Abstract
Circadian rhythms synchronize physiological processes with the light-dark cycle and are regulated by a hierarchical system initiated in the suprachiasmatic nucleus, a hypothalamic region that receives direct photic input. The suprachiasmatic nucleus then entrains additional oscillators in the periphery. Circadian rhythms are maintained by a molecular transcriptional feedback loop, of which brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1) is a key member. Disruption of circadian rhythms by deletion of the BMAL1 gene (Bmal1 knockout [KO]) induces a variety of disease states, including infertility in males, due to unidentified mechanisms. We find that, despite normal sperm function, Bmal1 KO males fail to mate with receptive females, indicating a behavioral defect. Mating is dependent on pheromone detection, as are several other behaviors. We determined that Bmal1 KO males also fail to display aggression and avoidance of predator scent, despite intact main olfactory function. Moreover, the vomeronasal organ, a specialized pheromone-responsive organ, was also functionally intact, as determined by calcium imaging in response to urine pheromone stimulus. However, neural circuit tracing using c-FOS activation revealed that, although Bmal1 KO males displayed appropriate activation in the olfactory bulb and accessory olfactory bulb, the bed nucleus of the stria terminalis and the medial preoptic area (areas responsible for integration of copulatory behaviors) failed to activate highly in response to the female scent. This indicates that neural signaling in select behavioral centers is impaired in the absence of BMAL1, likely underlying Bmal1 KO male copulatory defects, demonstrating the importance of the BMAL1 protein in the maintenance of neural circuits that drive pheromone-mediated mating behaviors.
Circadian rhythms are involved in a wide range of physiological processes, including metabolism, stress, aging, and fertility (1–4). They are regulated by a transcriptional feedback loop comprised of the core clock proteins, brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein (BMAL), circadian locomotor output cycles kaput (CLOCK), PERIOD, and CRYPTOCHROME. Light input via the optic nerve synchronizes the suprachiasmatic nucleus (SCN), the central pacemaker located in the hypothalamus, to external light cues. The SCN coordinates peripheral clocks, aligning clock gene expression in peripheral tissues with the day-night cycle. In most peripheral tissues, circadian rhythms are maintained in the absence of cues from the SCN through core clock feedback loops (5–7).
Mutant mice lacking the BMAL1 protein (also called MOP3 and ARNTL) have abnormal circadian rhythms (8). These Bmal1 knockout (KO) mice can still respond to photic input and have normal activity rhythms under a regular light-dark schedule but become arrhythmic in constant darkness (8). Bmal1 KO mice exhibit several physiological abnormalities, even when maintained in a regular light-dark schedule, including advanced aging, heart defects, diabetes, and increased reactive oxygen species accumulation (1–3). Additionally, Bmal1 KO males and females are completely infertile (4, 9). Bmal1 KO female infertility is caused by early embryo loss due to insufficient ovarian progesterone synthesis (4) as well as neuroendocrine defects causing abnormal estrous cycles and loss of the preovulatory LH surge (10). Conversely, the failure of male Bmal1 KO to sire pups is not yet fully understood. Whereas sperm counts in male Bmal1 KO mice are slightly decreased, sperm can still fertilize oocytes in vitro, suggesting that abnormal sperm function is not the primary cause of Bmal1 KO male infertility (9). In the absence of spermatogenic defects, another possible mechanism of male Bmal1 KO inability to produce litters is neuroendocrine dysfunction. The primary male sex steroid, T, is necessary for both spermatogenesis and the regulation of male mating behaviors (11). Bmal1 KO males have moderately decreased levels of T, which supports the possibility that T-dependent mating behaviors may be impaired.
Here we examine the following three possible mechanisms for the lack of fertility in Bmal1 KO males: 1) Bmal1 KO males may have a defect in the hypothalamic-pituitary-gonadal (HPG) axis that impacts the endocrine regulation of reproductive function; 2) Bmal1 KO males may have a defect in olfaction, such that they cannot detect pheromones critical for eliciting mating behavior; or 3) there may be a disruption in the neural circuit of mating behavior, such that one or more Bmal1 KO neural substrates cannot respond properly, or at all, to essential mating-related pheromonal cues. To tease apart these possibilities, we examine the role of Bmal1 in the male HPG axis by evaluating the hypothalamic and pituitary gene expression and reproductive hormone levels critical for reproductive success. We complement this with assessments of the ability of Bmal1 KO males to detect pheromones and analyses of the patterns of brain activation after exposure to receptive female pheromones. Through this work, we provide novel findings that establish the behavioral basis underlying infertility in the absence of BMAL1 function.
Materials and Methods
Mice
Bmal1 floxed mice were obtained from Jackson Laboratories. Global Bmal1 KO males were generated by crossing a Bmal1 floxed mouse with a ZP3-Cre mouse (12) that results in germline deletion of Exon 8 of the Bmal1 gene to produce a nonfunctional protein in all cells throughout the body. The floxed allele (13) deletes the same portion of the gene (most of the basic helix-loop-helix containing exon 8 as well as exon 9) that is deleted in the Bmal1 KO mouse reported previously (8). The Bmal1 KO line was validated by wheel-running experiments (data not shown), demonstrating arrhythmicity in constant darkness. Bmal1 KO males were obtained through heterozygous matings, and wild-type (WT) littermates were used as controls in all experiments. Males aged 8–12 weeks were used in all experiments and were group housed unless otherwise stated. Genotyping primers are shown in Table 1. Animals were housed in a 12-hour light, 12-hour dark cycle (lights off at 6:00 pm) with ad libitum access to food and water unless noted. Locomotor activity of Bmal1 KO mice is maintained in normal light-dark conditions (8). All procedures were approved by the University of California, San Diego, Animal Care and Use Committee.
Table 1.
Primers
| Gene | Forward Primer (5-′) | Reverse Primer (3-′) |
|---|---|---|
| GnRH | ACACTTGGTTGAGTCTTTCCA | TGGCTTCCTCTTCAATCAGAC |
| Kiss1 | CCCTTCCTCCCAGAATGATC | TCCCAGGCATTAACGAGTTC |
| Vasopressin | CTACTTCCAGAACTGCCCAAG | GCAGATGCTTGGTCCGAAG |
| Vasoactive intestinal peptide | ACTAGCCAGCTACAGCCAAC | TTCTGGCTTCCATCTCGGTG |
| Tyrosine hydroxylase | AAGATCAAACCTACCAGCCG | TACGGGTCAAACTTCACAGAG |
| Androgen receptor | CGACTATTACTTTCCACCCCAG | TGCTGGCACATAGATACTTCTG |
| Neurokinin B | CTGCTTCGGAGACTCTACG | GGTTGGCTGTTCCTCTTGC |
| Dynorphin | GTGTGCAGTGAGGATTCAGG | AGTCATCCTTGCCACGGAGC |
| Actin | ACCTTCTACAATGAGCTGCG | CTGGATGGCTACGTACATGG |
| Kiss1 primers (AVPV micropunches) | CAAAAGTGAAGCCTGGATCC | GTTGTAGGTGGACAGGTCC |
| Actin (AVPV micropunches) | GCTGTGCTATGTTGCTCTAGACTT | CATAGAGGTCTTTACGGATGTCAAC |
Statistics
Statistical analyses were performed using an unpaired two-tailed Student's t test, Mann-Whitney, one-way ANOVA, or two-way ANOVA, followed by Tukey post hoc analysis as indicated in the figure legends, with P ≤ .05 to indicate significance.
Blood serum collection and hormone measurement
Blood was collected immediately after the animals were killed via arterial collection. For hormone assays, 50 μL of tail vein blood was collected into a capillary tube. Blood was allowed to clot for 90 minutes at room temperature and then centrifuged at 2000 × g for 15 minutes. Serum was collected and stored at −80°C until the time of assay. FSH, LH, and T were measured at the University of Virginia Hormone and Ligand Core (RRID:SCR_004318). Intraassay and interassay coefficients of variation (CVs) for LH were 4.5 and 8.3, respectively, and the detection limit was 0.04 ng/mL. Intraassay and interassay CVs for FSH were 6.9 and 9.4, respectively, and the detection limit was 2.0 ng/mL. For T, intraassay and interassay CVs were 4.5 and 8.3, respectively.
GnRH and kisspeptin challenges
For GnRH and kisspeptin challenges, a baseline blood sample was first collected via the tail vein. One microgram per kilogram GnRH (Sigma; L7134 or 30 nm) or kisspeptin (Tocris; 4243) was then injected ip, and tail vein samples (10 μL) were collected after 5, 10, 15, 25, and 45 minutes. Blood was allowed to clot for 90 minutes and then centrifuged at 2000 × g for 15 minutes. Serum was collected and stored at −80 until the LH measurement. Samples were run in a singlet on MILLIPLEX (number MPTMAG-49K; Millipore) using a Luminex Magpix (LH: lower detection limit 5.6 pg/mL, intra-assay coefficient of variance 15.2, and inter-assay coefficient of variance 4.7%.
