Abstract
Adult Leydig cells are derived from proliferating stem/progenitor Leydig cells in the infant testis and subsequent differentiation to steroidogenic cells in adult mice. Leydig cell proliferation in the infant testis occurs primarily in response to increased levels of LH that induce Leydig cell expression of neuregulin 1 (NRG1). Depletion of NRG1 in Nrg1 mutant mice (Nrg1flox;flox;Cyp19a1Cre mice) dramatically reduces Leydig cell proliferation in the infant testes, leading to a reduction of testis weight, epididymial weight, and serum T in the adult mutant mice. The mutant mice are subfertile due to impaired sexual behavior and abnormal elongation of the spermatogenic cells. These defects were reversed by T treatment of the mutant mice in vivo. Furthermore, NRG1 alone induces the proliferation of Leydig cells in cultures of infant (d 10) testes obtained from mutant mice. Collectively these results show that LH induction of NRG1 directly drives the proliferation of Leydig cells in the infant testis, leading to an obligatory number of adult Leydig cells required for the production of sufficient androgen to support and maintain spermatogenesis and sexual behavior of adult male mice.
Androgens are essential for male sexual development, masculinization, and fertility (1–3). The production of androgens occurs mainly in Leydig cells, of which there are two subtypes: fetal Leydig cells (FLCs) and adult Leydig cells (ALCs) (4, 5). In the fetal testis, FLCs express enzymes including CYP11A1 and CYP17A1, which convert cholesterol to androstenedione, but do not express 17β-hydroxysteroid dehydrogenase 3 (HSD17B3) enzymes essential for converting androstenedione to active androgens (6, 7). Rather, fetal Sertoli cells express the enzymes that convert androstenedione to testosterone (7). After birth, the number of FLCs decreases in the infant testis, whereas the number of ALCs increases concomitantly with increasing levels of LH (8–10). ALCs express all enzymes that are required for the production of androgen from cholesterol and are located in the interstitial tissue of the adult testis (11, 12). Because LH can activate both the protein kinase A (PKA) and RAS-MAPK kinase (MEK)-1 pathways in ovarian cells (13) and Leydig cells (14) and because LH induces multiple factors, especially those that can activate the epithelial growth factor (EGF) receptor (15, 16) or the other erb-b2 receptor tyrosine kinase (ERBB) family members (17) in granulosa cells of ovulating follicles in ovary, the ability of LH to impact Leydig cell proliferation, differentiation, and function might involve multiple factors including the ligands for ERBB family.
Chen et al (2009) (18) reported that the proliferative activity of Leydig cells was high in stem Leydig cells and progenitor Leydig cells mostly observed in testes of mice at 1–3 weeks of age. The proliferation of Leydig cells ceases after the Leydig cells are fully differentiated to ALCs in testes of mice more than 90 days old (19). However, when some genes including Erbb2 are overexpressed in ALCs of adult testis, proliferation is restored and Leydig cell tumors develop (20–22). ERBB2 belongs to ERBB family that consists of ERBB1, ERBB2, ERBB3, and ERBB4, all of which, except for ERBB2, contain a ligand binding domain and all of which, except ERBB3, have a tyrosine kinase domain (23, 24). Because ERBB2 has a tyrosine kinase domain, it can form a heterodimer with other ErbB family members and activate signaling from the cell surface to the cytoplasm and nuclei (23, 24). In breast cancer cells, ERBB2 mainly forms heterodimers with ErbB3 due to the high expression of ligands for ERBB3; autoactivation of ERBB2 via a single-nucleotide substitution is related to the malignancy of breast cancer (25). Elevated expression of ERBB2 is associated with Leydig cell tumors (20); low expression in ALCs in the adult testis is associated with marginal proliferation (26). However, there is no report to determine the relationship between the proliferation of stem or progenitor Leydig cells in the infant testis and the expression of specific ligands for ERBB3 in these cells.
The neuregulins (NRG1, NRG2, NRG3, and NRG4) comprise a family of ligands specific for ERBB3 and ERBB4 but not ERBB1 (epidermal growth factor receptor) (27). Our previous studies showed that LH induces Nrg1 expression in granulosa cells of ovulating follicles and that NRG1 activated ERBB2/3 heterodimers to control the timing of meiotic progression of oocytes (17, 28, 29). Nrg1 expression was observed within 2 hours after LH stimulation and was controlled by the transcription factors, cAMP response element-binding protein and CCAAT/enhancer-binding protein, which were activated by the cAMP-PKA and ERK1/2 pathways, respectively (17). Therefore, because Nrg1 is an LH target gene and because the gene encodes the ligand for ErbB3, we hypothesized that NRG1 was also regulated in Leydig cells by LH to induce cell proliferation in infant testis. One research group, Abé and collaborators (30), recently reported the expression of NRG1 in Sertoli cells of the fetal testis, which impacted the proliferation and meiotic initiation of spermatogonia cells. In the present study, we document the cell-specific expression of NRG1 in HSD17B3-positive Leydig cells and show that its disruption in these cells using Nrg1flox/flox;Cyp19a1-Cre mutant mice leads to impaired proliferation and survival of the Leydig cells during testis development and reduced steroidogenesis and spermatogenesis in the adult testis.
Materials and Methods
Materials
Pregnant mare serum gonadotropin (equine chorionic gonadotropin [eCG]) and human chorionic gonadotropin (hCG) were purchased from Asuka Seiyaku. NRG1 was obtained from R&D Systems, Inc. T (T6147) and ganilelix acetate (SML0241), a GnRH antagonist, were obtained from Sigma. Ovine LH was a gift from the National Hormone and Pituitary Program (Torrance, California). DMEM-F12 medium and penicillin-streptomycin were from Invitrogen; fetal bovine serum was from Life Technologies Inc; oligonucleotide poly-(deoxythymidine) was from Invitrogen, avian myeloma virus reverse transcriptase was from Promega, routine chemicals and reagents were obtained from Sigma or Nakarai Chemical Co. Antineuregulin 1 antibody (catalog number ab53104) was purchased from Abcam. Antiproliferating cell nuclear antigen (PCNA) antibody (catalog number 2586) and antiphosphorylated ERK1/2 antibody (catalog number 4376) was purchased from Cell Signaling Technology, Inc. Anti-β-actin antibody (catalog number 128K4805) was from Sigma. Anti-CYP17a1 antibody (catalog number bs-3853) was purchased from Bioss. Anti-CRE recombinase antibody (catalog number MAB3120) was purchased from Millipore. Antiphosphorylated AKT antibody (catalog number 905–658) was purchased from Assay Designs. The anti-EGF domain of NRG1 and anti-HSD17B3 antibodies were generated in our previous studies (7, 28).
Generation of ALC-specific Nrg1KO mice
Mice lacking NRG1 in Leydig cells (LeyNrg1KO) were generated by crossing Cyp19a1-Cre transgenic mice (31) with Nrg1flox/flox mice (32), kindly provided by Dr Carmen Birchmeier (Max Delbrueck Center for Molecular Medicine, Berlin, Germany). In Cyp19a1-Cre transgenic mice, Cre driven by a −180-bp promoter region between exon 1 and exon 2 of human CYP19a1 is selectively expressed in round cells in the interstitial tissue of testis of mice at 1 week of age but not in the adult testis (Supplemental Figure 1, A and B), indicating that Cre is selectively expressed in Leydig cells of the infant testis. In Nrg1flox/flox mice, loxP sites were inserted into intron 2 and intron 5 of type III Nrg1 (accession number NM 178591.2). By the reaction of Cre recombinase, the functional EGF domain that is encoded by exons 3–5 of Nrg1 gene was deleted in the Cre-expressing cells. Using an antiserum against a unique peptide (PNEFTGDR) within the EGF domain of NRG1 (28), the positive signals were detected only within seminiferous tubes but not in the interstitial tissue of the testis in both young and adult male mice (Supplemental Figure 1D). Thus, the expression of functional NRG1 was selectively disrupted in the Leydig cells of the testis, especially undifferentiated ALCs, in the Nrg1flox/flox; Cyp19a1-Cre male mice.
Localization of Cre expression
We generated transgenic mice expressing Rfp in the Cre-positive cells by crossing Cyp19a1-Cre transgenic mice with Rfp knock-in mice. Testes were collected and fixed in 4% (wt/vol) paraformaldehyde/PBS overnight and embedded in paraffin and processed by routine procedures as described above. Sections were probed with the anti-Red Fluorescent Protein antibody (1:1000, ab-62341; Abcam). After washing by 0.3% (vol/vol) Triton X-100 in PBS (−), the antigens were visualized with Cy3-conjugated goat antirabbit IgG (1:200; Sigma), fluorescein isothiocyanate-conjugated goat antirabbit IgG (1:200; Sigma), and 4′,6-diamidino-2-phenylindole (DAPI; VECTESHIELD mounting medium with DAPI; Vector Laboratories). The digital images were captured using a Zeiss Axiphot microscope with a ×20 objective.
Animals
Animals were housed in the Experiment Animal Center at Hiroshima University under a 14-hour light, 10-hour dark schedule and provided with food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Hiroshima University.
