for double counting was used. The AMPKα2-expressing cells were not individually counted because of the diffuse nature of the silver grains.
Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Inc). Comparison among groups was determined by a Student's t test or a two-way ANOVA followed by Tukey post hoc multiple comparison tests. Data are presented as mean ± SEM, and an α-value (P) less than .05 (5%) was considered significant. Post hoc power calculation for each experiment was conducted and type II error was considered at 20% threshold. Photomicrographs were produced by capturing images with a digital camera (Axiocam; Zeiss) mounted directly onto the microscope using the Zen software. Adobe Photoshop CS6 image-editing software was used to integrate photomicrographs into plates.
Results
AMPKα2 mRNA is widespread in the hypothalamus
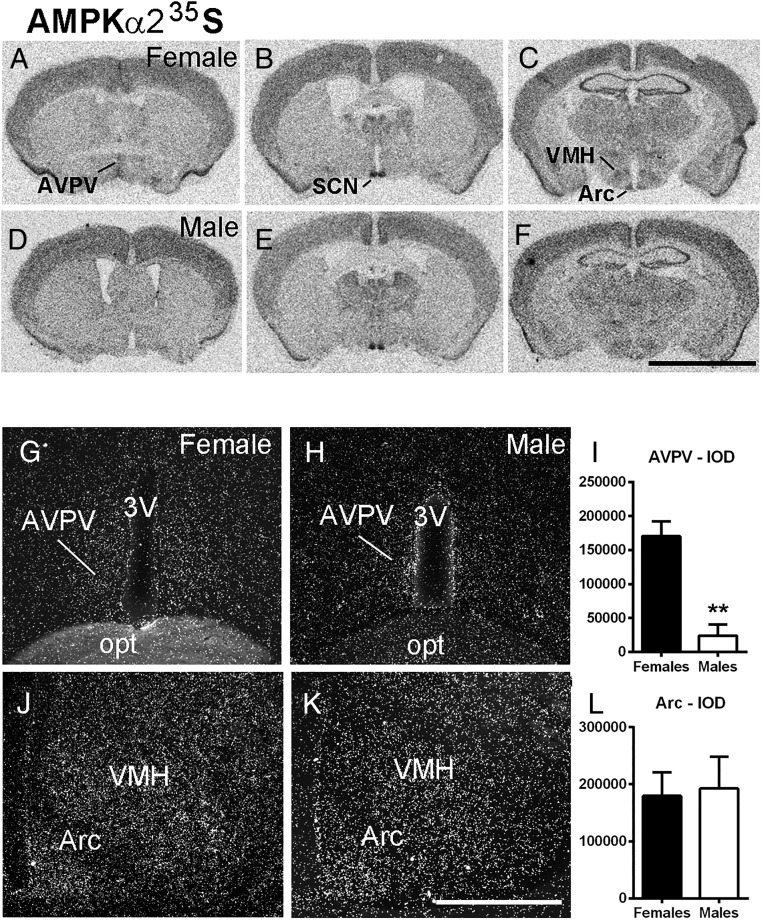
To determine the distribution of hypothalamic AMPKα2 mRNA, we performed single-label ISHH in control male (n = 4) and diestrous female mice (n = 7). We found dense expression of AMPKα2 mRNA in the suprachiasmatic nucleus, hippocampus, and piriform cortex in both sexes (Figure 1, A–F). Moderate AMPKα2 expression was found in the AVPV (Figure 1, A and D) of females, in the ventromedial nucleus of the hypothalamus, and in the Arc (Figure 1, C and F) in both sexes. Differences between sexes were not clear, except in sexually dimorphic nuclei. AMPKα2 was higher in the AVPV of female mice (P < .01, t = 5.48; df = 5) but not different in the Arc (P = .85, t = 0.18 df = 6, Figure 1, G–L).
Figure 1.
AMPKα2 mRNA is highly expressed in hypothalamic nuclei. A–F, Bright-field digital images showing hybridization signal in sections of the hypothalamus of a female (A–C) and a male (D–F) mouse. G, H, J, and K, Dark-field digital images showing the distribution of AMPKα2 mRNA in the AVPV and Arc of a female (G and J) and a male (H and K) mouse. I and L, Bar graphs showing quantification of hybridization signal (silver grains) using IOD comparing the AVPV and Arc of male and female mice. Note higher expression of AMPKα2 mRNA in the AVPV of females. opt, optic tract; 3V, third ventricle; SCN, suprachiasmatic nucleus; VMH, ventromedial nucleus of the hypothalamus. Scale bar, 3 mm (A–F); 200 μm (G, H, J, and K). Unpaired two-tailed t test (nonparametric) was used for the analysis. **, P < .01. F test to compare variance between groups showed no difference for data in panels I and L.