Preparation of stimulator female mice
Female mice aged 8 weeks were ovariectomized and implanted with a low-dose estrogen pellet (SILASTIC brand tubing; Dow Corning Corp; inner diameter 1.0 mm, outer diameter 2.16 mm) containing crystalline 17-β E2 (Sigma; catalog number 8875) dissolved in SILASTIC brand medical adhesive at 1 μg/20 g body weight. One day prior to testing, females were injected with 1 μg of estradiol benzoate (Sigma; catalog number 8515) in sesame oil (100 μL) followed by 500 μg of progesterone (Sigma; catalog number 8783) in sesame oil (100 μL) at 2:00 pm on the day of testing.
Chemoinvestigation
After lights out (6:00 pm/Zeitgeber time 12), an estrous female was placed into each male's cage, and the behavior was analyzed by video recording under dim red light. The total time of the anogenital sniffing was recorded for 5 minutes after the introduction of the estrous female.
Aggression
Male mice were singly housed for 2 weeks prior to experimentation. A resident-intruder paradigm was used, in which the intruder was placed into the home cage of the resident male. Resident aggression assays reflect the capacity of the test mouse (resident) to be aggressive toward an intruder male to defend their territory. Resident males were socially isolated for 2 weeks prior to the experimentation to increase aggressive responsiveness to intruders (14). In each aggression trial, opponent mice (intruders) were the same age or younger (but at least 7 wk of age) and the same weight or less than the resident male (14). Experiments were recorded, and the total time spent performing aggressive behaviors over a 15-minute period was recorded manually. Aggressive behaviors included wrestling, biting, and scratching. Animals were monitored carefully and trials were ended early if the aggressive time exceeded 2 minutes 30 seconds or if the animal showed an evidence of bleeding.
Mounting assay
Male mice were placed in an empty cage and provided with a hormonally primed estrus stimulator female mouse for 30 minutes (after 6:00 pm under dim red lights). The first two mating trials (1 wk apart) were considered training and not scored because experience has been demonstrated to facilitate rodent sexual behavior. This was done to ensure that the failure of Bmal1 KO mice to mount was not due to a lack of significant exposure to an estrus female (15). The third mating trial was videotaped and total number of mounts was scored manually from each session. All mating trials were performed between 6:00 and 8:00 pm because male mating behavior has been demonstrated to occur in this time frame.
Fear behavior
Male mice (naïve to predator scent) were habituated for 10 minutes in a clean cage. Mice were then presented with 60 μL of fox urine (16) (1:10 dilution in H2O, 0.45 μm filter sterilized) on a 1-in. square of Whatman paper encased in a plastic cassette. Behavior was recorded over a 15-minute period and quantified manually. Percentage of time spent in a predefined area near the fox scent or away from the fox scent was calculated.
Gonadectomy and T pellet implantation
Male mice aged 8–10 weeks were anesthetized with isoflurane, bilaterally gonadectomized (GDX), and implanted sc with a SILASTIC brand (Dow Corning Corp; internal diameter 1.02 mm; external diameter 2.16 mm) capsule-packed T (Sigma; T1500; 6 mm). These implants have been shown previously to produce elevated physiological levels of T (11.1 ± 0.8 ng/mL) (17). Animals were allowed to recover 2 weeks before the administration of behavioral tests, a time frame that has been previously reported to restore mating behaviors in castrated mice (18).
Anteroventral periventricular (AVPV) micropunches
Kisspeptin was measured in the AVPV of Bmal1 KO males by performing micropunches of the AVPV/periventricular nucleus (2 mm diameter punches from 400 μm thick brain sections) (described in reference 19) from intact female WT, male WT, and male Bmal1 KO mice. RNA was extracted using the RNeasy lipid tissue minikit (QIAGEN), and 500 ng of total RNA was reverse transcribed using the Omniscript reverse transcription kit (QIAGEN), and the cDNA was stored at −20°C until use in quantitative real-time PCR (qPCR).
To detect Kiss1 in micropunch cDNA using qPCR, quantitative RT-PCR was performed on each cDNA sample in duplicate using the Bio-Rad CFX Connect real-time system (Bio-Rad Laboratories) and the Quantitect SYBR Green PCR kit. Standard curves were generated for each product using a dilution series of cloned cDNAs for Kiss1 and β-actin to quantify the abundance of cDNA in each sample. The quantitative RT-PCR cycling parameters were as follows: one cycle of 95°C for 15 minutes, followed by 40 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Data collection was taken at the 60°C annealing phase of each cycle.
Quantitative RT-PCR
Quantitative RT-PCR was performed on Bio-Rad CFX Connect (Bio-Rad Laboratories) using primers described in Table 1. Briefly, whole pituitary or hypothalamus was homogenized in 1 mL Trizol (Invitrogen) and passed through a 26-gauge needle. RNA was extracted with 200 μL of chloroform and precipitated with 500 μL of isopropanol and 1 μL of linear acrylamide (Ambion) as a coprecipitate. The pellet was then resuspended in ribonuclease- and deoxyribonuclease-free water, and genomic DNA was removed with Turbo DNA-free (Ambion) following the manufacturer's instructions. One microgram of total mRNA was converted to cDNA using iScript cDNA synthesis kit (Bio-Rad Laboratories). Twenty micrograms of cDNA were loaded in each qPCR reaction. All primers were tested for efficiency and specificity (Table 1). To ensure the presence of a single product, a dissociation curve was performed after each run. Data were collected from threshold values from the Bio-Rad CFX Manager 3.0 software (Bio-Rad Laboratories).
Buried food test
The buried food test was performed according to previously published methods for evaluating mouse olfaction (20). Briefly, male mice were fasted overnight and then placed in a new cage with a surplus of sterile bedding. A single pellet of standard mouse chow was hidden from view under approximately 3 cm of bedding, and a single mouse was placed in the cage and the latency to find the food pellet was recorded. All experiments were performed in the morning after an overnight fast.
Territorial marking assay
Prior to the assay, male mice were exposed to estrous females in a mating trial for 30 minutes after 6:00 pm under dim red lights at least three times. Estrous urine was collected from ovariectomized females implanted with an estrogen pellet. One day prior to testing, 1 μg of estrogen suspended in sesame oil was injected sc at 9:00 am. On the following day at 2:00 pm, 500 μg of progesterone was injected. Male mice aged 8–12 weeks were placed individually in a sterile polycarbonate cages for 10 minutes to habituate. Mice were then moved to a clean polycarbonate cage for another 10 minutes. After this habituation period, a piece of Whatman paper was placed on the bottom of the cage and left for 10 minutes to establish baseline urine marking. Then the first piece of Whatman paper was removed and a new piece was placed in the cage. Fifty microliters of urine from an estrous female were spotted into the center of the Whatman paper and left for 10 minutes. The Whatman paper was then dried overnight and sprayed with 0.2% ninhydrin (Sigma) diluted in 100% EtOH and air dried until urine spots developed a purple color. Papers were photographed and images were converted to binary and percentage of the paper marked by urine was determined using Image J Fiji software.
Vomeronasal organ (VNO) neuron function
Transient increases in free Ca2+ concentration in dissociated VNO neurons were determined by ratiometric Fura-2 fluorescence as described (21, 22). Briefly, males were killed, and VNO tissue was collected and dissociated and loaded with Fura-2 to detect increases in CA2+, indicative of neuron firing. VNO neurons were perfused with freshly collected whole male urine followed by the high-molecular-weight fraction. The high-molecular-weight urine fraction was isolated using Amicon Ultra centrifugal filters (Millipore) to collect proteins larger than 10 kDa. Three WT and three Bmal1 KO mice were used to image a total of 2280 and 2370 vomeronasal sensory neurons, respectively.
c-FOS immunohistochemistry
Males were isolated from a female scent for at least 2 days prior to the experiment and, on the day of the experiment, singly housed in a clean cage for 4 hours prior to exposure to the female scent. Male mice were then exposed (at 6:00 pm under dim red light) to bedding (5 mL) and urine (60 μL) collected from hormonally primed estrous stimulator female mice, which was placed on the bottom of the cage to allow direct contact. Both WT and Bmal1 KO males visibly investigated and made nasal contact with the female bedding. After 90 minutes, males were killed and brains were collected in fixative (6:3:1 mixture of absolute alcohol, 37% formaldehyde, and glacial acetic acid). Brains were fixed overnight and then dehydrated in 70% ethanol and processed for paraffin sectioning. Brain sections were cut at 12 μm on a microtome, dried, and baked at for 1 hour 60°C. Sections were deparaffinized and rehydrated, endogenous peroxidases were quenched with 0.03% hydrogen peroxide, and antigens were retrieved with 10 mM sodium citrate (pH 6). Sections were blocked with goat serum and stained with c-FOS antibody (Table 2; Santa Cruz Biotechnology; catalog number sc-52 RRID:AB_10160513; 1:1000). Goat antirabbit secondary was applied for 1 hour, and then c-FOS positive cells were visualized with 3,3′-diaminobenzidine. Neuroanatomical landmarks were used to identify the region of interest (ROI) as depicted in the corresponding figure. Each biological replicate consisted of quantified c-FOS nuclei per the defined ROI for a minimum of three unilateral sections from each experimental group. c-FOS-positive cells were quantified by an experimenter blinded to the treatment group. The numbers from each biological replicate were then averaged across all the animals in that group. Cells were quantified in the regions specified using Image J Fiji software (RRID:SCR_002285). Briefly, images were converted to eight bits, the threshold was adjusted to include positively labeled cells, and the total number of labeled cells per the defined ROI were analyzed from the selected brain region. Particle detection was limited to a minimum of (50)2 pixels to discriminate c-FOS positive nuclei from noise. Quantified cells were marked and cross-referenced with the original image to ensure accuracy. Slides were coded to blind the researcher to treatment group during analysis.