GnRH treatment
In vivo GnRH-hCG treatments were done according to Kawamura et al (33) and Houk et al (34). Intact male mice were caged separately at least 1 day before they were subjected to the treatment. The mice were injected with ganirelix (75 mg/kg) each morning for 2 days. Some of the treated mice were further injected ip with hCG (10 IU) or saline.
T replacement
Three-month-old LeyNrg1KO were treated with T (50 ng/d) or saline (control) daily for 7 days. After 7 or 14 days from the start of the T treatment, the animals were used for analyses.
Mating test
The mating experiment was conducted using five 2-month-old males of each genotype (Nrg1flox/flox, wild type [WT], and Nrg1flox/flox;Cyp19a1-Cre, LeyNrg1KO). Adult WT female mice were placed in each cage for 6 months, and the number of pups in each litter was recorded.
Sexual behavior analyses and in vivo fertilization test
Sexual behavior analyses were done according to Kayasuga et al (35). Each male mouse was isolated 1 hour before the test and tested for 1 hour with immature females injected ip with 4 IU of eCG followed 48 hours later with 5 IU hCG to ensure high sexual receptivity. The incidence of ejaculation (percentage of animals that ejaculated) was determined by the existence of vaginal plugs in the females after each test. At 30 hours after the hCG injection, the putative fertilized eggs were recovered from oviduct, and then pronuclei formation was observed in each egg.
Assay for number and morphology of sperm
The assay for sperm number and morphology was done according to Nakada et al (36). Briefly, the cauda of epididymis of both genotypes were cut by a 27-gauge syringe to release sperm in 500 μL of Human Tubal Fluid medium, and the total number of sperm was counted. These sperm were also used as the sample for the morphology analysis. Collected sperm were smear stained with hematoxylin and eosin, and the frequencies of morphological abnormalities were checked in each mouse.
Assay for sperm motility using computer-assisted sperm assay (CASA) system
For the study of sperm motility, a CASA was performed (37). Sperm were incubated for 2 hours at 37°C under 5% CO2 in air, and motility was assessed. Ten microliters of sperm samples were loaded into 100-μm-deep sperm analysis chambers. At least three fields were recorded for each sample analyzed using a CASA system (SMAS; DETECT). Tracks and kinematic parameters were recorded.
Organ culture
Testes were cultured according to Delbès et al (38) and Zhang et al (30). Testes recovered from mice at 10 days age were cut into two fragments, and the fragments were placed on filters (pore size 0.45 μm, catalog number140652; Thermo Fisher Scientific). The filters were floated on 1 mL of DMEM-F12 containing 0.1% (vol/vol) fetal bovine serum in a tissue culture dish and then were incubated at 35°C for 72 hours. LH and/or recombinant EGF domain of NRG1 were added to the culture dishes. The medium was changed every 24 hours.
Percoll density gradient centrifugation
For preparing adult Leydig cells, germ cells, and Sertoli cells, Percoll density gradient centrifugation was performed as previously described by Shima et al (7) and Niedziela and Lerchl (39). Briefly, 10 decapsulated testes from male mice were incubated for 10 minutes at 37°C with gentle stirring in 50 mL DMEM (Nacalai Tesque) supplemented with 0.1% BSA (Sigma) and 0.5 mg/mL collagenase (GIBCO). Centrifugation was performed using a discontinuous density gradient of Percoll (20%, 30%, 50%, and 60%; GE Healthcare UK Ltd). The gradient was centrifuged at 2500 × g for 60 minutes at 4°C. After the centrifugation, the gradient was fractionated into plastic tubes, and each fraction was centrifuged at 200 × g for 10 minutes at 4°C and then washed twice with cold DMEM and finally centrifuged again at 200 × g for 10 minutes at 4°C. Germ cells at different developmental stages including spermatozoa were fractioned at the interphase between 20% and 30% of Percoll. Sertoli cells were between 30% and 50% of Percoll, and Leydig cells were present through the 50% Percoll zone.
RNA extraction and quantitative PCR analyses
Total RNA was obtained from whole testes using the RNAeasy minikit (QIAGEN Sciences) according to the manufacturer's instructions. Total RNA was reverse transcribed using 500 ng poly-(deoxythymidine) and 0.25 U avian myeloblastosis virus-reverse transcriptase at 42°C for 75 minutes and 95°C for 5 minutes. Quantitative real-time PCR analyses were performed as previously (40). Briefly, cDNA and primers shown in Supplemental Table 1 were added to 15 μL total reaction volume of the Power SYBR Green PCR master mix (Applied Biosystems). PCRs were then performed using the StepOne real-time PCR system (Applied Biosystems). Conditions were set to the following parameters: 10 minutes at 95°C followed by 45 cycles each of 15 seconds at 95°C and 1 minute at 64°C. L19 was used as a control for reaction efficiency and variations in concentrations of mRNA in the original reverse transcriptase reaction.
Western blot analyses
Whole testes were lysed with radioimmunoprecipitation assay buffer (20 mM Tris, pH 7.5; 150 mM NaCl, 1% [vol/vol] Nonidet P-40, 0.5% [wt/vol] sodium deoxycholate, 1 mM EDTA, and 0.1% [wt/vol] sodium dodecyl sulfate) containing complete protease inhibitors (Roche). Western blot analyses were performed according to our previous study (17). Briefly, extracts (10 μg protein) were resolved by SDS-PAGE (12.5%) and transferred to polyvinyl difluoride membranes (GE Bioscience). Membranes were blocked in Tris-buffered saline and Tween 20 (10 mM Tris, pH7.5; 150 mM NaCl; and 0.05% [vol/vol] Tween 20) containing 5% (wt/vol) nonfat Carnation instant milk (Nestle Co). Blots were incubated with primary antibodies (anti-NRG1 antibody was used at 1:1000 dilutions and anti-β-actin antibody was used at 1:10 000) overnight at 4°C. After washing in Tris-buffered saline and Tween 20 (10 mM Tris, pH7.5; 150 mM NaCl; and 0.05% [vol/vol] Tween 20), enhanced chemiluminescence detection was performed using the enhanced chemiluminescence system according to the manufacture's specifications (GE Bioscience) and appropriate exposure of the blots to Fuji X-ray film (Fujifilm). The intensity of the bands was analyzed using a Gel-Pro analyzer (Media Cybernetics).
Immunohistochemistry
Testes were collected and fixed in Bouin's solution (Sigma) overnight, dehydrated in 70% (vol/vol) ethanol, and embedded in paraffin. The fixed sections (4 μm) embedded in paraffin were deparaffinized in xylene washes and quenched with 3% hydrogen peroxide in methanol. The sections were incubated with 20% (vol/vol) nonimmune goat serum/PBS to block nonspecific sites followed by incubation with primary antibody overnight at 4°C. The positive signals were visualized using VECTASTAINE life avidin biotin complex rabbit IgG kit (Vector Laboratories) according to the manufacture's recommendations.
Immunofluorescence
Testes were collected and fixed in 4% (wt/vol) paraformaldehyde/PBS overnight and embedded in paraffin and processed by routine procedures as described above. Sections were probed with the primary antibodies. (The primary antibodies were used at 1:100 except for anti-PCNA antibody [1:2000].) After washing by 0.3% (vol/vol) Triton X-100 in PBS (−), the antigens were visualized with Cy3-conjugated goat antirabbit IgG (1:200; Sigma), fluorescein isothiocyanate-conjugated goat antirabbit IgG (1:200; Sigma), Cy3-conjugated goat antirat IgG (1:200; Sigma), or Cy3-conjugated goat antimouse IgG (1:200; Sigma) and DAPI (VECTESHIELD mounting medium with DAPI; Vector Laboratories). Digital images were captured using a Keyence BZ-9000 microscope (Keyence Co) with a ×20 objective.
Morphology of seminiferous tubes
Testes were collected and fixed in Bouin's solution (Sigma) overnight and embedded in paraffin and processed by routine procedures as described above. Sections were stained in periodic acid-Schiff with hematoxylin as a nuclear stain. The identification of sperm and staging of spermatogenesis in the seminiferous tubules was done according to Oakberg (41) and Russell et al (42).
Terminal deoxynucleotidyl transferase-mediated biotinylated deoxyuridine triphosphate nick end-labeling (TUNEL) assay
Analyses of apoptotic cells (containing fragmented DNA) in testes were performed by the TUNEL method (in situ cell death detection kit POD; Roche Diagnostics) according to the manufacturer's instructions. Paraffin-embedded tissue sections (4 μm) of mouse testes were deparaffinized and treated with 30% H2O2/methanol to block endogenous peroxidase activity. After washing with PBS, the slides were incubated with 20 μg/mL proteinase K (Sigma) in 10 mM Tris-HCl (pH 7.4) at room temperature for 30 minutes. After washing with PBS, the positive control sample (but not the experimental samples) was treated with deoxyribonuclease (5 μg/mL) for 15 minutes at 4°C. Then all tissue samples were rinsed in PBS and incubated with the TUNEL reaction mixture at 37°C for 30 minutes. The tissues were rinsed in PBS and incubated in DAB (POD substrate) for 10 minutes. The number of TUNEL-positive cells and the total number of cells were counted in the interstitial region of the testes.
Measurement of T
The concentration of T was measured using a rodent T ELISA kit (Endocrine Technology) according to the manufacturer's instructions. Serum samples (50 μL each) were analyzed using a microplate reader to determine the amount of substrate converted at 450 nm (Bio-Rad Laboratories).