AMPKα2 is expressed in a subset of Kiss1 neurons
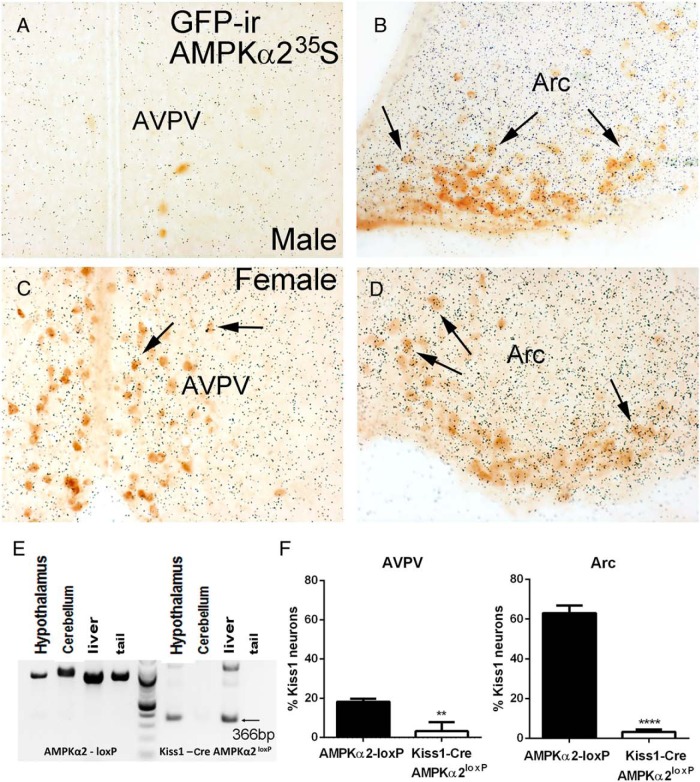
The degree of colocalization of AMPKα2 mRNA in Kiss1 neurons was determined by dual-label ISHH/IHC in the hypothalamic sections of Kiss1-Cre/GFP males (n = 4) and diestrous females (n = 7). We found virtually no expression of AMPKα2 in the few Kiss1 neurons of the male AVPV. About 20% (18.2% ± 0.9%) of the AVPV Kiss1 neurons of females coexpressed AMPKα2 mRNA. In the Arc, about 45% (42% ± 7.5%) of the Kiss1 neurons of the males and 65% (63% ± 2.2% in females) of Kiss1 neurons of the females coexpressed AMPKα2 mRNA (Figure 2, A–D).
Figure 2.
Expression of AMPKα2 in Kiss1 neurons and validation of the Kiss1-Cre AMPKα2loxP mouse model. A–D, Bright-field digital images showing the coexpression of AMPKα2 (silver grains) and Kiss1 (GFP immunoreactive [ir] cells) in the AVPV and Arc of male (A and B) and female (C and D) mice. E, PCR product showing DNA recombination in hypothalamus and liver of Kiss1-Cre AMPKα2loxP female mice (366 bp band, 100 bp DNA ladder). F, Graphs showing percentage of Kiss1 cells expressing AMPKα2 mRNA in control and Kiss1-Cre AMPKα2loxP female mice (n = 4/group). Unpaired two-tailed t test was used for the analysis. **, P < .05, ****, P < .0001 (t = 25.61, df = 4). F test to compare variance between groups showed no difference between groups for AVPV and Arc.
Kiss1-Cre AMPKα2loxP mice showed no changes in body weight and reproductive function
To determine whether AMPKα2 in Kiss1 cells is required for the control of body weight and reproductive function, we crossed the Kiss1-Cre/GFP and AMPKα2-floxed mice. For validation of the mouse model and Cre-induced DNA recombination, DNA was extracted from hypothalamus, cerebellum, liver, and tail, and DNA recombination was assessed using AMPKα2 primers. Only hypothalamus and liver showed a detectable PCR band (366 bp in addition to ∼960 bp for the control allele), indicating successful genomic recombination (Figure 2E). As expected, very few GFP+ neurons (one or two per brain) of Kiss1-Cre AMPKα2loxP mice had hybridization signal above background (Figure 2F).
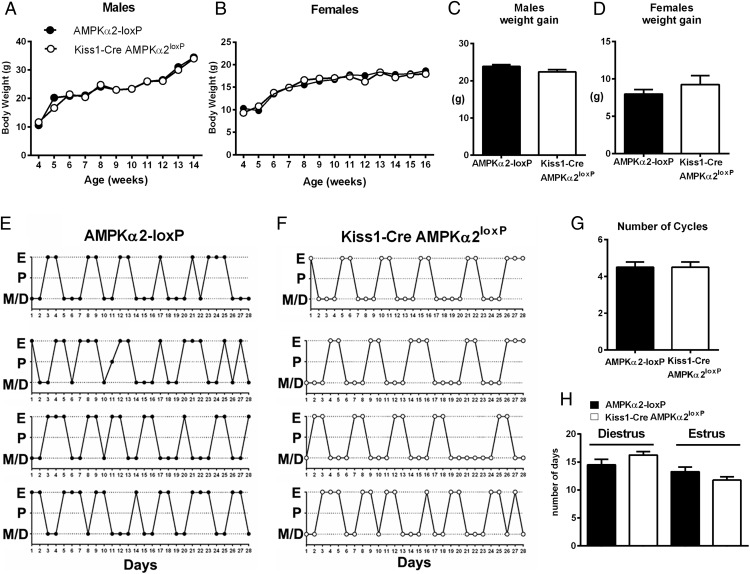
Three cohorts of Kiss1-Cre AMPKα2loxP females (n = 32 total) were evaluated and compared with control wild-type littermates (n = 40 total). Due to the AMPKα2 role in metabolism, the expression of Kiss1 in the mouse liver and the deleterious effects of metabolic dysfunction in the reproductive axis (10, 30, 31), we evaluated changes in body weight after the deletion of AMPKα2 in Kiss1 cells from 3 to 15 weeks of age in both males and females. No difference between genotypes was observed (n = 6–8 males, n = 9–12 females, Figure 3, A and B). At 14 (males) or 16 (females) weeks of age, no difference in weight gain was observed (P > .05, data analyzed by two tailed t test; males: t = 1.79 df = 9, and females: t = 0.95 df = 8).