Table 2.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| c-Fos (N terminus) | c-FOS antibody (4) | Santa Cruz Biotechnology, sc-52 | Rabbit, polyclonal | 1:1000 |
Results
Bmal1 KO males are infertile and fail to mate with receptive females
We determined that Bmal1 KO males were unable to mate by evaluating the presence of a copulatory plug in WT females after cohabitation with either a WT or Bmal1 KO male. Although females housed with a WT male showed a plug after an average of 4 days, Bmal1 KO males never plugged any females, corroborating previous data (2, 9, 23, 24) (Figure 1A).
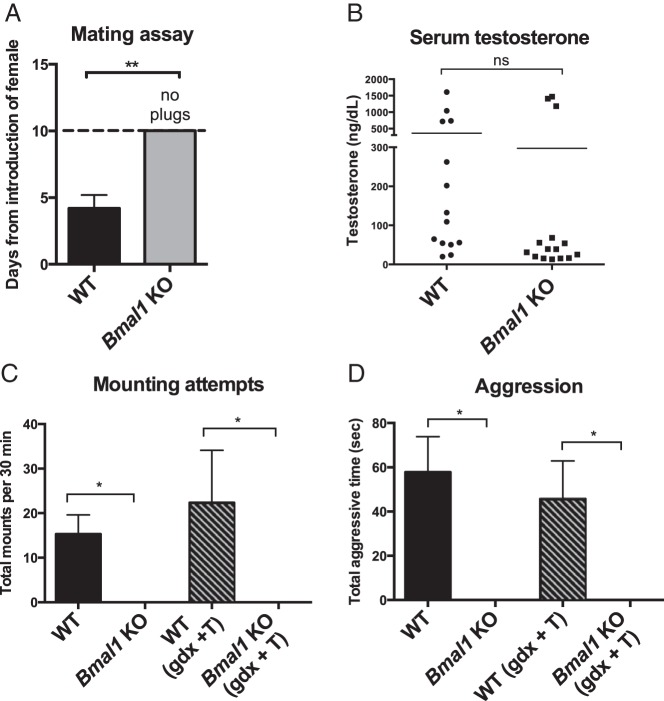
Figure 1.
Mating and aggressive behavior in Bmal1 KO males. A, Male mating assay: days until appearance of copulatory plug after introduction of an estrous WT female (n = 4, Student's t test). B, Serum T measurement in WT and Bmal1 KO males (n = 10–12, Mann-Whitney test). C, Mating behavior: total number of mounting attempts in 30 minutes during a mating trial with a receptive WT female (n = 9 intact, 3–4 GDX + T, one way ANOVA followed by Tukey post hoc). D, Aggression (resident): aggressive behavior during introduction of a WT male into the test subject's (resident's) home cage (n = 5–6 intact, 3–4 GDX + T). *, P < .05; **, P < .01.
Because vaginal plugs were not identified in any of the female WT mice paired with male Bmal1 KO mice, this indicated that Bmal1 KO males did not successfully mate with females. Therefore, we directly examined Bmal1 KO mounting behavior. Bmal1 KO males were paired with receptive (estrous) females for 30 minutes and the total number of mounts was recorded. During a 30-minute behavioral trial, WT males mounted an average of 15 times, whereas Bmal1 KO males displayed no mounting behavior (Figure. 1C).
Given that sexual and aggressive behaviors are positively linked and both behaviors are mediated by pheromones (21, 25, 26), we next investigated whether Bmal1 KO males exhibited impaired aggressive behavior in addition to their impaired sexual behavior. Normally, WT males will defend their home territory by displaying aggressive behavior to protect their territory. To test this behavior, a WT male is placed into the home cage of the test animal in an assay called the resident paradigm. When WT males were placed in the home cage of a WT male, they displayed, on average, 58 seconds of aggressive behavior. However, when Bmal1 KO males were placed into the home cage of a WT male, no aggression was observed (Figure 1D).
Because T is a critical mediator of male reproduction (both for regulating sperm production and mating behavior), we next measured serum T concentrations. Serum T was lower in Bmal1 KO males (Figure 1B). Although lower than in WT males, the T levels observed in the KO mice were still within the range sufficient to facilitate spermatogenesis in mice (9). Importantly, T is normally secreted in a pulsatile manner, and serum measurements taken at only a single time point may not provide a full representation of T secretion (27), so we cannot conclusively state that T secretion is normal in Bmal1 KO males. To ensure that inadequate T levels were not responsible for the failure to mate, an additional cohort of WT and Bmal1 KO males was gonadectomized and administered exogenous T to normalize T levels (GDX + T). GDX + T treatment evoked mating behavior in WT males but did not rescue mounting behavior in the Bmal1 KOs (Figure 1C), indicating that the failure of Bmal1 KO males to mount is not due to low circulating T. GDX + T also failed to rescue aggressive behaviors in the resident aggression paradigm (Figure 1D).
Fear response to predator scent is also absent in Bmal1 KO males
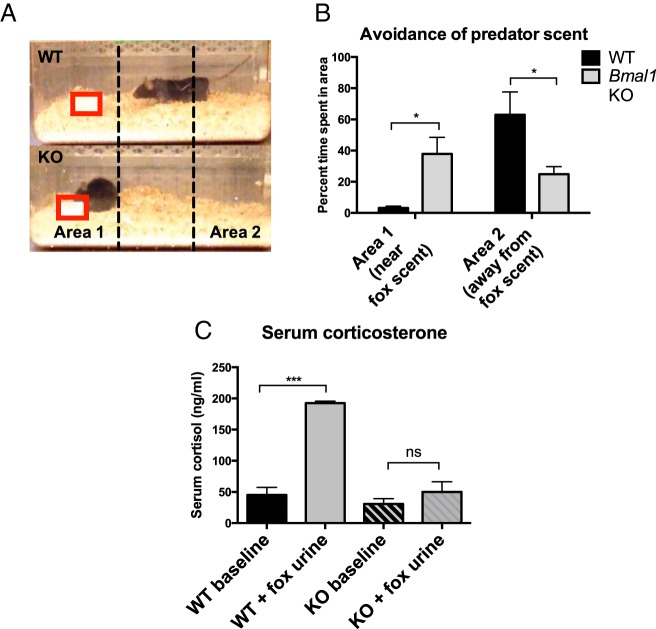
The failure of Bmal1 KO males to mate or display aggression, two behavioral responses driven by pheromonal stimuli, indicates that Bmal1 KO males may have a defect in the ability to detect or respond to pheromonal cues. Another behavioral response mediated by pheromones is the fear response to predator scent. Upon exposure to predator scent (diluted fox urine), molecules (kairomones) from predator urine bind to receptors in the VNO and activate a neural circuit that induces risk assessment behaviors and avoidance (26, 28). To examine whether Bmal1 KO males displayed normal avoidance behavior, we housed WT or Bmal1 KO mice with fox urine (predator scent) and monitored risk assessment behavior. Bmal1 KO males did not display avoidance or risk assessment behavior in response to predator scent; rather, they directly investigated the scent without apparent caution and without retreating. In contrast, WT mice displayed strong avoidance behavior, investigating the scent briefly before retreating into the opposite corner of the cage. WT males spent 63% of the time in the area away from the fox scent and only 3% of time in the same area as the scent. Bmal1 KO males spent 25% time in the area away from the scent and 38% time near the scent (Figure 2, A and B). We measured serum cortisol, a hormone that increases in response to stress, after exposure to fox urine and found that although WT males displayed greater than 4-fold increases in serum corticosterone (192.6 ng/mL fox urine exposed vs 45.1 ng/mL basal), corticosterone did not significantly increase in Bmal1 KO males (50.1 ng/mL fox urine exposed vs 30.7 ng/mL basal) (Figure 2C).
Figure 2.