Statistics
Statistical analyses of data from three or four replicates for comparison were carried out by either a Student's t test or a one-way ANOVA followed by a Student's t test (Statview; Abacus Concepts, Inc). In the in vitro culture studies, the data obtained from three different cultures were carried out by a two-way ANOVA (Statview; Abacus Concepts, Inc).
Results
Temporal changes in the expression and localization of NRG1 in mouse testis after birth
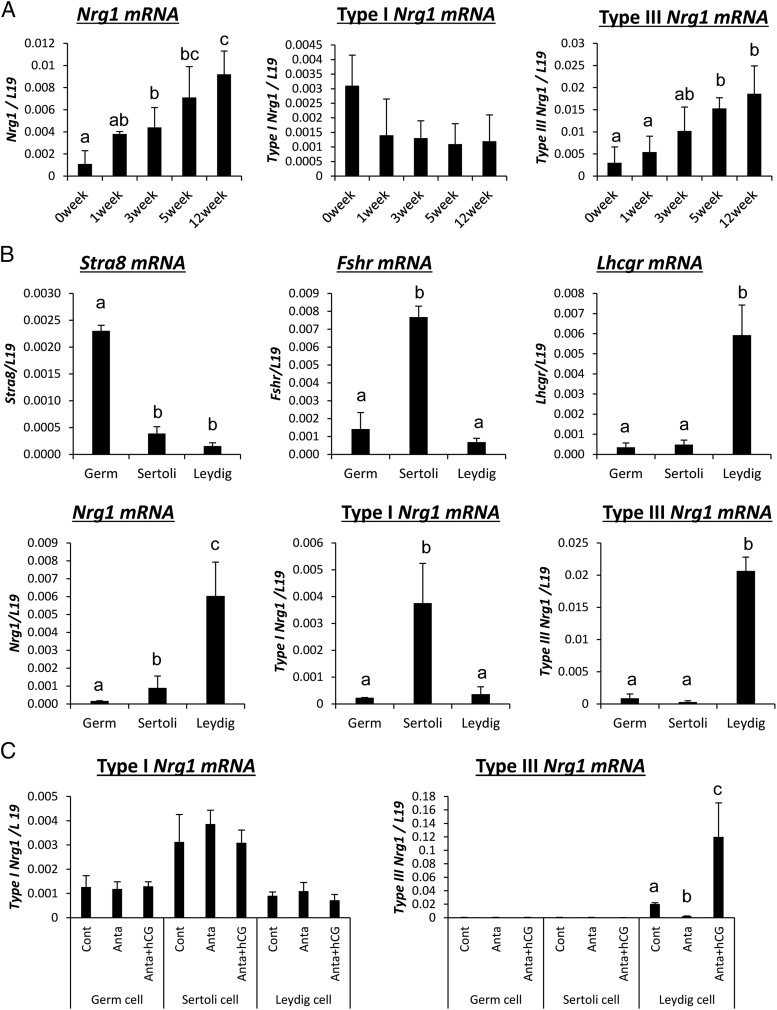
The mouse Nrg1 gene has three different transcriptional start sites, encoding types I, II, and III mRNAs and protein. Therefore, we made three primer sets to identify each type of Nrg1 transcript in mouse testes by RT-PCR. The expression of type I Nrg1 was stably detected in testes of mice from birth (0 wk) to adult (12 wk) (Figure 1A). By contrast, type III Nrg1 increased significantly in an age-dependent manner and type II Nrg1 was not amplified by our primer set (Supplemental Figure 2A and Figure 1A). To clarify which type of cells in adult testis expressed each type of Nrg1 transcript, testicular tissue was separated into germ cells, Sertoli cells, and Leydig cell fractions by Percoll density gradient centrifugation according to Shima et al (7) and Niedziela et al (39). In the germ cell-enriched fraction, Stra8 mRNA, a known marker of germ cells, was strongly detected; however, the expressions of Nrg1 was low. In Sertoli cells, not only Fshr mRNA but also type I Nrg1 mRNA were highly expressed; however, the expression level of type I Nrg1 was not altered by a GnRH antagonist and/or hCG injection. In Leydig cells, type III Nrg1 and Lhcgr mRNAs were strongly detected. Moreover, in mice treated with the GnRH antagonist, the expression of type III Nrg1 mRNA was significantly decreased (Figure 1, B and C), and the suppressive effects were reversed by the additional hCG treatment (Figure 1C). An anti-NRG1 antibody recognized NRG1 in Leydig cells localized in the testicular interstitial tissue of hCG-injected mice (Supplemental Figure 2B), indicating that LH acts on Leydig cells to induce the expression of type III Nrg1 in testis.
Figure 1.
Kinetic changes of type III Nrg1 expression in LH/hCG-stimulated Leydig cells in mouse testes. A, The kinetic changes of Nrg1 (total, type I, or type III) expression in the testis collected from 0 to 12 weeks of age. Levels of mRNA were normalized to that of L19. Values are represented as the mean ± SEM of three replicates. Different superscripts denote significant differences in each cell (P < .05). B, The expression of each type of Nrg1 or specific markers of each type of testis cells in germ cells, Sertoli cells, or Leydig cells of the adult testis. Testes were collected from 12-weeks-old mice, and then percoll density gradient centrifugation was performed for the preparation of each type of cell in the testis. Levels of the gene expression were normalized to that of L19. Values are represented as the mean ± SEM of three replicates. Different superscripts denote significant differences in each cells (P < .05). C, The expression of type I Nrg1 and type III Nrg1 in each type of cell in the testis after GnRH antagonist treatment and/or hCG injection. Immature mice at 21 days of age were treated daily for 2 days with a GnRH antagonist (25 μg/d) and then received an ip injection of 10 IU hCG. Before GnRH antagonist treatment (control) and at 0 (anta) and 4 hours (hCG) after hCG, testes were collected, and then percoll density gradient centrifugation was performed for the preparation of each type of cell in the testis. The mRNA levels of type I Nrg1 and type III Nrg1 were analyzed by real-time PCR using specific primers that recognize the each specific domain. Levels of the gene expression were normalized to that of L19. Values are represented as the mean ± SEM of three replicates. Different superscripts denote significant differences in each cell (P < .05).
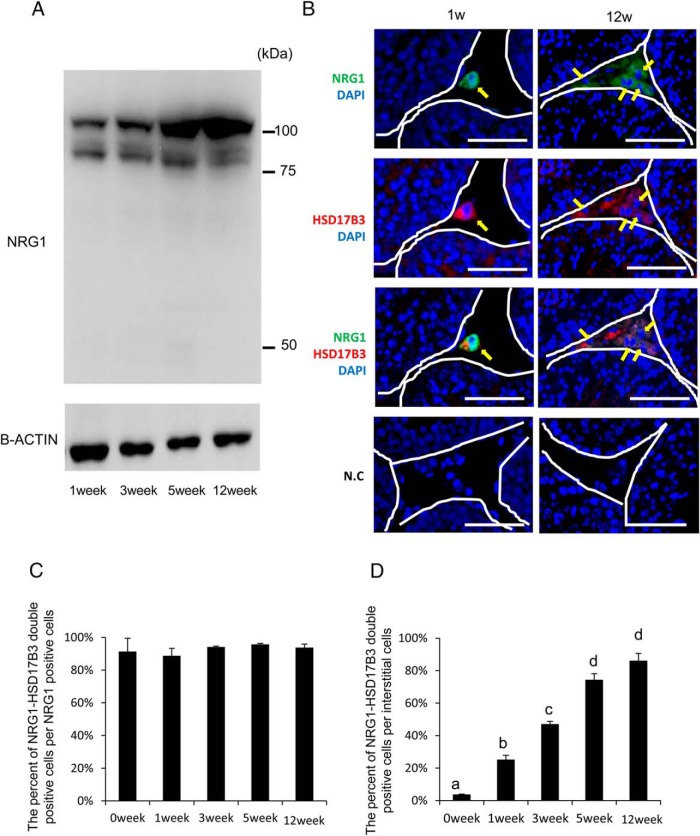
The intensity of the NRG1 upper band also increased in parallel with the induction of type III Nrg1 mRNA (Figure 2A). However, the lower band of NRG1 was not dramatically changed (Figure 2A). The cellular localization of NRG1 in mouse testes was examined by double immunofluorescence using anti-NRG1 C-terminal and anti-HSD17B3 antibodies that specifically recognize functional Leydig cells in interstitial tissue (7). At both 1 and 12 weeks of age, positive signals for the anti-NRG1 C terminus and anti-HSD17B3 were observed in the interstitial tissue (Figure 2B, arrows). The percentage of double-positive cells per NRG1 positive cells was about 90% and this percentage did not change during development of the testis after birth (Figure 2C). However, the percentage of double-positive cells per interstitial cells was significantly increased in an age-dependent manner (Figure 2D). The localization of ERBB3, one of the NRG1 receptors, was analyzed in the testes of mice at 1 and 12 weeks of age. The ERBB3-positive signals colocalized with anti-HSD17B3-positive cells, indicating that NRG1 is expressed in and acts on ALCs at 12 weeks (Supplemental Figure 3).
Figure 2.