Figure 3.
Selective deletion of AMPKα2 in Kiss1 neurons causes no changes in body weight and estrous cycles. A and B, Graphs showing progression of body weight of male and female Kiss1-Cre AMPKα2loxP (n = 8 males, n = 12 females) and control mice (n = 6 males, n = 9 females). Data were analyzed using a repeated-measures ANOVA. C and D, Graphs showing weight gain from weeks 4–14 in males and females (F test of variance did not show difference; t = 1.788, df = 9 for males, and t = 0.9411, df = 8 for females). No differences between genotypes were noticed, but differences in weight gain along time in both groups were detected. E and F, Illustration of estrous cycles of Kiss1-Cre AMPKα2loxP and control mice. G and H, Bar graphs showing number of cycles (G), number of days in diestrous phase, and number of days in estrous phase (H) in 28 days. No differences were detected. Data in C, D, G, and H were analyzed using a two-tailed t test. F test to compare variance between groups showed no differences. D, diestrus; E, estrus; M, metestrus; P, proestrus.
Control and mutant mice were assessed for sexual maturation. No difference in age at pubertal completion (44.4 ± 0.62 d in controls, n = 17 vs 44.9 ± 0.75 d in AMPKα2 deleted female mice, n = 9; data analyzed by two tailed t test, t = 0.4709 df = 24) was detected. The AMPKα2-deleted mice (16–20 wk of age) also showed normal estrous cycles compared with control littermates (Figure 3, E and F). No difference in the number of cycles, the number of days spent in diestrus, or the number of days spent in the estrous phase was found (Figure 3, G and H). Male and female Kiss1-Cre AMPKα2loxP mice were fertile, with no difference in the number of pups delivered compared with controls (P = .92, n = 9 controls, 6.1 ± 0.7 pups vs n = 15 mutant, 6.2 ± 0.73 pups, data analyzed by two tailed t test, t = 0.08148 df = 22).
Kiss1-Cre AMPKα2loxP females showed normal E2 feedback regulation
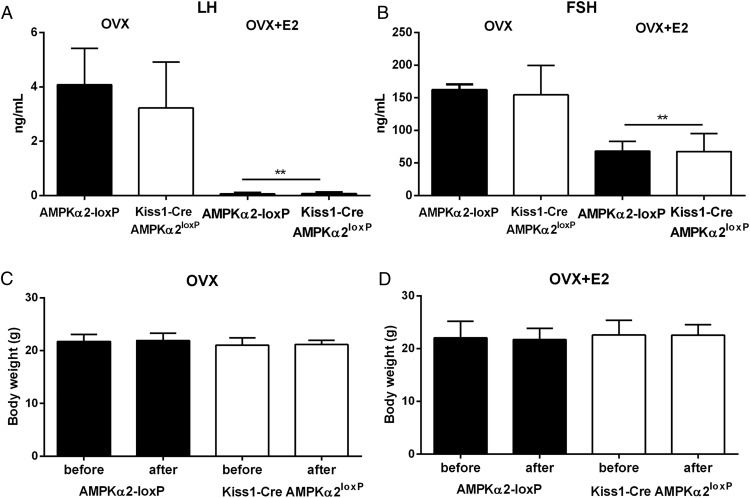
We next assessed whether AMPKα2 in Kiss1 neurons is required for E2 feedback regulation. Groups of mice were ovariectomized and treated with oil (OVX, n = 4 control and n = 8 experimental) or E2 (OVX+E2, n = 6 control and n = 9 experimental) in SILASTIC brand capsules (Dow Corning Inc). E2-induced change in uterine weight was used as control for the experimental protocol. A similar decrease in uterus weight was observed in both groups after a bilateral ovariectomy (0.026 ± 0.003 g in control vs 0.038 ± 0.004 g in mutant mice, P > .05; t = 1.950, df = 9), and a similar increase in uterus weight was apparent after E2 treatment (0.156 ± 0.012 g in control vs 0.124 ± 0.008 g in mutant mice, P > 0. 05; t = 2.146, df = 12). Data were analyzed using a two-tailed t test. Comparing both genotypes, we observed a similar increase in LH and FSH serum levels in OVX mice (P = .45 for LH and P = .78 for FSH) and a decrease in LH and FSH serum levels in OVX+E2 mice (P = .85 for LH and P = .95 for FSH; Figure 4, A and B). No change in body weight in OVX and OVX+E2 groups was observed during the period analyzed (P = .78 for OVX and P = .98 for OVX+E2; Figure 4, C and D). E2-associated changes in Kiss1 mRNA in the AVPV and Arc were also unaffected in AMPKα2-deleted mice (Figure 5). Data were analyzed using a two-way ANOVA and Sidak's multiple comparisons test. Only differences among treatment were noticed. No differences in the interaction or between genotypes were observed.
Figure 4.