Fear behavior in Bmal1 KO male mice. A and B, Fear behavior: time spent in marked area near (area 1) predator scent (fox urine) or away (area 2) from predator scent. Fox urine placement is marked by a red box (n = 6, one way ANOVA). C, Serum corticosterone was measured by an ELISA after exposure to predator scent for 10 minutes (n = 5 for scent exposed groups, n = 2 for baseline groups, one way ANOVA followed by Tukey post hoc). *, P < .05; **, P < .01; ***, P < .001.
HPG axis function is mildly impaired in Bmal1 KO males
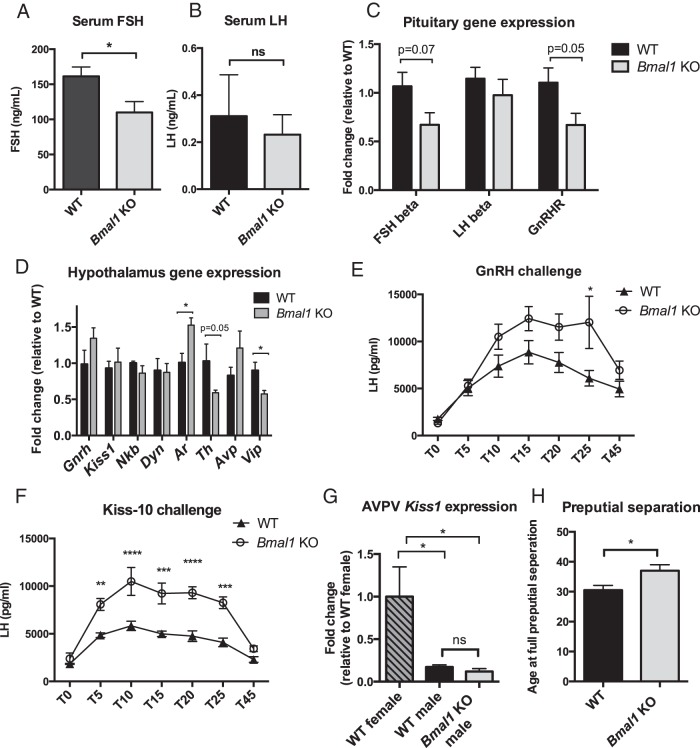
We then investigated other aspects of the HPG axis to determine whether the loss of Bmal1 affects hypothalamic, pituitary, and testis function. FSH, a pituitary hormone responsible for Sertoli cell function in the testis, was significantly decreased in both Bmal1 KO serum (161 ng/mL WT vs 110 ng/mL Bmal1 KO) (Figure 3A) and whole pituitary (399 μg/pit WT vs 225 μg/pit Bmal1 KO) (data not shown). Serum LH, the pituitary hormone responsible for T secretion, was unchanged in WT vs Bmal1 KO (0.310 ng/mL ± 0.18 vs 0.232 ± 0.08 ng/mL, n = 6) (Figure 3B), as measured in serum and whole pituitary (870 μg/pituitary ± 101 vs 808 ± 109μg/pituitary, n = 5–6) (data not shown). We also analyzed gene expression in the pituitary. FSH-β expression also was decreased by 33% in Bmal1 KO males, correlating with the decrease in FSH pituitary and serum hormone levels. GnRH receptor mRNA expression was also decreased by 33% in Bmal1 KO males. However, LH mRNA levels were not significantly different, in agreement with our serum and pituitary protein levels (Figure 3C).
Figure 3.
Hypothalamic and pituitary function in Bmal1 KO males. A, Male serum FSH (n = 6, Student's t test). B, Male serum LH (n = 6, Student's t test). C, Pituitary gene expression: mRNA expression of FSHβ, LHβ, and GnRH receptor mRNAs in the pituitary of WT and Bmal1 KO males (n = 5, Student's t test). D, Hypothalamic gene expression (n = 4–6, Student's t test). E, GnRH challenge: LH response to exogenous injection of GnRH (n = 5–6, two way ANOVA followed by Sidak post hoc). F, Kisspeptin challenge: LH response to injection of kisspeptin peptide (kiss-10) (n = 4, two way ANOVA followed by Sidak post hoc). G, AVPV kisspeptin expression: mRNA expression of kisspeptin in the AVPV, a marker of brain masculinization (n = 3, one way ANOVA followed by Tukey pot hoc). H, Preputial separation: postnatal day when preputial separation occurs in male mice (marker of puberty) (n = 8–9, Student's t test). *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001.
We examined the hypothalamic expression of several genes related to male fertility and circadian rhythms (Figure 3D). Levels of Gnrh1 mRNA, a hormone that causes the release of both LH and FSH, were not significantly different between WT and Bmal1 KO mice. Similarly, kisspeptin (Kiss1), a hormone important for GnRH release, was unchanged. Vasoactive intestinal peptide (Vip), a core peptide of the SCN, was decreased by 1.3-fold in Bmal1 KO male hypothalami (WT 0.90 ± 0.11 vs KO 0.58 ± 0.24). We also hypothesized that tyrosine hydroxylase (Th), the rate-limiting enzyme for catecholamine synthesis, including dopamine, may be affected because dopamine levels increase in the hypothalamus before and during mating. Tyrosine hydroxylase was decreased in Bmal1 KO hypothalami by 2-fold (WT 1.03 ± 0.23 vs KO 0.59 ± 0.10, P = .05 by a t test). Androgen receptor expression, the receptor that binds T and is important for mediating T response in the brain, was increased 1.5-fold in Bmal1 KO male hypothalami (WT 1.01 ± 0.13 vs KO 1.53 ± 0.12), suggesting potential up-regulation, possibly in response to lower circulating T.
Pituitary function is altered in Bmal1 KO mice
We next challenged the HPG axis of Bmal1 KO mice using exogenous GnRH, a potent inducer of LH release, or exogenous kisspeptin, a potent stimulator of GnRH neurons. These challenges test the ability of the gonadotrope cells in the pituitary to respond to GnRH stimulus and test the ability of GnRH neurons themselves to respond to kisspeptin. In each case, we measured LH response over a time course after injection, and, surprisingly, we found that Bmal1 KO males exhibited increased LH secretion in response to both GnRH and kisspeptin, suggesting increased sensitivity to these hormones (Figure 3, E and F).
Brain masculinization of Bmal1 KO male mice
The hypothalamus is a sexually dimorphic region of the brain, and appropriate masculinization is necessary to maintain male-specific behaviors, including mounting and aggression (29). To determine whether decreased male-like behaviors (such as mounting and aggression) in Bmal1 KO males might be due to improper masculinization of the brain, we examined sexually dimorphic expression of kisspeptin. AVPV kisspeptin, a known marker of sexual dimorphism in the brain, is found at high levels only in females and in males deprived of T during perinatal development. We found that Bmal1 KO males express appropriately male-like levels of kisspeptin in the AVPV (Figure 3G).
Improper brain masculinization would also impair the initiation of puberty, which is sexually dimorphic in its onset, with females normally entering puberty before males. We therefore analyzed puberty onset in Bmal1 KO males because lack of pubertal development is also known to affect fertility. Bmal1 KO males entered puberty approximately 4 days later than WT males, as measured by preputial separation (Figure 3H); however, Bmal1 KO mice also display a growth delay and entered puberty upon reaching the same body weight as WT mice (WT = 14.6 ± 0.7 vs KO = 17 ± 0.8, n = 4 WT, n = 8 KO, not significant by a Student's t test). It is known that the onset of puberty is dependent on body weight (30), and thus, it is probable that the observed delay in puberty is related to the growth delay and unlikely to be the cause of infertility.
Collectively these data indicate that the HPG axis is mildly impaired in Bmal1 KO males, with altered pituitary function and decreases in T production. However, these defects are not sufficient to explain the complete lack of copulatory behavior in Bmal1 KO males because testicular histology is normal and sperm are competent. Additionally, exogenous T replacement did not rescue any copulatory or aggressive behavior, indicating that the low circulating T in Bmal1 KO males is not responsible for these behavioral defects.