NRG1 is expressed in HSD17B3-positive Leydig cells (ALCs) in mouse testes. A, Western blot analysis of NRG1 protein levels in testes collected from 1 to 12 weeks of age. The membrane was incubated with a commercial anti-NRG1 polyclonal antibody (Abcam) that recognizes the IgG repeat and EGF domain of NRG1. β(B)-Actin was used as a loading control. Results are representative of three separate experiments. B, Cross-sections of mouse testes were stained with antibodies to visualize either NRG1 (green) or HSD17B3 (red), a marker of adult Leydig cells, at postnatal week 1 (infant) and 12 weeks (adult). White lines delineate the border of the seminiferous tubules. Scale bars correspond to 100 μm. Arrows (yellow) indicate NRG1-positive cells and/or HSD17B3-positive cells in the seminiferous tubules and interstitial tissue. N.C., negative controls without primary antibody. C, The percentage of NRG1-HSD17B3 double-positive cells per NRG1 positive cells in the interstitial tissue of testes at each age. The number of positive cells in a section per testis was counted and a total of three different samples were analyzed. Values are mean ± SEM of three different samples. D, The percentage of NRG1-HSD17B3 double-positive cells per all of interstitial cells. The number of positive cells or total number of interstitial cells in each section per testis was counted and a total three different samples were analyzed. Values are mean ± SEM of three different samples. Different superscripts denote significant differences in each cells (P < .05).
The characteristics of male fertility in LeyNrg1KO
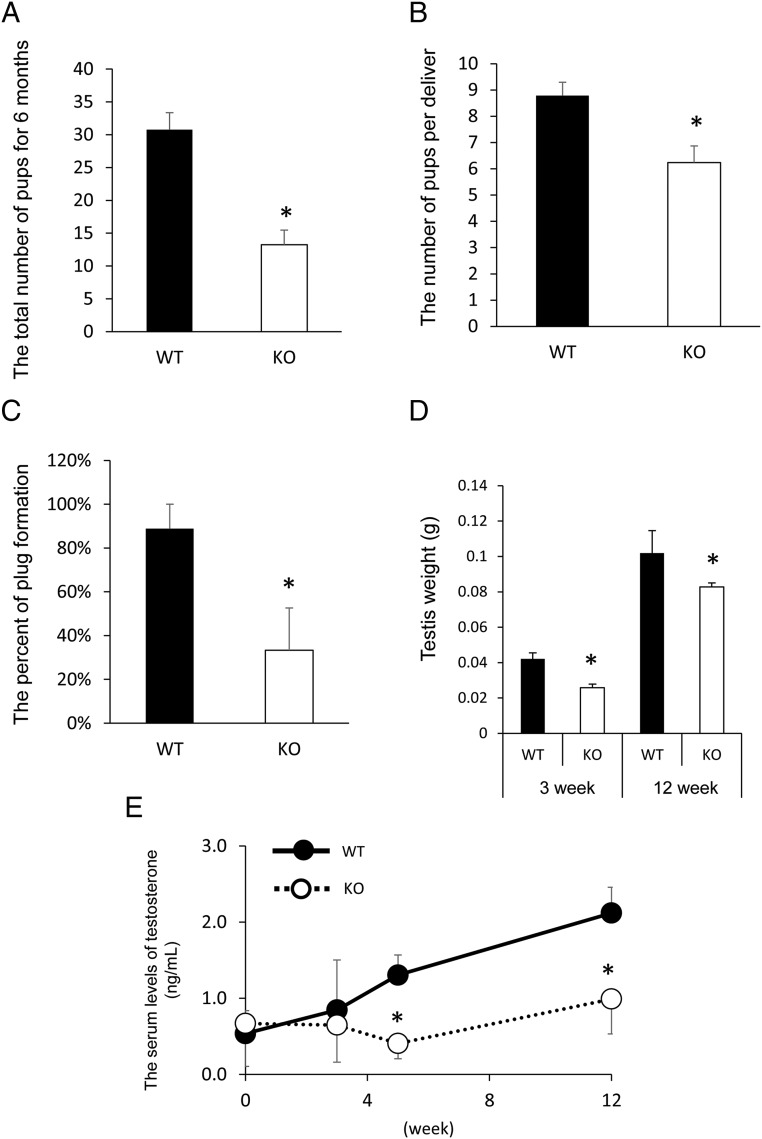
The fertility of the mutant male mice was tested by mating each Nrg1flox/flox; Cyp19a1-Cre male mouse (Leydig cell specific-Nrg1 KO: LeyNrg1KO) with a WT fertile female for 6 months. The total number of pups born during 6 months was significantly lower when mutant males were housed with WT female mice as compared with that in the WT mating pair (Figure 3A). The number of pups born was also significantly lower in the mutant/WT pairs (6.1 ± 0.7) than that in WT/WT pairs (8.8 ± 0.6) (P < .05) (Figure 3B). The percentage of females plugged by LeyNrg1KO males was also significantly lower than that in the WT mating pairs when each adult male mouse (12 wk age) was caged with a hormone-stimulated female mouse, respectively (Figure 3C). The testes of LeyNrg1KO mice were smaller and weighed less than the testes of WT mice (Figure 3D and Supplemental Figure 4). The serum levels of T in the mutant male are similar to those in WT mice at 0–3 weeks of age; however, T levels increased in WT mice at 5 weeks of age. This increase was not observed in the mutant male mice in which the levels of T were significantly lower than those in WT mice (Figure 3E).
Figure 3.
Analyses of male fertility in LeyNrg1KO mice. A, The total number of pups born in each LeyNrg1KO (KO) male and WT female mating pair or each Nrg1flox/flox (WT) male and WT female mating pair was determined during a 6-month breeding period. Five pairs were analyzed in each genotype. *, Significant difference between genotypes (P < .05). B, The average number of pups born per litter during 6 months of breeding was calculated for each LeyNrg1KO (KO) male and WT female or Nrg1flox/flox (WT) male and WT female mating pairs. Five pairs were prepared in each genotype. *, Significant difference between genotypes (P < .05). C, Plug formation in each WT female mated with either an LeyNrg1KO (KO) male or Nrg1flox/flox (WT) male. Each male was tested for 1 hour with an eCG-hCG-treated female. Tests were done using three male mice of each genotype. *, Significant difference between genotypes (P < .05). D, Testis weight in 3- or 12-week-old WT and LeyNrg1KO (KO) male mice. Values are represented as the mean ± SEM of five testes. *, Significant differences observed between genotypes at each age (P < .05). E, Serum levels of T in WT or LeyNrg1KO (KO) mice (n = 3 animals at each age for each genotype). Values are represented as the mean ± SEM of three replicates. *, Significant differences observed at 5 or 12 weeks age between genotypes (P < .05).
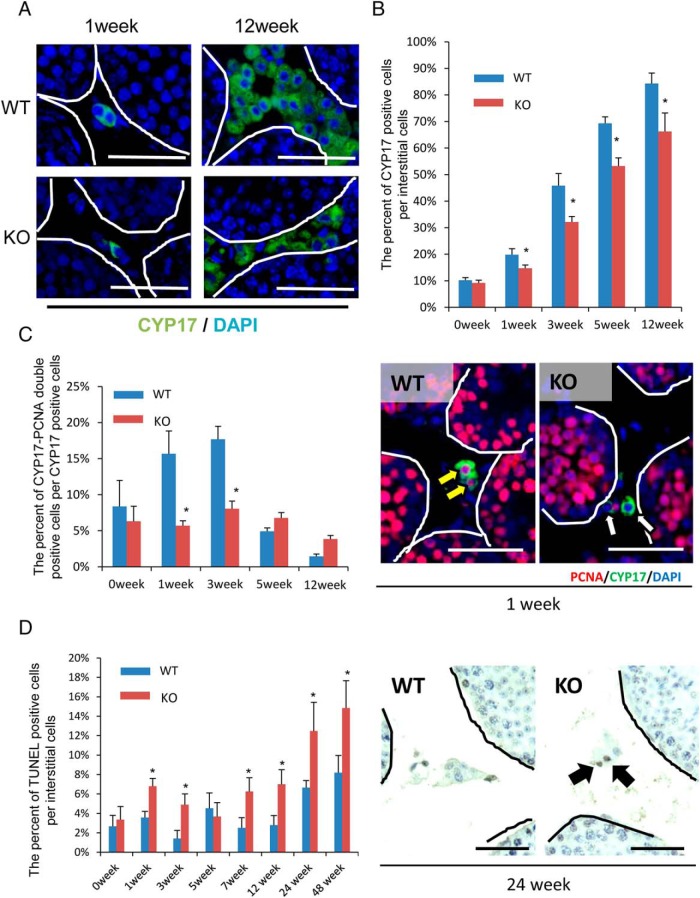
In 12 week-old adult WT testes, cells immunopositive for CYP17A1 were observed throughout the interstitial tissue, whereas the level of CYP17A1 was much less in the adult LeyNrg1KO testes (Figure 4A). The percentage of CYP17A1-positive cells per interstitial cells was significantly higher in WT than LeyNrg1KO mice and increased to about 90% in the adult testis of WT but only to 70% in LeyNrg1KO mice (Figure 4B). These results indicate that the proliferation and/or survival of Leydig cells is reduced by the depletion of NRG1. Strikingly, the percentage of PCNA-CYP17AI double-positive cells per CYP17A1-positive cells in the interstitial cells was high at 1 and 3 weeks after birth, whereas the percentage declined by 12 weeks in WT. However, the percentage of PCNA-CYP17A1 double-positive cells did not change in the LeyNrg1KO from 1 to 12 weeks after birth, and the percentage was significantly lower than that of WT at 1 week and 3 weeks of age (Figure 4C). Additionally, the percentage of TUNEL-positive cells per interstitial cells was significantly higher in LeyNrg1KO than that of WT, especially in older mice (Figure 4D), indicating greater apoptosis.