Selective deletion of AMPKα2 in Kiss1 neurons causes no changes in E2 feedback regulation. A and B, Bar graphs showing levels of LH and FSH in Kiss1-Cre AMPKα2loxP and control mice after OVX and OVX+E2. C and D, Bar graphs showing body weight before and after OVX and OVX+E2 in Kiss1-Cre AMPKα2loxP (n = 8 OVX, n = 9 OVX+E2) and control (n = 4 OVX and n = 6 OVX+E2) mice. No differences between groups were observed for gonadotropins and body weight. An unpaired, two-tailed t test was used for the analysis. F test to compare variance between groups showed no differences. In panel A, LH of OVX (t = 0.7783, df = 9) and LH of OVX+E2 (t = 0.1440, df = 10) are shown. In panel B, FSH of OVX (t = 0.2782, df = 9) and FSH of OVX+E2 (t = 0.06039, df = 12) are shown. In panel C, change in body weight of OVX control (t = 0.1802, df = 4) and change of body weight of OVX mutant (t = 0.2720, df = 15) are shown. In panel D, change in body weight of OVX+E2 control (t = 0.1179, df = 9) and change of body weight of OVX+E2 mutant (t = 0.4467, df = 12) are shown.
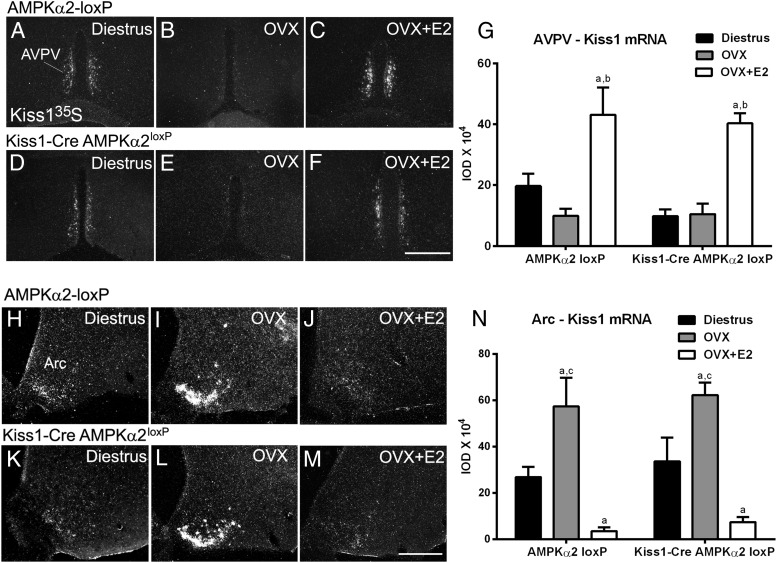
Figure 5.
No changes in E2-induced changes in Kiss1 mRNA expression in the AVPV and the Arc of Kiss1-Cre AMPKα2loxP mice (n = 4–6/group). A–F, Dark-field images showing Kiss1 mRNA in the AVPV of Kiss1-Cre AMPKα2loxP and control mice on diestrus, after OVX and OVX+E2. G, Bar graphs showing quantification of hybridization signal using IOD comparing the diestrous, OVX, and OVX+E2 females. H–M, Dark-field images showing Kiss1 mRNA in the Arc of Kiss1-Cre AMPKα2loxP and control mice in diestrus, after OVX and OVX+E2. N, Bar graphs showing quantification of hybridization signal using IOD comparing diestrous, OVX, and OVX+E2 females. a, P < .05 compared with diestrous females; b, P < .05 compared with OVX females; c, P < .05 compared with OVX+E2 females. Data were analyzed using a two-way ANOVA and Sidak's multiple comparisons test. Only differences among treatment were noticed. No differences in the interaction or between genotypes were observed.
Kiss1-Cre AMPKα2loxP mice are unresponsive to acute metabolic challenges
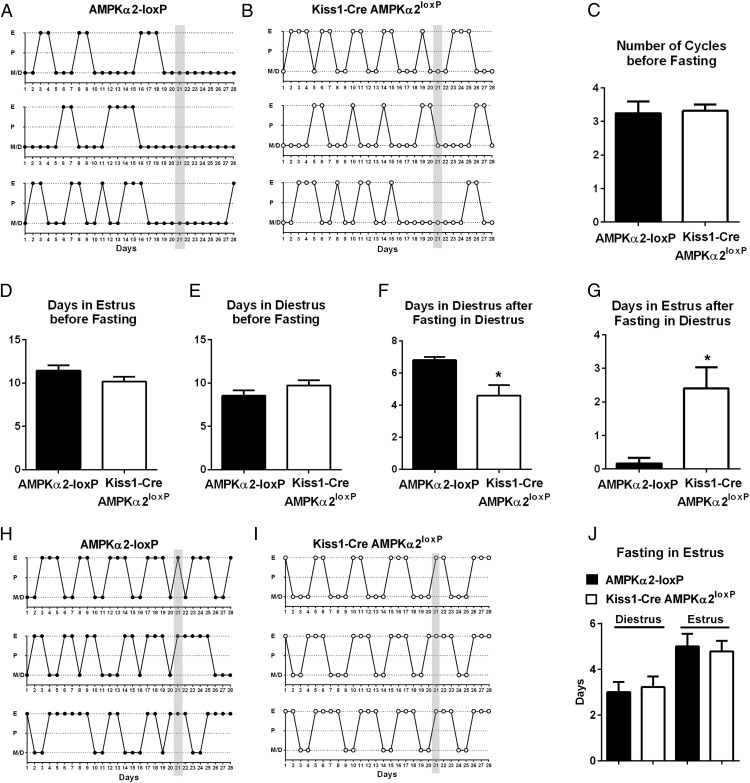
The AMPK signaling pathway is activated in states of negative energy balance (32–34). To assess whether AMPKα2 in Kiss1 neurons is required for the reproductive adaptations to negative energy balance in adult females, we evaluated changes in estrous cyclicity after acute overnight fasting (35, 36). Mice were initially evaluated for cyclicity (n = 12 controls and n = 22 Kiss1-Cre AMPKα2loxP mice, for 3 wk). No difference in the number of estrous cycles, days in estrus, and days in diestrus was observed before fasting (Figure 6, A–E). After 3 weeks, the mice were fasted in diestrus (Figure 6, A and B) or in estrus day (Figure 6, H and I). In control mice, fasting in diestrus extended the time spent in diestrus (n = 6, Figure 6, A, F, and G), but no effect was observed when mice were fasted in estrus day. However, no disruption in the estrous cycle was observed when Kiss1-Cre AMPKα2loxP mice were fasted in either the diestrus (n = 10) or estrus (n = 12) phase (Figure 6, B, I, and J).