Olfaction and response to female pheromones in Bmal1 KO males
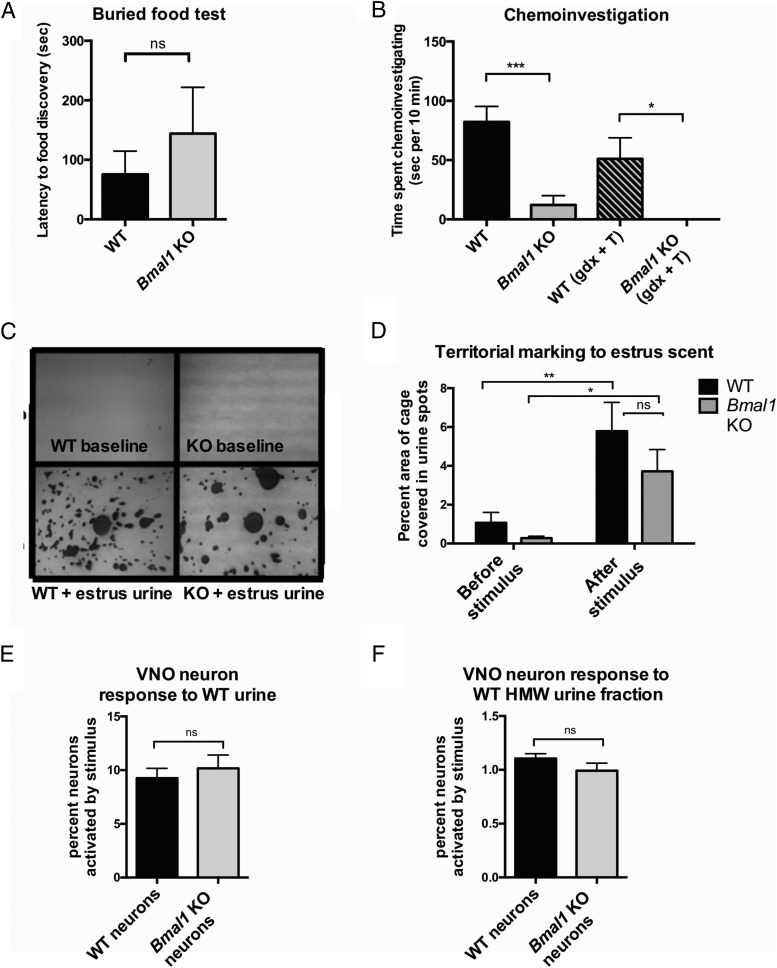
Whereas our examination of HPG axis function collectively suggests slight dysregulation of the HPG axis in Bmal1 KO males, it is unlikely that these subtle differences are responsible for the complete failure of mating behavior. Because it is known that the ability to detect olfactory cues (pheromones) is critical for the induction of mating behavior (31), we next tested whether Bmal1 KO males showed any defects in olfactory function or pheromone detection. We first verified the general olfactory function of Bmal1 KO males by testing whether they could uncover food hidden from view (buried food test). Bmal1 KO males were able to find food pellets hidden under cage bedding similar to WT males, demonstrating that they could respond to general olfactory cues (Figure 4A).
Figure 4.
Olfactory function in Bmal1 KO males. A, Buried food test: latency to discovery of buried food pellet (n = 7, Student's t test). B, Chemoinvestigation: time spent performing anogenital sniffing upon introduction of receptive female (n = 3–9, one way ANOVA). C and D, Territorial marking: urine marks in response to a spot of receptive female estrous urine scent on Whatman paper (n = 6–8, Student's t test). E, VNO function: isolated vomeronasal neuron calcium flux response to whole urine from a WT male (n = 3, Student's t test). F, VNO function: isolated vomeronasal neuron response to high molecular weight (HMW) fraction of urine from a WT male in culture (n = 3 Student's t test, P < .05). **, P < .01; ***, P < .001.
We next investigated the chemoinvestigatory behavior of Bmal1 KO males to determine whether Bmal1 KO males could respond to olfactory cues from a receptive female. The first step prior to mating is anogenital sniffing, which allows the male to gather pheromonal cues from the female. A WT or Bmal1 KO male was paired with an estrous female, and total time spent engaging in anogenital sniffing was recorded. WT males spent an average of 82 seconds investigating the estrous female, whereas Bmal1 KO males displayed chemoinvestigatory behavior for only 12 seconds. Although Bmal1 KO males did chemoinvestigate for a short period briefly after introduction of the estrous female, they did not persist in this behavior, as did the WT males (Figure 4B). As in mounting behavior, GDX + T replacement did not rescue chemoinvestigatory behavior, demonstrating that lack of circulating T is not responsible for the decrease in this behavior (Figure 4B).
To determine whether Bmal1 KO males could respond at all to estrous female urine, we next examined territorial marking behavior. WT males typically mark territory with urine spots in response to the estrous female scent. We found that Bmal1 KO males robustly marked their territory when presented with the estrous female scent in a similar fashion to WT males, demonstrating the ability of Bmal1 KO males to successfully detect female scent (Figure 4, C and D).
VNO function in Bmal1 KO males