Figure 4.
The proliferation and survival of Leydig cells is suppressed in LeyNrg1KO mice. A, Immunofluorescence staining for CYP17A1, a marker of Leydig cells in testes of WT and LeyNrg1KO (KO) mice at 1 and 12 weeks of age. Scale bars correspond 100 μm. B, The percentage of CYP17A1-positive interstitial cells per total number of interstitial cells. The number of positive cells per section per testis was evaluated (n = 3 animals at each age for each genotype). Values are represented as the mean ± SEM of three sections. *, Significant differences at each age between genotypes (P < .05). C, The percentage of PCNA-CYP17A1 double-positive interstitial cells per CYP17A1-positive interstitial cells (n = 3 animals at each age for each genotype). Values are represented as the mean ± SEM of three sections. *, Significant differences at each age between genotypes (P < .05). Scale bars correspond to 5 μm. D, The percentage of TUNEL-positive interstitial cells per all of interstitial cells. The number of positive cells per section per testis was evaluated (n = 3 animals at each age for each genotype). Values are represented as the mean ± SEM of three sections. *, Significant differences at each age between genotypes (P < .05).
NRG1 promotes the proliferation of ALCs in infant testis in vitro in response to LH
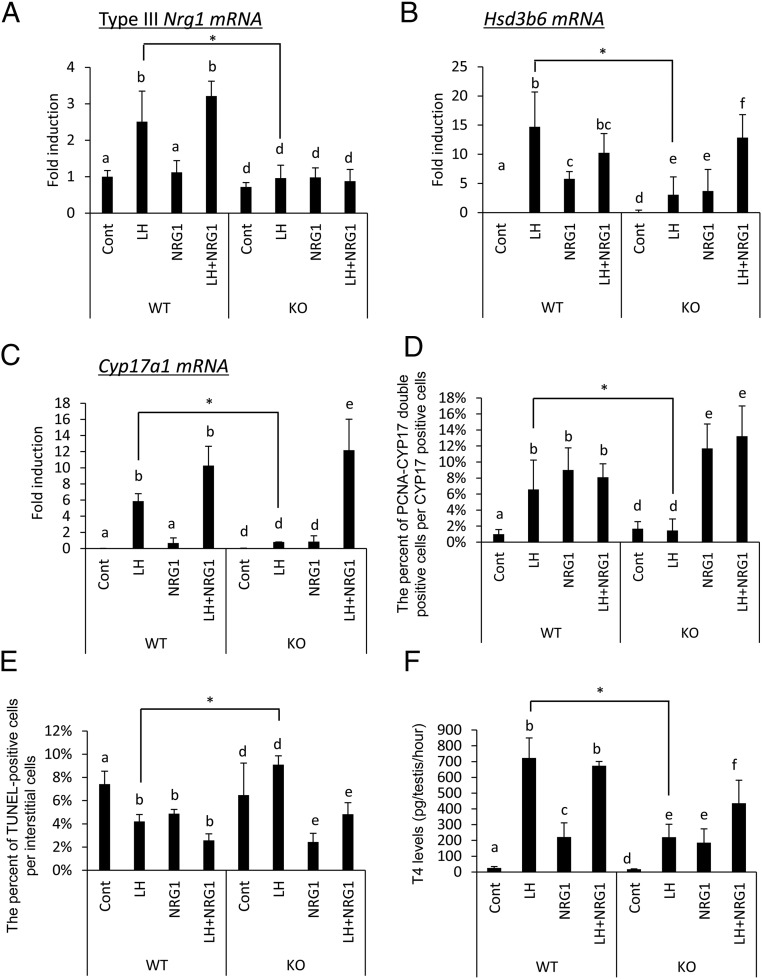
To determine whether NRG1 directly regulates both the proliferation and survival of Leydig cells in LH-stimulated testes, we cultured testes from day 10 WT and mutant mice with either LH or NRG1 alone or in combination. In WT testes, Nrg1 was increased by LH but not NRG1, and the addition of NRG1 to the LH-containing medium did not affect the Nrg1 expression (Figure 5A). The expression levels of Cyp17a1 and Hsd3b6 were dramatically increased by LH but not by NRG1. The combinational treatment with LH and NRG1 did not enhance the expression levels of Cyp17a1 and Hsd3b6 as compared with those in the testis cultured with LH alone (Figure 5, B and C). The proliferation of Leydig cells (PCNA and CYP17A1 double positive cells) was significantly induced by either LH or NRG1 alone (Figure 5D). The induction of apoptosis was also significantly decreased by treatment with either LH or NRG1 alone (Figure 5E). The T levels in the culture media were significantly increased by either LH or NRG1; however, the response to LH was greater than that that to NRG1, and no additional effect was detected by costimulation with LH and NRG1 (Figure 5F).
Figure 5.
NRG1 is essential for the proliferation of ALCs in infant testis cultured with LH. A–C, Testes from postnatal day 10 mice of both genotypes, WT and LeyNrg1KO (KO), were cultured for 3 days in media (control [cont]), LH (100 ng/μL), NRG1 (50 ng/μL), and LH+NRG1. The levels of mRNA encoding Nrg1 type III (a), Hsd3b6 (b), or Cyp17a1 (c) were analyzed by real-time PCR and normalized to that of L19. The value of controls (cont; no treatment) is set as 1. The data are presented as fold induction (mean ± SEM of three different experiments). The data were statistically analyzed by a two-way ANOVA. If a significant difference was observed in each factor, the comparison analysis was done by a Student's t test. Different superscripts denote significant differences among the treatment groups in each genotype (P < .05). *, Significant differences observed in each treatment group between genotypes. D and E, Testes from postnatal day 10 mice of both genotypes were cultured for 3 days with media alone (control [cont]), LH (100 ng/μL), NRG1 (50 ng/μL), and LH+NRG1. The number of PCNA-CYP17A1 double-positive (d) and TUNEL-positive (e) cells per section per each testis was analyzed (n = 3 for each treatment group and genotype). Values are presented as the mean ± SEM of three different cultured testes. The data were statistically analyzed by a two-way ANOVA. If the significant difference was observed in each factor, the comparison analysis was done by a Student's t test. Different superscripts denote significant differences among the treatment groups in each genotype (P < .05). *, Significant differences observed in each treatment group between genotypes. F, T (T4) levels in the culture medium after incubating testes from postnatal day 10 mice of both genotypes for 3 days with media alone (control [Cont]), LH (100 ng/μL), NRG1 (50 ng/μL), and LH+NRG1. The data were statistically analyzed by a two-way ANOVA. If the significant difference was observed in each factor, the comparison analysis was done by a Student's t test. Different superscripts denote significant differences among the treatment groups in each genotype (P < .05). *, Differences observed in each treatment group between genotypes.
In the testes of the mutant mice, the induction of Nrg1 expression was not observed in any treatment groups (Figure 5A). The expression of Hsd3b6 was induced by LH and NRG (Figure 5B). The strong induction of Hsd3b6 was observed when the tissues were cultured with both LH and NRG1 (Figure 5B). The expression level of Cyp17a1 was not induced by either LH or NRG1. However, a dramatic induction of Cyp17a1 was detected by the combinational treatment with LH and NRG1 (Figure 5C). The percentage of PCNA and CYP17A1 double-positive cells per CYP17A1 single-positive cells was not significantly increased by LH treatment. Rather, NRG1 alone increased the number of PCNA and CYP17A1 double-positive cells (Figure 5D). The percentage of TUNEL-positive cells in testis cultured with LH was similar to that in control (without any hormone), whereas the addition of NRG1 reduced the rate of TUNEL-positive cells in the interstitium of the mutant testes (Figure 5E). Either LH or NRG1 increased T production; however, the maximum levels were observed when the mutant testes were cultured with both LH and NRG1 (Figure 5F). These results indicated that the proliferation and survival of Leydig cells in the infant testis were dependent on the LH-induced expression and functions of NRG1 and that the production of T increased in relationship to the number of the Leydig cells within the testis.