Figure 6.
Acute fasting disrupted the estrous cycles of control mice but not of Kiss1-Cre AMPKα2loxP mouse. A and B, Illustration of estrous cyclicity of Kiss1-Cre AMPKα2loxP (n = 6) and control (n = 10) mice before and after fasting in diestrus (d 21, gray bar). C–E, Bar graphs showing the number of cycles, the number of days spent in diestrus, and the number of days spent in the estrus stage before fasting. No differences between genotypes were observed. F and G, Bar graphs showing the number of days spent in diestrus (F) and the number of days spent is estrus (G) after fasting during the diestrous stage. Note that mutant mice did not show anestrus in response to fasting, as observed for control mice. H and I, Illustration of estrous cyclicity of Kiss1-Cre AMPKα2loxP (n = 6) and control (n = 12) mice before and after fasting on estrus (d 21, gray bar). No difference was observed comparing genotypes. An unpaired, two-tailed t test was used for the analysis. F test to compare variance between groups showed no differences. *, P < .05. In panel C, t = 0.1804, df = 29; in panel D, t = 1.444, df = 28; in panel E, t = 1.306, df = 28; in panel F, t = 2.374, df = 13; in panel G, t = 2.652, df = 14; in panel J diestrus, t = 0.3274, df = 13; in panel J estrus, t = 0.2973, df = 12.
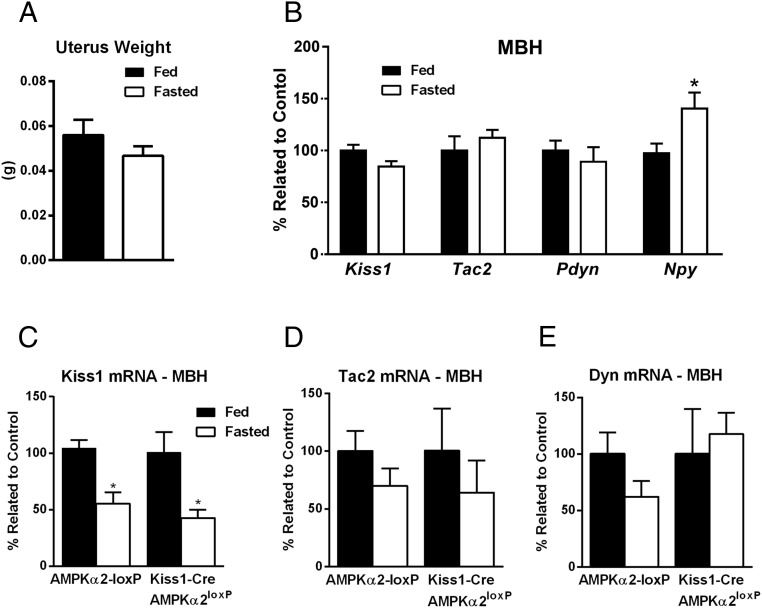
Previous studies in female rats have shown that food restriction (48–72 h of fasting) decreases hypothalamic Kiss1 mRNA levels (16, 17, 37). Because scarce data are available on Kiss1 gene expression after an acute fasting paradigm in female mice, we initially performed the test in control animals, applying the experimental design used to evaluate changes in the estrous cycles, ie, overnight fasting during diestrus phase. In two independent cohorts analyzed (n = 5–6 per group in both cohorts), we found that overnight fasting in normally cycling females euthanized in diestrus is not sufficient to reduce uterus weight or to change Kiss1 expression in control mice (cohort 1: P = .31, t = 1.068, df = 8 for the Arc and P = .24, t = 1.29, df = 6 for the AVPV; cohort 2: P = .08, t = 2.01, df = 9 for the Arc). In addition, no changes in either Tac2 or Pdyn gene expression were detected, whereas Npy expression, used as positive control for the experimental protocol, was increased in fasted females (Figure 7, A and B). Data were analyzed using a two-tailed t test.
Figure 7.
Kiss1 mRNA is decreased in male but not in female mice after overnight fasting. A, Bar graphs showing uterus weight in fed and overnight fasted control (n = 5 fed, n = 6 fasted) diestrous mice. B, Bar graphs showing relative expression of Kiss1, Tac2, Pdyn, and Npy genes in mediobasal hypothalamus (MBH) of fed and overnight fasted control (n = 5 fed, n = 6 fasted) diestrous female mice. C and D, Bar graphs showing relative expression of Kiss1 (C), Tac2 (D), and Pdyn (E) genes in mediobasal hypothalamus of fed and overnight fasted Kiss1-Cre AMPKα2loxP (n = 6 fed, n = 5 fasted) and control (n = 6 fed, n = 4 fasted) male mice. D, diestrus; E, estrus; M, metestrus; P, proestrus. *, P < .05. Data in panels A and B were analyzed using a two-tailed t test, and an F test of variance showed no differences between groups. Data in panels C–E were analyzed using a two-way ANOVA and Sidak's multiple comparisons test. Only differences among treatment were noticed. No differences in the interaction or between genotypes were observed.