The inability of Bmal1 KO males to mate with receptive female mice, display aggression toward an intruder, and display fear response to predator scent (all pheromone/kairomone mediated behaviors) suggests that Bmal1 KO have defective pheromone detection and/or response. In mice, pheromonal cues can be detected by both the main olfactory epithelium (MOE) located in the posterior nasal cavity and the VNO located in the nasal septum. Given that Bmal1 KO males demonstrated intact MOE function evidenced by food detection and territorial marking (Figure 4, A, C, and D), we examined whether the VNO neurons of Bmal1 KO males might not respond to pheromonal cues. We isolated and cultured VNOs from both WT and Bmal1 KO males and loaded them with Fura2 (a calcium indicator indicative of neuron firing) to determine whether VNO neurons from Bmal1 KO males were activated when presented with a stimulus. Bmal1 KO VNO neurons responded similarly to WT VNO neurons when presented with either whole urine (from a WT C57BL/6 male) (Figure 4E) or the high-molecular-weight fraction that contains the major urinary protein pheromones (Figure 4F), indicating that VNO neurons in Bmal1 KO males are fully responsive to the odor proteins from urine that were tested. These data demonstrate that the pheromone-related behavioral defects observed in Bmal1 KO mice do not originate within the VNO.
Neural circuit of copulatory behavior is impaired in Bmal1 KO males
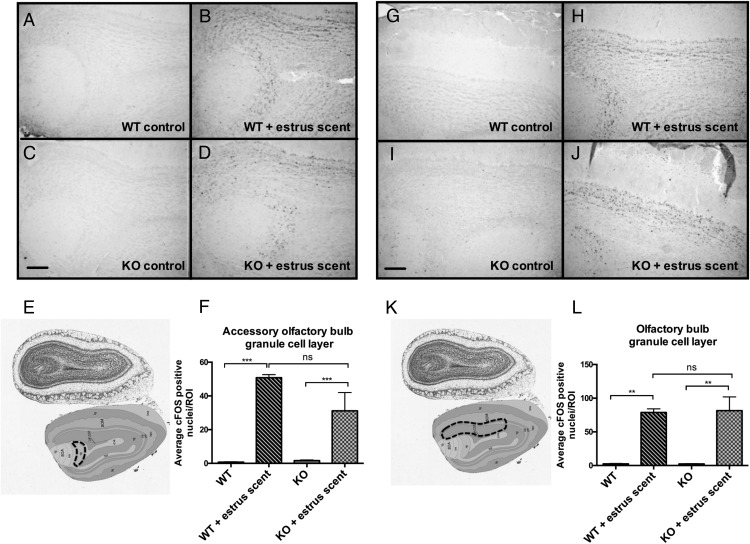
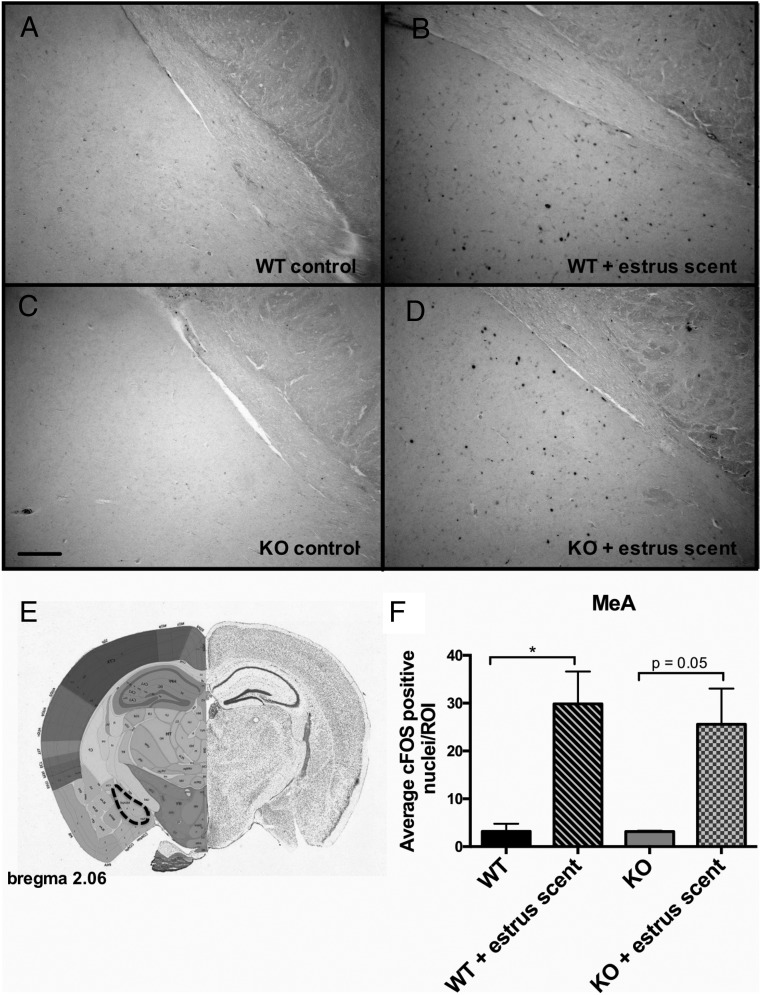
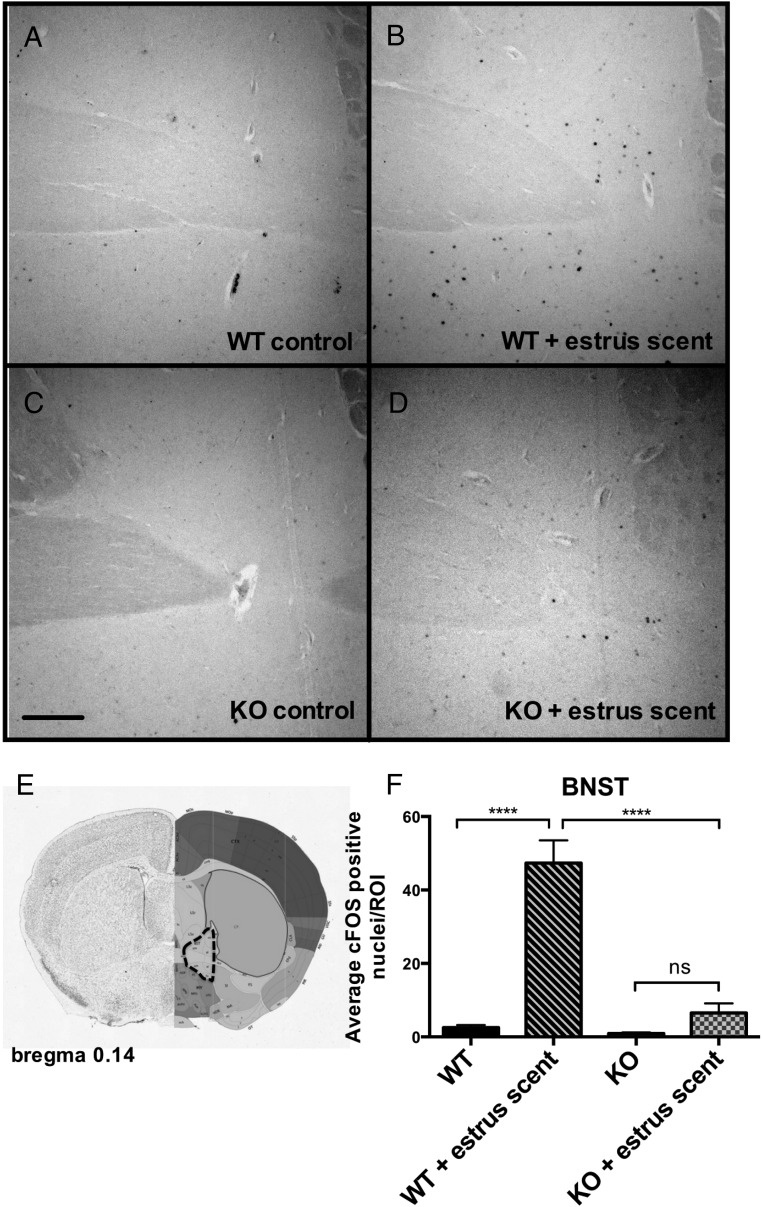
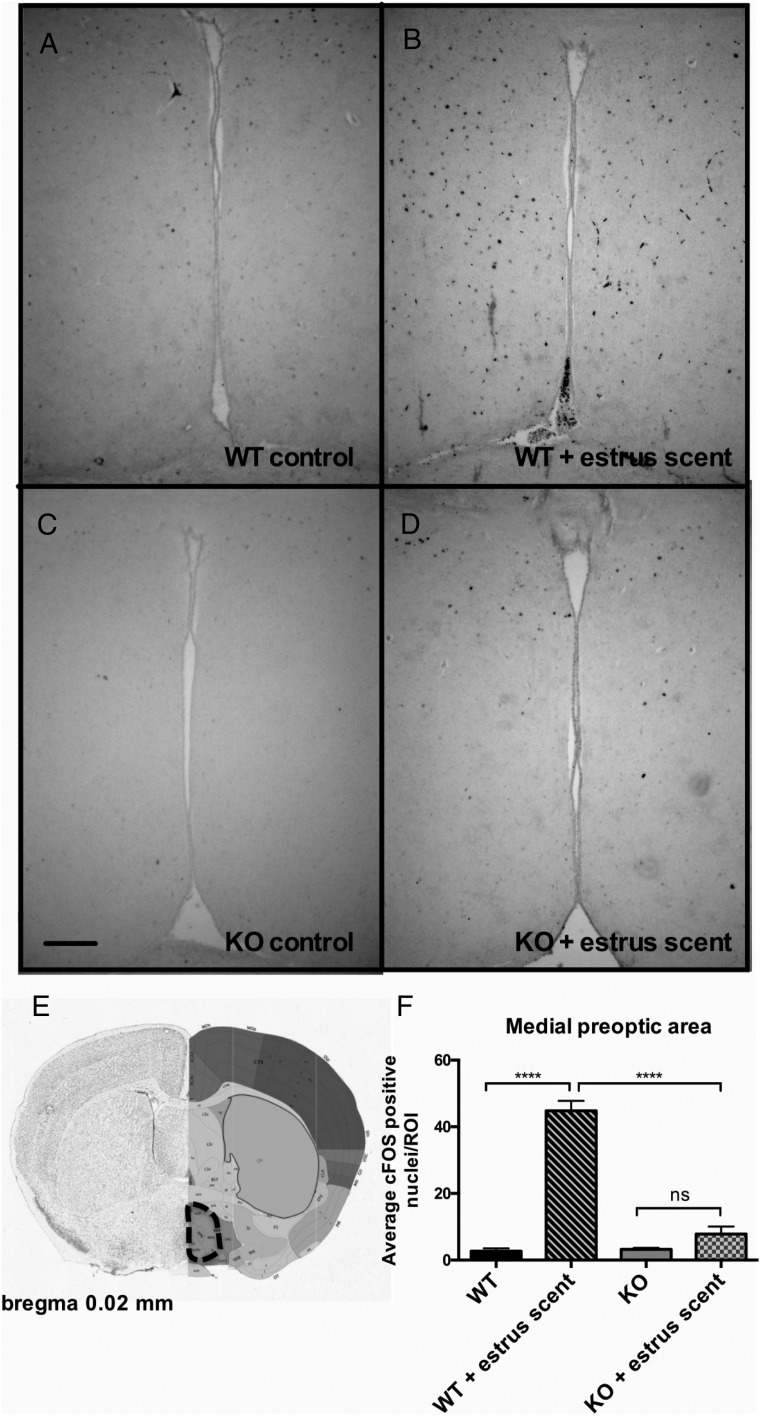
We reasoned that, whereas the VNO was functional, there might be a downstream defect in signal propagation within the neural circuits responsible for pheromone-induced behaviors. The neural circuit of male mating behavior is well characterized; pheromones are detected by either the MOE or the VNO, and the signals are then propagated to the brain to initiate sexual behavior. Pheromonal signals from the MOE project to the olfactory bulb (OB), whereas signals from the VNO project to the accessory olfactory bulb (AOB), and these signals converge in the bed nucleus of the stria terminalis (BNST), which then innervates the medial preoptic area (MPOA), a site regarded as the major integrative site for the regulation of sexual behavior (32, 33). Projections from the OB and AOB also innervate the medial amygdala, which then projects information back to the hypothalamus (34). An inability to detect pheromones eliminates both copulatory and aggressive behaviors (29). To assess the functionality of the neural circuit of mating in Bmal1 KO males, we analyzed the pattern of c-FOS induction, a marker a neural activity, in select brain regions after exposure to estrous female scent. We found robust c-FOS induction in both the OB and the AOB (Figure 5). This further strengthens the evidence that the defect does not lie within either the MOE or the VNO and that the signaling between the MOE and VNO and the immediate targets (the OB and the AOB, respectively) is intact. We next analyzed c-FOS activation in the medial amygdala, which receives direct projections from both the VNO and the MOE, and found that c-FOS induction was comparable between Bmal1 KO and WT groups in response to the estrous scent (Figure 6). Next, we measured c-FOS patterns in the BNST, which receives input from the amygdala and is considered an important part of the neural circuit that modulates male mating behavior. We found significantly less c-FOS activation in the Bmal1 KO males compared with WT controls (Figure 7). Finally, we examined the MPOA, a hypothalamic site regarded as the major integrative site for the initiation of male sexual behavior and found significantly less c-FOS induction in the MPOA of Bmal1 KO males exposed to the estrous scent compared with WT (Figure 8). These findings suggest that defective neural circuit signaling is responsible for the failure of copulatory behavior exhibited by the Bmal1 KO males.