Reduced levels of elongated sperm in the LeyNrg1KO mice
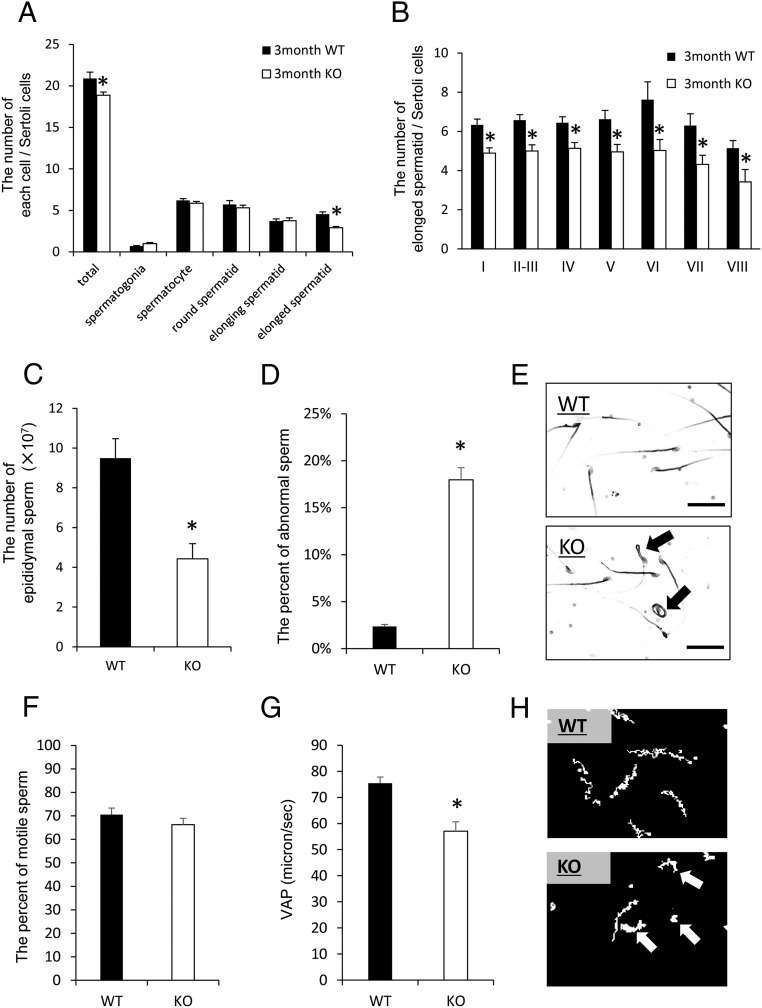
The spermatogenic cycle within the seminiferous tubules of the LeyNrg1KO mice showed a pattern similar to that observed in WT mice (Supplemental Figure 6A). Additionally, the number of Sertoli cells did not differ between WT and LeyNrg1KO mice (Supplemental Figure 6B), suggesting that the disruption of NRG1 in ALCs did not alter the cellular organization of the seminiferous tubules or the systematic differentiation of male germ cells. However, the total number of male germ cells per Sertoli cells in each seminiferous tubule was significantly lower in the LeyNrg1KO mice as compared with that in the WT mice (Figure 6A). The process of spermatogenesis from spermatogonia to spermatozoa is complex and involves multiple changes in sperm morphology (43). In the LeyNrg1KO mice, the number of elongated spermatids was significantly lower than that in the WT; however, the number of sperm at other stages did not differ between genotypes (Figure 6, A and B). TUNEL analyses indicated that apoptosis occurred selectively in spermatids within the lumen of the seminiferous tubules in the LeyNrg1KO mice (Supplemental Figure 6, D and E), indicating that the differentiation from elongating spermatids to elongated spermatids (mature sperm) was abnormal in LeyNrg1KO mice.
Figure 6.
Abnormal spermatogenesis occurs during the elongation stage in LeyNrg1KO mice. A, The number of germ cells at each stage of spermatogenesis per Sertoli cell in 3-month-old WT and LeyNrg1KO (KO) mice. Paraffin sections of each testis were stained in periodic acid-Shiff, and germ cells at each stage of spermatogenesis were counted according to Oakberg et al (1956) (41). Values are represented as the mean ± SEM of three different testes in each genotype. *, Significant differences observed in total number of spermatogenic cells and those at the stage of elongation between genotypes. B, The number of elongated spermatids per Sertoli cell at each developmental stage of the seminiferous tubule. Values are represented as the mean ± SEM of three different testes in each genotype. *, Significant differences observed between genotypes. C and D, The number of sperm and the percentage of sperm with abnormal morphology in the epididymis of 3-month-old WT or LeyNrg1KO (KO) mice. Values are presented as the mean ± SEM (n = 3 male mice of each genotype. *, Significant differences observed between genotypes. E, The morphology of sperm collected from 3-month-old WT or LeyNrg1KO (KO) mice. Scale bars correspond to 10 μm. Black arrows represent abnormal sperm tail morphology. F and G, The percentage of motile sperm (f) and the average path velocity (VAP) of sperm (g) in sperm recovered from the epididymis of 3-month-old WT or LeyNrg1KO (KO) mice. Sperm were cultured for 2 hours in 500 μL of modified Human Tubal Fluid medium and analyzed by the CASA system (Neill and Olds-Clarke, 1987 [37]). Values are represented as the mean ± SEM (n = 3 samples for each genotype. *, Significant differences observed between genotypes. H, The motility of sperm recovered from WT and LeyNrg1KO (KO) male mice analyzed by the CASA system (Neill and Olds-Clarke, 1987 [37]).
The number of sperm collected from the epididymis was also significantly lower in the mutant mice as compared with that in WT mice (Figure 6C), abnormal morphologies, such as jackknifed-like sperm tails or folded sperm tails, were observed in the mutant sperm (Figure 6E), and the percentage of sperm with abnormal morphology was significantly higher in mutant mice than WT (Figure 6D). CASA was used for quantitative comparisons of sperm motility (37). CASA-generated sperm tracks showed clear differences between genotypes. Although the motility of sperm did not differ between genotypes (Figure 6F), the patterns of motility were different. WT sperm exhibited a straight path of motility, whereas the mutant sperm showed nonprogressive motility including a loop-like pattern (Figure 6H, arrows). Average path velocity of mutant sperm was 58 μm/sec, a speed significantly lower than 78 μm/sec observed for WT sperm (Figure 6G).
T restores mating behavior and spermatogenesis in LeyNrg1KO mice
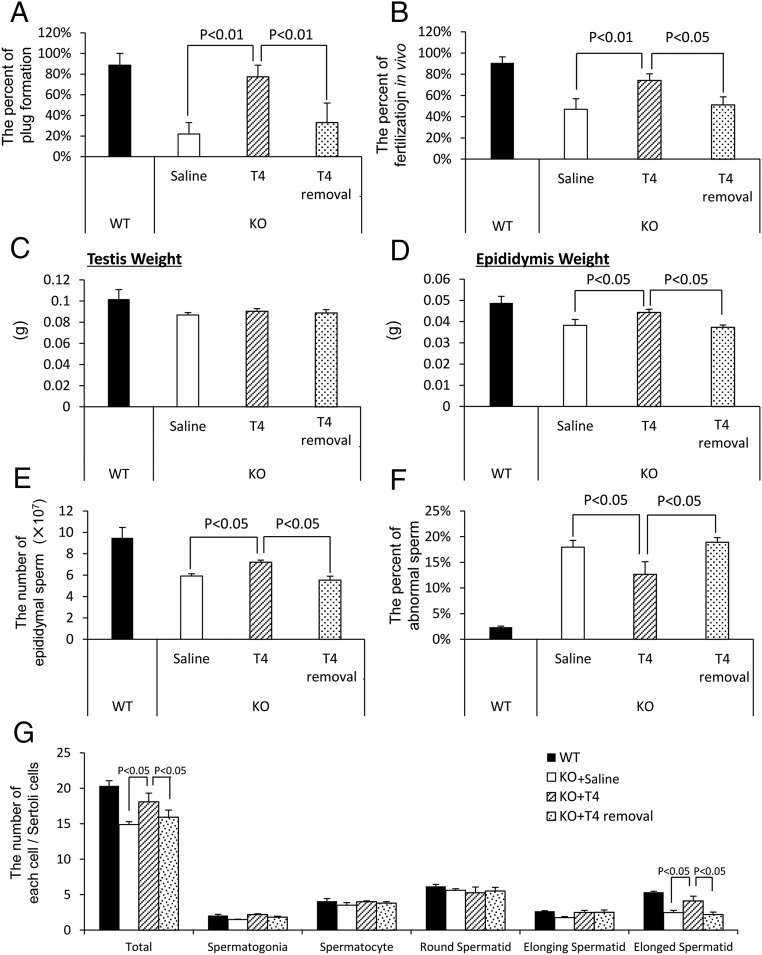
To determine whether the reproductive defects observed in the mutant mice were due to a reduced androgen production by the ALCs, T was injected into 3-month-old LeyNrg1KO mice daily for 1 week. This treatment increased mutant male mating behavior and plug formation (Figure 7A) and in vivo fertilization (Figure 7B) to levels observed in WT mice. Although the weight of the testis was not increased by the treatment with T (Figure 7C), the restoration of normal fertility was associated with an increased weight of the epididymis (Figure 7D), increased numbers of normal sperm collected from the epididymis (Figure 7E), and a reduced number of abnormal sperm (Figure 7F). Additionally, the total number of sperm cells and the number of elongated spermatid increased significantly by the T treatment (Figure 7G), indicating that the abnormal spermatogenesis in LeyNrg1KO mice was due to the low levels of T. These positive effects of T in the mutant mice were reversed within a week after the cessation of T treatment.
Figure 7.