To assess whether food restriction affects gene expression in Kiss1 neurons of males (38), mice were euthanized after an overnight fasting, and the mediobasal hypothalamus was used for qPCR analysis (n = 5–6/genotype including fed mice as control). Increased NPY mRNA was observed in both groups, suggesting that the fasting paradigm had the expected effect on the expression of relevant genes (data not shown). Both genotypes showed a fasting-induced decrease in Kiss1 mRNA expression, and no changes in either Tac2 mRNA or Dyn mRNA expression were observed (Figure 7, C–E). Data were analyzed using a two-way ANOVA and Sidak's multiple comparisons test. Only differences between treatments (fed vs fasted) were detected. No differences in the interaction or between genotypes were observed.
Discussion
In this study, we show that subsets of AVPV and Arc Kiss1 neurons coexpress the AMPKα2 catalytic subunit. Deletion of AMPKα2 in Kiss1 cells causes no changes in body weight and reproductive function. Males and females are fertile and produce normal litter size. The AMPKα2-deleted and control females have similar gonadotropin levels and Kiss1 mRNA expression in response to estradiol feedback assays. However, after an acute metabolic challenge (overnight fasting), control females remained in diestrus for about 6 days, but AMPKα2-deleted mice showed only a mild, nonsignificant disruption of the estrous cycle, suggesting a role for the α2 subunit as an energy sensor in the reproductive neural circuitry.
In most mammals, reproductive function is controlled by the availability of energy stores (39, 40). During negative energy balance, GnRH pulsatility is suppressed and fertility is decreased to preserve energy for basic physiological processes and survival (10). The reproductive axis therefore must respond to changes in circulating levels of metabolic cues and adapt to the immediate energy availability and demands. These cues originate from circulating hormones (eg, leptin, insulin, and ghrelin) or oxidable fuels (eg, glucose and fatty acid) (10, 30, 41–44). However, the neural circuitry and molecular pathways associated with the integration of these complex systems have been difficult to dissect, mainly due to the existence of highly redundant pathways originated from evolutionary constraints to allow species survival.
The AMPK is a metabolic-sensing enzyme that is activated by conditions of cellular ATP depletion, ie, hypoxia, glucose deprivation, excess exercise, and multiple forms of energy expenditure. Once activated, AMPK regulates energy balance by changing cellular activity or the responses to humoral cues with opposing effects in metabolic regulation (3, 5, 34, 45). In particular, leptin, insulin and ghrelin all modify AMPK activity. Whether the effects of these hormones via AMPK also affect the reproductive neuroendocrine axis has not been demonstrated.
Leptin has an inhibitory effect in hypothalamic AMPK, potentially by decreasing α2 subunit activity. A similar effect was described for insulin (5), and constitutive overexpression of hypothalamic AMPK decreases leptin or insulin action in food intake (5). Leptin-deficient mice and humans are obese and infertile, and states of low leptin decrease the activity of the reproductive neuroendocrine axis (30, 46–49). Leptin-deficient mice display decreased Kiss1 mRNA expression, suggesting that leptin may act upon Kiss1 neurons to control the reproductive axis (17, 38, 50–52). However, we have demonstrated that direct leptin action in Kiss1 neurons is neither required nor sufficient for reproductive function (53, 54). Likewise, whereas the lack of insulin signaling in the brain disrupts fertility, only a mild delay for sexual maturation was observed in mice lacking insulin receptor in Kiss1 neurons (44, 55, 56). These studies suggest that the ability of Kiss1 neurons to sense and translate the metabolic demands to the reproductive neuroendocrine axis is independent from leptin or insulin signaling. In addition, the lack of effect of deletion of AMPKα2 in Kiss1 cells on body weight, sexual maturation, and fertility indicates that the α2-subunit is not required in conditions of adequate energy availability. However, AMPKα2 proved to be necessary for the reproductive adaptations to acute metabolic distress. The lack of AMPKα2 in Kiss1 cells resulted in a maladaptive response and a disconnection between energy availability and reproductive function. Because of the well-defined role of Kiss1 neurons in female reproductive function (14, 15, 51, 57, 58), we postulate that this effect is mediated by AMPKα2 in hypothalamic Kiss1 neurons. However, additional scrutiny will be necessary to assess whether Kiss1 cells in the liver are associated with the AMPK action in the metabolic control of female reproductive function.
The signals associated with AMPK recruitment are not known. Whereas levels of leptin and insulin decrease, ghrelin increases in conditions of negative energy balance (59, 60). Ghrelin is an important signal for meal initiation. It promotes the storage of lipids as fat, induces glucagon release, and suppresses insulin secretion and sensitivity (61–63). At least part of ghrelin's effect in metabolism requires AMPK in hypothalamic neurons and ghrelin's stimulation of AMPK requires the α2-catalytic subunit (4, 20) .
In the brain, ghrelin decreases the pulsatile release of LH (41, 64–66). The exact sites of ghrelin action in the reproductive neural circuitry are not known, but the ghrelin receptors (GHSRs) are expressed in AVPV and Arc Kiss1 neurons (23). Both Kiss1 populations are highly responsive to changing levels of sex steroids (57), and we have recently shown that Ghsr gene expression is also modulated by E2 in Kiss1 Arc neurons (23). A high number (∼80%) of Kiss1 cells coexpresses GHSR mRNA in the Arc of females in a high estradiol milieu, whereas only about 20% of AVPV Kiss1 neurons coexpress GHSR. This degree of colocalization is strikingly similar to the findings of the current study, suggesting that ghrelin could potentially act through Kiss1 neurons to regulate the HPG axis by recruiting AMPK (37). Further studies are necessary to test this model.