Figure 5.
c-FOS expression in the granule cell layer of the OB and AOB in WT vs Bmal1 KO males after exposure to estrous female scent. A, WT control OB. B, WT + estrous female scent OB. C, Bmal1 KO control OB. D, Bmal1 KO + estrous female scent OB. E, Dotted outline indicates quantified portion. Images were obtained from the Allen Mouse Brain Atlas (http://mouse.brain-map.org). F, Quantification of c-FOS-positive cells in defined area. G, WT control AOB. H, WT + estrous female scent AOB. I, Bmal1 KO control AOB. J, Bmal1 KO + estrous female scent AOB. K, Dotted outline indicates quantified portion. Images obtained from the Allen Institute web site. L, Quantification of c-FOS-positive cells in defined area (n = 3 animals per treatment group). *, P < .5, **, P < .01, ***, P < .001, by ANOVA followed by Tukey post hoc. Scale bar, 100 μm. Image reproduced with permission from the Allen Institute.
Figure 6.
Expression of c-FOS in the medial amygdala in WT vs Bmal1 KO males after exposure to estrous female scent. A, WT control. B, WT + estrous female scent. C, Bmal1 KO control. D, Bmal1 KO + estrous female scent. E, Dotted outline indicates quantified portion. Images were obtained from the Allen Mouse Brain Atlas (http://mouse.brain-map.org). F, Quantification of c-FOS-positive cells in defined area (n = 4 animals per treatment group). *, P < .5, **, P < .01, ***, P < .001, by ANOVA followed by Tukey post hoc. Scale bar, 100 μm. Image reproduced with permission from the Allen Institute.
Figure 7.
Expression of c-FOS in the BNST in WT vs Bmal1 KO males after exposure to estrous female scent. A, WT control. B, WT + estrous female scent. C, Bmal1 KO control. D, Bmal1 KO + estrous female scent. E, Dotted outline indicates quantified portion. Images obtained from the Allen Mouse Brain Atlas (http://mouse.brain-map.org). F, Quantification of c-FOS-positive cells in defined area (n = 4 animals per treatment group). *, P < .5, **, P < .01, ***, P < .001, by ANOVA followed by Tukey post hoc. Scale bar, 100 μm. Image reproduced with permission from the Allen Institute.
Figure 8.
Expression of c-FOS in the MPO in WT vs Bmal1 KO males after exposure to estrous female scent. A, WT control. B, WT + estrOus female scent. C, Bmal1 KO control. D, Bmal1 KO + estrous female scent. E, Dotted outline indicates quantified portion. Images obtained from the Allen Mouse Brain Atlas (http://mouse.brain-map.org). F, Quantification of c-FOS-positive cells in defined area (n = 4 animals per treatment group). *, P < .5, **, P < .01, ***, P < .001, by ANOVA followed by Tukey post hoc. Scale bar, 100 μm. Image reproduced with permission from the Allen Institute.
Discussion
Bmal1 KO males and females both fail to produce litters, yet the primary mechanisms regulating fecundity appear to differ between males and females. Here we show that Bmal1 KO males display behavioral abnormalities leading to their infertility, including a decreased amount of chemoinvestigation, and a lack of mounting and aggression. Additionally, these animals fail to respond to predator scent. We further show that HPG axis function is largely intact and that the male Bmal1 KO failure to copulate is likely attributed to abnormal brain circuitry, specifically at the level of the BNST and MPOA.
Because T is decreased in Bmal1 KO males, we normalized T levels and repeated copulation and aggression experiments because T is required for both of these behaviors (11). Restoration of normal T levels did not restore either copulatory or aggressive behaviors, suggesting that absence of sufficient circulating T in adulthood is not the primary cause of these behavioral deficiencies. We also considered the possibility that early postnatal T levels are not sufficient to properly masculinize the brain, thereby lowering copulation and aggression. Male mice normally experience a surge of T shortly after birth that is required to organize the brain for future adult masculine behaviors (35). We examined two major markers of brain masculinization in the Bmal1 KO males, puberty and Kiss1 expression in the AVPV, and found both of these to be male-like. Additionally, Bmal1 KO males demonstrated appropriate territorial urine marking in response to female scent stimulus, a male-specific behavior. Furthermore, the presence of yet another nondimorphic behavior, the lack of response to fear stimulus, suggests that the behavioral abnormalities in the Bmal1 KO mice are caused by a common brain abnormality preventing the processing of input olfactory stimuli. We examined olfactory function, both the MOE by the ability to forage hidden food and the VNO by direct assessment of neuron response to stimulus. Both of these functions were intact, and thus, we reasoned that the signal must be disrupted downstream of the detection of olfactory cues. The neural circuitry for male response to female scent is well characterized, and as expected, there was a lack of neuronal activation in several key areas important for generating a behavioral response to the female scent, the BNST and the MPOA. Additionally, the failure of Bmal1 KO mice to avoid predator scent (fox urine) indicates that the processing circuit regulating the fear response to predator kairomones is impaired. Although we did not measure neural activation patterns after exposure to a fox urine stimulus, the presence of normal olfaction (ability to uncover hidden food) suggests that the Bmal1 KO males can detect the odor but that the downstream neural signaling is affected. The ability of Bmal1 KO males to uncover food hidden from view indicates that they have some function of the main olfactory system, although this does not eliminate the possibility that pheromone detection in the MOE is defective.
There are numerous possibilities for the failure of neuronal activation in the Bmal1 KO males. It is yet unclear how circadian rhythms may regulate the neural circuits of mating and aggressive behaviors. Loss of rhythmicity in the SCN has been shown to increase depression-like behaviors in mice, providing the possibility that coordination of circadian rhythms by the SCN is important for regulating behavior (36). The SCN projects directly to the MPOA and the BNST, providing the opportunity for regulation through circadian signals from the SCN. Future research might include investigating the effects of circadian rhythm disruptions in the SCN, MPOA, or BNST on mating and aggression to identify the cellular populations where rhythms are important for maintaining these behaviors.
It is also possible that loss of rhythmicity induces oxidative stress in the brain, which could damage the neural signaling cascades. Recent evidence demonstrates that oxidative stress is increased in Bmal1 KO mice, and as a result, synapses are degraded and brain connectivity is decreased (37), possibly explaining the failure of Bmal1 KO males to complete the neural circuits that regulate copulatory behaviors. A similar mechanism could be attributed to abnormal aggression and fear behaviors.
Mating behavior in rodents is a demonstrable circadian behavior, with males showing decreased latency to initiating copulation during the dark phase. SCN ablation does not abolish mating behavior in male mice but does abolish the circadian rhythm of sexual behavior (38). Whereas it is possible that the Bmal1 KO males simply lose the preference to mate during the timing of our mating assay (6–8 pm/Zeitgeber time 12–14), this is unlikely due to the absence of copulatory plugs, even when males are continuously paired with females over the course of 10 days. Therefore, it is likely that the loss of reproductive behavior is due to more than simply loss of rhythmicity in the SCN but possibly more pleotropic effects on other brain regions regulating this behavior. Similarly, olfactory function displays circadian rhythmicity, and olfactory sensitivity varies over the 24-hour period (39). However, if decreased olfactory sensitivity were responsible for the failure to initiate copulatory behaviors, it is likely that Bmal1 KO males would eventually sire litters when continuously paired with a female, which was not observed in either our study or previous studies of the Bmal1 KO male (2, 9).
We also show that tyrosine hydroxylase, the rate-limiting enzyme involved in dopamine synthesis, is reduced in the hypothalami of Bmal1 KO males (Figure 3D). Dopamine is a critical mediator of copulation, with a release of dopamine in the medial preoptic are critical for mating (40), so a reduction in tyrosine hydroxylase could indicate that the dopamine release preceding mating may be impaired. Indeed, other groups have shown that circadian rhythms are critical for mediating reward and addiction behavior (41), and several genes in the ventral tegmental area are dysregulated in clockδ19/δ19 mutant mice. Additionally, in the striatum, dopamine transporter, dopamine receptors, and tyrosine hydroxylase display circadian expression (42). Thus, it is plausible that abnormal reward processing contributes to the lack of investigation and mounting in Bmal1 KO males. However, it is not currently thought that the dopaminergic system regulates fear response, so although this may be a contributing factor, it is unlikely to be the sole cause for all of the behavioral abnormalities in Bmal1 KO males.