Exogenous T restores mating behavior and spermatogenesis in LeyNrg1KO mice. A, Mating behavior (plug formation) of Nrg1flox/flox (WT) male or LeyNrg1KO (KO) male mice was analyzed either at 1 week after the daily injections of saline (control) or T (T4) or at 1 week after cessation of hormone treatment (T4 removal). At each time interval, each male was housed separately with a WT eCG-hCG-treated female and plug formation evaluated (n = 3 male mice in each treatment group. Values are presented as the mean ± SEM of three replicates. B, The percentage of oocytes containing two pronuclear per total number of oocytes recovered from the oviducts of WT females at 16 hours after hCG injection and mated with WT or mutant male mice. Values are presented as the mean ± SEM of three different female mice. C and D, Testis weight (c) and epididymal weight (d) determined for WT and each treated group of LeyNrg1KO (KO) male mice. Values are mean ± SEM of three animals. E and F, The number of sperm (E) and the percentage of abnormal sperm (F) recovered from the epididymis. Values are mean ± SEM of three animals. G, The number of germ cells at each stage of spermatogenesis per Sertoli cell in WT and each treated group of LeyNrg1KO (KO) male mice. Values are represented as the mean ± SEM of three different testes in each genotype.
Discussion
Our studies in which the gene encoding neuregulin 1 (Nrg1) was selectively depleted in murine Leydig cells provide new insights into the roles and interactions of LH and NRG1 in regulating specific testicular functions, namely Leydig cell proliferation during testis development via the ERK1/2 pathway, survival of fully differentiated Leydig cell (adult Leydig cells, ALCs) in the adult testis by the AKT pathway, and the sperm maturation by maintenance of T production by ALCs.
The proliferation of Leydig cells that is induced by LH is restricted to a narrow time interval (postnatal wk 1–3) before the Leydig cells are fully differentiated into ALCs (18). This proliferation provides the full complement of ALCs in the adult testis in which proliferation no longer occurs (44). Rather, in ALCs of the adult testis, LH induces the expression of genes involved in androgen production (45). Thus, levels of serum androgen in adult male mice is determined both by LH-induced proliferation of Leydig cells during early testis development and by LH-induced expression of steroidogenic genes in ALCs of the adult testis. Our results extend those observed previously showing the following: 1) the proliferation of Leydig cells occurs during the differentiation process from stem to progenitor of Leydig cells in the infant testis but not at later times of differentiation in adult testis (46); 2) either a PKA inhibitor or MEK1/2 inhibitor suppressed not only LH mediated proliferation of Leydig cells but also the expression of genes involved in androgen production (47); 3) disruption of Mek1/2 in Leydig cells reduced number of ALCs in the mature testis without altering the level of cAMP (48); and thus 4) MEK1/2-ERK1/2 signaling is essential for the proliferation of Leydig cells. Our studies clearly show that LH induction of NRG1 contributes to the proliferation Leydig cells during early testis development.
Specifically, in this study, we document that type III NRG1 is induced by LH in HSD17B3-positive Leydig cells and that disruption of Nrg1 in these cells reduced their proliferative activity during early development in the infant testis. In ovarian granulosa cells and in brain Schwan cells, it is known that NRG1 binds to ERBB3 to form the heterodimer with ERBB2 and that the heterodimer activates the MEK1-ERK1/2 signaling pathway (17, 49). ERBB3 is expressed in rat and mouse Leydig cells (29, 50), suggesting that it likely mediates the phosphorylation of ERK1/2 observed in Leydig cells of adult testes; this is greatly diminished in the Nrg1 mutant mice (Supplemental Figure 5A). Collectively, these results suggest that LH induction of NRG1 but not LH itself directly activates MEK1-ERK1/2 signaling via ERBB3 in Leydig cells. That NRG1-ERK1/2 signaling mediates the proliferation of Leydig cells is supported further by the organ cultures of mouse testes. Specifically, we show that either LH or NRG1 induces the proliferation of Leydig cells in the infant (d10) WT testis; however, the Leydig cell proliferation was induced by NRG1 but not by LH in the LeyNrg1KO testes. Conversely, LH but not NRG1 increased androgen production in the organ cultures. Therefore, LH directly increases the androgen production but does not directly induce the proliferation of Leydig cells; the expression of NRG1 is required for the activation of ERK1/2 signaling to induce the proliferation of Leydig cells selectively during early testis development.
Expression of NRG1 persists in fully differentiated Leydig cells, ALCs in the adult testis, and based on results from the mutant mice, we conclude that NRG1 promotes the survival, but not proliferation, of ALCs in the adult testis. Specifically, disruption of Nrg1 in ALCs reduced the cell survivability of ALCs of adult testis. The addition of NRG1 to in vitro cultures of LeyNrg1KO testis decreased the high level of TUNEL-positive Leydig cells to a normal rate observed in the WT testis cultured with LH. The role of NRG1 in the cell survival has been reported by numerous groups and the mechanism of NRG1-induced cell survival is dependent on the phosphorylation of AKT (51, 52). In the present study, fewer Leydig cells were immune positive for phosphorylated AKT in both infant and adult LeyNrg1KO testis compared with WT (Supplemental Figure 5B). Thus, the induction of NRG1 by LH is not only a key factor to promote the proliferation of Leydig cells during development but is also a survival factor that maintains ALC viability in the adult testis.
As a result of the low number and reduced viability of ALCs in adult testis of LeyNrg1KO mice, testis production and serum levels of T were reduced compared with those of WT mice. T activates the androgen receptor (AR) in Sertoli cells in which this pathway is required for the completion of spermatogenesis in the testis (3) and in neuronal cells that maintain sexual behavior (1, 3). The Nrg1 mutant mice exhibited reduced numbers of elongated spermatids in the seminiferous tubules, low numbers of normal sperm in the epididymis, and TUNEL-positive apoptotic spermatids in the lumen region of seminiferous tubules (Supplemental Figure 6, C–E). This phenotype is similar to that reported in which male rats treated with a CYP11A1 inhibitor suppressed T production and increased apoptosis and premature detaching of spermatids from Sertoli cells (53) and in mice hypomorphic for Ar in which the expression level of AR in Sertoli cells was much lower than WT and the number of elongated sperm was reduced (54). Collectively these results indicate that reduced T production in the mutant Nrg1 ALCs promotes apoptosis of spermatids during elongating stage via the abnormal change of adhesion between spermatid and Sertoli cells.
Of relevance, the impaired elongation of spermatids and fertility is rescued in the Nrg1 mutant mice by treatment with exogenous T for 7 days, indicating that T coming from the ALCs is the source of this steroid and that AR present in Sertoli cells mediates androgen action at this specific stage of spermatogenesis. Although Nrg1 is expressed in Sertoli cells of the fetal testis and is required for the proliferation of spermatogonia cells (30), Sertoli cell-derived NRG1 does not appear to be required for the final stage of spermatogenesis. Therefore, the present study is the first report that the expression of NRG1 in Leydig cells is required for the optimal production of androgen in the adult testis. Both studies indicate that NRG1 regulates specific functions in somatic cells of the testis to regulate the specific stages of spermatogenesis from the proliferation of spermatogonia cells to elongating spermatids.
The injection of flutamide, an AR antagonist, immediately after birth negatively affected the sexual behavior of adult male mice, and the phenotype was not rescued by treatment with exogenous androgen in the adult animals (55, 56). Removing the testes of adult mice also reduces the frequency of copulation; however, the decrease of copulation is rescued by the treatment with exogenous T for 5–10 days (57). These differences have been explained by the androgen-mediated differentiation of brain immediately after birth. That is, androgen produced from fetal Leydig cells of the fetal testis stimulates the brain, which determines the sex differentiation of the brain. In the present study, the injection of exogenous androgen to mature LeyNrg1KO improved the sexual behavior, suggesting the abnormal sexual behavior of LeyNrg1KO was caused by the low serum levels of androgen due to the low number of ALCs. Although NRG1 is essential for the development of the brain (58), the rescue experiment in this study excludes the possibility that Nrg1 is deleted in other cell types of the testis and other tissue including the brain.
In conclusion, the present study documents that Nrg1 is expressed and regulated in HSD17B3-positive Leydig cells by LH and that NRG1 acts in the Leydig cells but not in spermatogenic cells to maintain cell proliferation, survival, and T production. Specifically, disruption of Nrg1 in the Leydig cells impaired the proliferation of undifferentiated ALCs, leading ultimately to reduce T production. As a result, the Nrg1 mutant mice exhibit abnormal spermatogenesis, specifically the transition to elongated spermatids, and reduced copulatory behavior. Thus, NRG1 is a novel LH-regulated factor produced by immature and mature testicular interstitial cells that impacts Leydig cells functions, spermatogenesis, and sexual behavior.
Acknowledgments
Ovine LH was kindly provided by Dr A. F. Parlow (the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Disease, Torrance, California). We grateful to Dr Shosei Yoshida, Dr Kenshiro Hara, and Dr Yoshiaki Nakamura (Division of Germ Cell Biology, National Institute for Basic Biology, Okazaki, Japan,) for kindly helping the detection of stage of spermatogenesis.
Current address for Y.S.: Department of Anatomy, Kawasaki Medical School, Kurashiki, Japan.
This work was supported in part by a Grant-in-Aid for Scientific Research (Grants 24688028, 25132708, and 16H05017 (to M.S.) and Grant 15J05331 (to T.U.) from the Japan Society for the Promotion of Science and National Institutes of Health Grant NIH-HD-076980 (to J.S.R.).