Rodents and primates in acute metabolic distress exhibit transient anestrus and decreased pulsatile LH secretion (10, 30, 67–69). Several groups have suggested that in rats these effects are caused by decreases in Kiss1 mRNA expression and peptide production (16, 17, 37). In the present study, we observed that an acute metabolic challenge (overnight fasting) in normally cycling females prolonged the diestrus phase but did not change the levels of hypothalamic Kiss1 mRNA. It is important to emphasize that our findings are not comparable with previous published data. Other laboratories have demonstrated that 48–72 hours of fasting or chronic caloric restriction in female rats decreases hypothalamic Kiss1 gene expression (16, 17, 37, 70). In the present study, we subjected the mice to an overnight (∼16 h) food restriction. This approach represented a clear nutritional stress because it induced a prolonged diestrus phase in control mice but was not sufficient to reduce hypothalamic Kiss1 mRNA expression. Prolonged food restriction or control of E2 levels (eg, OVX and E2 replacement) may be necessary to detect a change. Notably, however, female mice with lack of AMPKα2 in Kiss1 cells were insensitive to the metabolic challenges showing normal cyclicity after acute fasting. This finding indicates that the adaptive response to acute nutritional stress was disrupted in the mutant mice.
We further reproduced previous findings in males (38) and found that change in hypothalamic Kiss1 gene expression remains intact in AMPKα2-deleted mice. Together with the data obtained in females, these findings suggest that the AMPK role mediating the acute effect of fasting in Kiss1 neuronal response is associated with changes in cell activity, not genomic expression. This is in line with previous studies showing that pharmacological manipulations of AMPK in hypothalamic slices alter the activity of GnRH neurons (71).
Glucose availability or alterations in the glucosensing response are also associated with the metabolic regulation of reproductive function (72, 73). Glucoprivation induced by fasting, intracerebroventricular injections of insulin, or 2-deoxyglucose all inhibit GnRH neurons and disrupt LH secretion and cyclicity. Acute blockade of AMPK decreased the inhibition of GnRH neuronal activity induced by low glucose levels (74), indicating that AMPK is an important mediator of glucosensing in GnRH cells. Whether this mechanism is reproduced in Kiss1 neurons still needs to be demonstrated.
Our present findings show that the AMPKα2 catalytic subunit in Kiss1 cells is dispensable for body weight and reproductive function in mice but is required for the reproductive adaptations to conditions of acute metabolic distress. Further studies are necessary to define the specific metabolic cue(s) that directly communicate states of acute energy depletion to Kiss1 neurons.
Acknowledgments
We thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for the hormone assays.
This work was supported by the National Institutes of Health Grant R01-HD-069702 (to C.F.E.), the National Science Foundation Graduate Research Fellowship (to J.L.C.), the Reproductive Endocrinology and Infertility Fellowship REI-MI (to E.M.), the National Council for Scientific and Technological Development (to B.C.B.), and Sao Paulo Research Foundation Grant FAPESP 2013/06281-1-Brazil (to M.A.T.). The University of Virginia Ligand Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMPK
- AMP-activated protein kinase
- Arc
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- DEPC
- diethylpyrocarbonate
- E2
- estradiol
- eGFP
- enhanced GFP
- GFP
- green fluorescence protein
- GHSR
- ghrelin receptor
- HPG
- hypothalamo-pituitary-gonadal
- IHC
- immunohistochemistry
- IOD
- integrated OD
- ISHH
- in situ hybridization histochemistry
- OVX
- ovariectomy
- qPCR
- quantitative PCR
- R26
- ROSA26
- SSC
- sodium chloride sodium citrate buffer.