Although we speculate that defects in the HPG axis are not responsible for the loss of sexual and aggressive behaviors in Bmal1 KO males, we do find subtle defects that indicate Bmal1 is playing a role in modulating the HPG axis. There is a cohort of evidence indicating that circadian rhythms regulate fertility through interactions with the HPG axis. GnRH neurons in the hypothalamus release pulsatile GnRH to stimulate LH and FSH release from the pituitary, which interact with the gonads to regulate spermatogenesis, oogenesis, and ovulation (43). GnRH neurons fire rhythmically in the hypothalamus to stimulate the release of FSH and LH. Bmal1 and Per2 exhibit circadian expression patterns in GnRH neurons both in vivo and in vitro (44, 45). In vitro, expression of a dominant-negative clock protein abrogates Per2 rhythmicity and pulsatile GnRH secretion, suggesting that the molecular clock machinery contributes to GnRH pulse generation. There is also a circadian-timed increased in GnRH firing rate under estrogen-permissive conditions at the onset of darkness that stimulates the preovulatory LH surge, and the LH surge is abolished in both Bmal1 KO females and clockδ19/δ19 mutant females (10, 46). Pituitary-specific knockout of Bmal1 does not ablate the LH surge, suggesting that the contribution of Bmal1 in the generation of the LH surge lies upstream of the pituitary (10).
Here we have shown that FSH transcription, pituitary FSH protein, and serum FSH hormone content were decreased, indicating that loss of Bmal1 affects FSH release in males. Given that FSH is important for Sertoli cell proliferation (47), this is fitting with previously reported data (9) that Bma1l KO testes are slightly smaller. We saw no difference in LH content (pituitary mRNA expression, pituitary protein content, or serum levels), but LH increased significantly more in Bmal1 KO compared with WT after stimulation with either kisspeptin or GnRH injection, suggesting that the pituitary is hypersensitive. We speculate that perhaps the loss of Bmal1 affects GnRH neuron coordination and endogenous LH pulses may be disrupted. This could result in an accumulation of LH in storage granules, which would account for the increased release in the presence of exogenous stimuli. Given that the LH surge in female mice is known to be a circadian phenomenon (23), it is not wholly unexpected for the circadian clock machinery to be involved in regulating LH release. Alternatively, negative feedback regulating LH release in response to GnRH could be disrupted in Bmal1−/− males, resulting in an increased sensitivity to secretagogue stimulus.
We have demonstrated a role for Bmal1 in male fertility at both the neuroendocrine and the behavioral level. We show that loss of Bmal1 causes a decrease in FSH mRNA and protein and a dysregulation of pituitary response to GnRH and kisspeptin stimulus. Loss of Bmal1 causes the elimination of three pheromone-dependent behaviors: copulation, aggression, and predator avoidance. Bmal1 KO males have a functional olfactory system but lack neural activation in key brain regions involved in male mating including the BNST and the POA. These data identify a new role for the circadian protein, Bmal1, in the integration of pheromonal input and demonstrate new functions of Bmal1 as a mediator of mating, aggression, and fear behaviors. These results have important implications for roles of disrupted circadian rhythms in regulating neural circuits of a wide variety of behaviors.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK044838, R01 HD072754, R01 HD082567 (to P.L.M.), and R01 HD065856 (to A.S.K.) as well as National Science Foundation Grant IOS-1457226 (to A.S.K.). It was also supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Grant P50 HD012303 as part of the National Centers for Translational Research in Reproduction and Infertility (to P.L.M.). P.L.M. was also partially supported by Grants P30 DK063491, P30 CA023100, and P42 ES010337. E.L.S. and S.L.S. were partially supported by Gant T32 HD007203. E.L.S. was also partially supported by the Lalor Foundation and Grant T32 DK007044. D.D.C. was partially supported by Grants T32 GM008666 and T32 DK007541 and was a student in the University of California, San Diego, Biological Sciences Graduate Program. S.J.S. was also partially supported by Gant F32 HD066849. The Center for Research in Reproduction, Ligand Assay and Analysis Core, University of Virginia, is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant P50 HD028934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AOB
- accessory olfactory bulb
- AVPV
- anteroventral periventricular
- BMAL
- brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein
- BNST
- bed nucleus of the stria terminalis
- CLOCK
- circadian locomotor output cycles kaput
- CV
- coefficient of variation
- GDX
- gonadectomized
- HPG
- hypothalamic-pituitary-gonadal
- KO
- knockout
- OB
- olfactory bulb
- MOE
- main olfactory epithelium
- MPOA
- medial preoptic area
- qPCR
- quantitative real-time PCR
- ROI
- region of interest
- SCN
- suprachiasmatic nucleus
- VNO
- vomeronasal organ
- WT
- wild type.
References
- 1. Paschos GK, Ibrahim S, Song WL, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kondratov RV, Kondratova AA, Gorbacheva VY, et al. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leliavski A, Shostak A, Husse J, et al. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014;155:133–142. [DOI] [PubMed] [Google Scholar]
- 4. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology. 2009;150:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. King DP, Zhao Y, Sangoram AM, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gekakis N, Staknis D, Nguyen HB, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. [DOI] [PubMed] [Google Scholar]
- 7. Kume K, Zylka MJ, Sriram S, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. [DOI] [PubMed] [Google Scholar]
- 8. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvarez JD, Hansen A, Ord T, et al. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu A, Zhu L, Blum ID, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154:2924–2935. [DOI] [PubMed] [Google Scholar]
- 11. Ogawa S, Robbins A, Kumar N, et al. Effects of testosterone and 7α-methyl-19-nortestosterone (MENT) on sexual and aggressive behaviors in two inbred strains of male mice. Horm Behav. 1996;30:74–84. [DOI] [PubMed] [Google Scholar]
- 12. Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. [DOI] [PubMed] [Google Scholar]
- 13. Storch KF, Paz C, Signorovitch J, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kikusui T. Analysis of male aggressive and sexual behavior in mice. Methods Mol Biol. 2013;1068:307–318. [DOI] [PubMed] [Google Scholar]
- 15. McGill TE, Manning A. Genotype and retention of the ejaculatory reflex in castrated male mice. Anim Behav. 1976;24:507–518. [DOI] [PubMed] [Google Scholar]
- 16. Fendt M, Endres T, Lowry CA, et al. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–1156. [DOI] [PubMed] [Google Scholar]
- 17. Poling MC, Kim J, Dhamija S, et al. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153:1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGinnis MY, Mirth MC, Zebrowski AF, et al. Critical exposure time for androgen activation of male sexual behavior in rats. Physiol Behav. 1989;46:159–165. [DOI] [PubMed] [Google Scholar]
- 19. Semaan SJ, Dhamija S, Kim J, et al. Assessment of epigenetic contributions to sexually-dimorphic Kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology. 2012;153:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8:Unit 8.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chamero P, Marton TF, Logan DW, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. [DOI] [PubMed] [Google Scholar]
- 22. Kaur A, Dey S, Stowers L. Live cell calcium imaging of dissociated vomeronasal neurons. Methods Mol Biol. 2013;1068:189–200. [DOI] [PubMed] [Google Scholar]
- 23. Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. [DOI] [PubMed] [Google Scholar]
- 24. Boden MJ, Kennaway DJ. Reproductive consequences of circadian dysfunction: Fertility in the Bmal1 null mouse. Reproduction. 2010;139:1077–1090. [DOI] [PubMed] [Google Scholar]
- 25. Petrulis A. Chemosignals and hormones in the neural control of mammalian sexual behavior. Front Neuroendocrinol. 2013;34:255–267. [DOI] [PubMed] [Google Scholar]
- 26. Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243:E257–E263. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci. 2014;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr Opin Neurobiol. 2011;21:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colledge WH, Mei H, d'Anglemont de Tassigny X. Mouse models to study the central regulation of puberty. Mol Cell Endocrinol. 2010;324:12–20. [DOI] [PubMed] [Google Scholar]
- 31. Wang Z, Balet Sindreu C, Li V, et al. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang CF, Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82:261–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reyes R, Mendoza J, Ballesteros J, et al. Male chemosignals inhibit the neural responses of male mice to female chemosignals. Brain Res Bull. 2004;63:301–308. [DOI] [PubMed] [Google Scholar]
- 34. Hashikawa K, Hashikawa Y, Falkner A, et al. The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol. 2016;38:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. [DOI] [PubMed] [Google Scholar]
- 36. Landgraf D, Long JE, Proulx CD, et al. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Musiek ES, Lim MM, Yang G, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eskes GA. Neural control of the daily rhythm of sexual behavior in the male golden hamster. Brain Res. 1984;293:127–141. [DOI] [PubMed] [Google Scholar]
- 39. Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci. 2006;26:12219–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wersinger SR, Rissman EF. Dopamine activates masculine sexual behavior independent of the estrogen receptor α. J Neurosci. 2000;20:4248–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McClung CA, Sidiropoulou K, Vitaterna M, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung S, Lee EJ, Yun S, et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell. 2014;157:858–868. [DOI] [PubMed] [Google Scholar]
- 43. Christensen A, Bentley GE, Cabrera R, et al. Hormonal regulation of female reproduction. Horm Metab Res. 2012;44:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology. 2010;91:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1–7 cell line. J Neurosci. 2003;23:11202–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller BH, Olson SL, Turek FW, et al. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnston H, Baker PJ, Abel M, et al. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. [DOI] [PubMed] [Google Scholar]