References
- 1. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. [DOI] [PubMed] [Google Scholar]
- 3. Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. [DOI] [PubMed] [Google Scholar]
- 5. Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. [DOI] [PubMed] [Google Scholar]
- 6. Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol. 2006;574:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397:4–14. [DOI] [PubMed] [Google Scholar]
- 8. Knuth UA, Friesen HG. Starvation induced anoestrus: effect of chronic food restriction on body weight, its influence on oestrous cycle and gonadotrophin secretion in rats. Acta Endocrinol (Copenh). 1983;104:402–409. [DOI] [PubMed] [Google Scholar]
- 9. Loucks AB, Heath EM. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78:910–915. [DOI] [PubMed] [Google Scholar]
- 10. Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–E832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cameron JL. Nutritional determinants of puberty. Nutr Rev. 1996;54:S17–S22. [DOI] [PubMed] [Google Scholar]
- 12. Frisch RE. Fatness, menarche, and female fertility. Perspect Biol Med. 1985;28:611–633. [DOI] [PubMed] [Google Scholar]
- 13. Ronnekleiv OK, Ojeda SR, McCann SM. Undernutrition, puberty and the development of estrogen positive feedback in the female rat. Biol Reprod. 1978;19:414–424. [DOI] [PubMed] [Google Scholar]
- 14. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 15. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuzaki T, Iwasa T, Kinouchi R, et al. Fasting reduces the kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr J. 2011;58:1003–1012. [DOI] [PubMed] [Google Scholar]
- 17. Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–1097. [DOI] [PubMed] [Google Scholar]
- 18. Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. [DOI] [PubMed] [Google Scholar]
- 19. Minokoshi Y, Kim Y-B, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. [DOI] [PubMed] [Google Scholar]
- 20. Komori T, Doi A, Nosaka T, et al. Regulation of AMP-activated protein kinase signaling by AFF4 protein, member of AF4 (ALL1-fused gene from chromosome 4) family of transcription factors, in hypothalamic neurons. J Biol Chem. 2012;287:19985–19996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frazao R, Lemko HM, da Silva RP, et al. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab. 2014;306:E606–E614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garfield AS, Patterson C, Skora S, et al. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153:4600–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leshan RL, Opland DM, Louis GW, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patterson CM, Leshan RL, Jones JC, Myers MG., Jr Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frazao R, Cravo RM, Donato J, Jr, et al. Shift in Kiss1 cell activity requires estrogen receptor α. J Neurosci. 2013;33:2807–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. [DOI] [PubMed] [Google Scholar]
- 30. Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci. 2013;70:841–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song WJ, Mondal P, Wolfe A, et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr. 2014;34:31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. [DOI] [PubMed] [Google Scholar]
- 34. Lopez M, Nogueiras R, Tena-Sempere M, Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat Rev Endocrinol. 2016;12(7):421–432. [DOI] [PubMed] [Google Scholar]
- 35. Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. [DOI] [PubMed] [Google Scholar]
- 36. Nappi RE, Rivest S. Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neuronal system in cycling female rats: an evaluation at the transcriptional level. Endocrinology. 1997;138:1374–1384. [DOI] [PubMed] [Google Scholar]
- 37. Forbes S, Li XF, Kinsey-Jones J, O'Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–147. [DOI] [PubMed] [Google Scholar]
- 38. Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. [DOI] [PubMed] [Google Scholar]
- 39. Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol Endocrinol Metab. 1996;270:E1–E19. [DOI] [PubMed] [Google Scholar]
- 40. Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernandez-Fernandez R, Martini AC, Navarro VM, et al. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254–255:127–132. [DOI] [PubMed] [Google Scholar]
- 42. Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm. 2008;77:285–300. [DOI] [PubMed] [Google Scholar]
- 43. Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317. [DOI] [PubMed] [Google Scholar]
- 44. Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. [DOI] [PubMed] [Google Scholar]
- 45. Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. [DOI] [PubMed] [Google Scholar]
- 47. Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. [DOI] [PubMed] [Google Scholar]
- 48. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. [DOI] [PubMed] [Google Scholar]
- 49. Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–877. [DOI] [PubMed] [Google Scholar]
- 50. Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. [DOI] [PubMed] [Google Scholar]
- 51. Castellano JM, Navarro VM, Fernandez-Fernandez R, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. [DOI] [PubMed] [Google Scholar]
- 52. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cravo RM, Frazao R, Perello M, et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One. 2013;8:e58698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Donato J, Jr, Lee C, Ratra DV, Franci CR, Canteras NS, Elias CF. Lesions of the ventral premammillary nucleus disrupt the dynamic changes in Kiss1 and GnRH expression characteristic of the proestrus-estrus transition. Neuroscience. 2013;241:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qiu X, Dowling AR, Marino JS, et al. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Evans MC, Rizwan M, Mayer C, Boehm U, Anderson GM. Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J Neuroendocrinol. 2014;26:468–479. [DOI] [PubMed] [Google Scholar]
- 57. Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57:288–298. [DOI] [PubMed] [Google Scholar]
- 58. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Higgins SC, Gueorguiev M, Korbonits M. Ghrelin, the peripheral hunger hormone. Ann Med. 2007;39:116–136. [DOI] [PubMed] [Google Scholar]
- 60. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 61. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. [DOI] [PubMed] [Google Scholar]
- 62. Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. [DOI] [PubMed] [Google Scholar]
- 63. Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. [DOI] [PubMed] [Google Scholar]
- 64. Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780–785. [DOI] [PubMed] [Google Scholar]
- 65. Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 2004;89:5718–5723. [DOI] [PubMed] [Google Scholar]
- 66. Kluge M, Schussler P, Schmidt D, Uhr M, Steiger A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J Clin Endocrinol Metab. 2012;97:E448–E451. [DOI] [PubMed] [Google Scholar]
- 67. Cagampang FR, Maeda K, Yokoyama A, Ota K. Effect of food deprivation on the pulsatile LH release in the cycling and ovariectomized female rat. Horm Metab Res. 1990;22:269–272. [DOI] [PubMed] [Google Scholar]
- 68. Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology. 1991;128:1532–1540. [DOI] [PubMed] [Google Scholar]
- 69. Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab. 1991;73:35–41. [DOI] [PubMed] [Google Scholar]
- 70. True C, Kirigiti MA, Kievit P, Grove KL, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol. 2011;23:1099–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nagatani S, Bucholtz DC, Murahashi K, et al. Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology. 1996;137:1166–1170. [DOI] [PubMed] [Google Scholar]
- 73. Ohkura S, Tanaka T, Nagatani S, Bucholtz DC, Tsukamura H, Maeda K, Foster DL. Central, but not peripheral, glucose-sensing mechanisms mediate glucoprivic suppression of pulsatile luteinizing hormone secretion in the sheep. Endocrinology. 2000;141:4472–4480. [DOI] [PubMed] [Google Scholar]
- 74. Roland AV, Moenter SM. Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol. 2011;25:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]