Disclosure Summary: The authors have nothing to disclose.
Appendix
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Neuregulin 1 | Anti-NRG1 antibody | Abcam, ab53104 | Rabbit polyclonal | WB:1000 | |
| IF:100 | |||||
| RFP | Anti-RFP antibody | Abcam, ab-62341 | Rabbit polyclonal | 1:200 | |
| PCNA | Anti-PCNA antibody | Cell signaling, 2586 | Mouse monoclonal | 2000 | |
| Phosphorylated ERK1/2 | Antiphosphorylated ERK1/2 antibody | Cell signaling, 4376 | Rabbit monoclonal | 100 | |
| β-Actin | Anti-β-actin antibody | Sigma Chemical Co, A5316 | Mouse monoclonal | 10 000 | |
| CYP17a1 | Anti-CYP17a1 antibody | Bioss, bs-3853 | Rabbit polyclonal | 300 | |
| CRE recombinase | Anti-CRE recombinase antibody, clone 2D8 | Millipore, MAB3120 | Mouse monoclonal | 100 | |
| Phosphorylated AKT | Antiphosphorylated AKT antibody | Assay Designs, number 905-658 | Mouse monoclonal | 100 | |
| EGF domain of NRG1 | PNEFTGDR | Anti-EGF domain of NRG1 antiserum | Kawashima I, et al (28) | Rabbit polyclonal | 5000 |
| HSD17B3 | Anti-HSD17B3 antibody | Shima Y, et al (7) | Rat monoclonal | 100 |
Abbreviations: IF, immunofluorescence; WB, Western blot.
Footnotes
- ALC
- adult Leydig cell
- AR
- androgen receptor
- CASA
- computer-assisted sperm assay
- DAPI
- 4′,6-diamidino-2-phenylindole
- eCG
- equine chorionic gonadotropin
- ERBB
- erb-b2 receptor tyrosine kinase
- FLC
- fetal Leydig cell
- hCG
- human chorionic gonadotropin
- HSD17B3
- 17β-hydroxysteroid dehydrogenase 3
- MEK
- MAPK kinase
- NRG
- neuregulin
- PCNA
- proliferating cell nuclear antigen
- PKA
- protein kinase A
- TUNEL
- terminal deoxynucleotidyl transferase-mediated biotinylated deoxyuridine triphosphate nick end labeling
- WT
- wild type.
References
- 1. Yeh S, Tsai M-Y, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99(21):13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci 2002;115(17):3491–3496. [DOI] [PubMed] [Google Scholar]
- 3. Chang C, Chen Y-T, Yeh S-D, et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griswold SL, Behringer RR. Fetal Leydig cell origin and development. Sex Dev. 2009;3(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Shaughnessy PJ, Fowler PA. Endocrinology of the mammalian fetal testis. Reproduction. 2011;141(1):37–46. [DOI] [PubMed] [Google Scholar]
- 6. O'Shaughnessy PJ, Baker PJ, Heikkilä M, Vainio S, McMahon AP. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis—androstenedione is the major androgen secreted by fetal/neonatal Leydig cells. Endocrinology. 2000;141(7):2631–2637. [DOI] [PubMed] [Google Scholar]
- 7. Shima Y, Miyabayashi K, Haraguchi S, et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol Endocrinol. 2013;27(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kerr J, Knell C. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988;103(3):535–544. [DOI] [PubMed] [Google Scholar]
- 9. Clark AM. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63(6):1825–1838. [DOI] [PubMed] [Google Scholar]
- 10. Baker P, O'Shaughnessy P. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122(2):227–234. [DOI] [PubMed] [Google Scholar]
- 11. Roosen-Runge EC, Anderson D. Development of the interstitial cells in the testis of the albino rat. Cells Tissues Organs. 1959;37(1–2):125–137. [DOI] [PubMed] [Google Scholar]
- 12. Mendis-Handagama SM, Risbridger GP, de Kretser DM. Morphometric analysis of the components of the neonatal and the adult rat testis interstitium. Int J Androl. 1987;10(3):525–534. [DOI] [PubMed] [Google Scholar]
- 13. Richards JS, Hedin L, Caston L. Differentiation of rat ovarian thecal cells: evidence for functional luteinization. Endocrinology. 1986;118(4):1660–1668. [DOI] [PubMed] [Google Scholar]
- 14. Moyle WR, Ramachandran J. Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology. 1973;93(1):127–134. [DOI] [PubMed] [Google Scholar]
- 15. Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-LC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. [DOI] [PubMed] [Google Scholar]
- 16. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. [DOI] [PubMed] [Google Scholar]
- 17. Noma N, Kawashima I, Fan H-Y, et al. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2011;25(1):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Ge R-S, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu G-X, Lin H, Chen G-R, et al. Deletion of the Igf1 gene: suppressive effects on adult Leydig cell development. J Androl. 2010;31(4):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hekimgil M, Altay B, Yakut BD, Soydan S, Ozyurt C, Killi R. Leydig cell tumor of the testis: comparison of histopathological and immunohistochemical features of three azoospermic cases and one malignant case. Pathol Int. 2001;51(10):792–796. [DOI] [PubMed] [Google Scholar]
- 21. Pfister D, Richter S, Thüer D, Giedl J, Heidenreich A, Klotz T. Her-2/neu expression in testicular cancer—a retrospective analysis in 57 cases. Urology 2010;76(5):1266.e6–e9. [DOI] [PubMed] [Google Scholar]
- 22. Mandoky L, Geczi L, Bodrogi I, Toth J, Bak M. Expression of HER-2/neu in testicular tumors. Anticancer Res. 2003;23(4):3447–3451. [PubMed] [Google Scholar]
- 23. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. [DOI] [PubMed] [Google Scholar]
- 24. Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. [DOI] [PubMed] [Google Scholar]
- 25. Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100(15):8933–8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shin I, Kim HJ, Nah WH, Park HJ, Gye MC, Park HY. Expression of activated HER2 in human testes. Fertil Steril. 2011;95(8):2725–2728. [DOI] [PubMed] [Google Scholar]
- 27. Mei L, Xiong W-C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawashima I, Umehara T, Noma N, et al. Targeted disruption of Nrg1 in granulosa cells alters the temporal progression of oocyte maturation. Mol Endocrinol. 2014;28(5):706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimada M, Umehara T, Hoshino Y. Roles of epidermal growth factor (EGF)-like factor in the ovulation process. Reprod Med Biol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Eto K, Honmyou A, Nakao K, Kiyonari H, Abé S. Neuregulins are essential for spermatogonial proliferation and meiotic initiation in neonatal mouse testis. Development. 2011;138(15):3159–3168. [DOI] [PubMed] [Google Scholar]
- 31. Fan H-Y, Shimada M, Liu Z, et al. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135(12):2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang X, Arber S, William C, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30(2):399–410. [DOI] [PubMed] [Google Scholar]
- 33. Kawamura K, Kumagai J, Sudo S, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA. 2004;101(19):7323–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Houk CP, Pearson EJ, Martinelle N, Donahoe PK, Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology. 2004;145(3):1269–1275. [DOI] [PubMed] [Google Scholar]
- 35. Kayasuga Y, Chiba S, Suzuki M, et al. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185(2):110–118. [DOI] [PubMed] [Google Scholar]
- 36. Nakada K, Sato A, Yoshida K, et al. Mitochondria-related male infertility. Proc Natl Acad Sci USA. 2006;103(41):15148–15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neill J, Olds-Clarke P. A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res. 1987;18(2):121–140. [DOI] [PubMed] [Google Scholar]
- 38. Delbès G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal leydig cell development via estrogen receptor α. Endocrinology. 2005;146(5):2454–2461. [DOI] [PubMed] [Google Scholar]
- 39. Niedziela M, Lerchl A. Isolation method of Leydig cells from mature male Djungarian hamsters (Phodopussungorus) and their steroidogenic activity in vitro. Andrologia. 1999;31(3):157–161. [PubMed] [Google Scholar]
- 40. Shimada M, Yanai Y, Okazaki T, et al. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21(10):2487–2502. [DOI] [PubMed] [Google Scholar]
- 41. Oakberg EF. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99(3):391–413. [DOI] [PubMed] [Google Scholar]
- 42. Russell LD, Ettlin RA, Sinha Hikim AP. Histological and histopathological evaluation of the testis. Cache River Press 1990:62–193. [Google Scholar]
- 43. Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55(4):548–573. [DOI] [PubMed] [Google Scholar]
- 44. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15(1):172–183. [DOI] [PubMed] [Google Scholar]
- 45. Zhang F-P, Pakarainen T, Zhu F, Poutanen M, Huhtaniemi I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145(3):1453–1463. [DOI] [PubMed] [Google Scholar]
- 46. Mendis-Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65(3):660–671. [DOI] [PubMed] [Google Scholar]
- 47. Hirakawa T, Ascoli M. The lutropin/choriogonadotropin receptor-induced phosphorylation of the extracellular signal-regulated kinases in Leydig cells is mediated by a protein kinase A-dependent activation of ras. Mol Endocrinol. 2003;17(11):2189–2200. [DOI] [PubMed] [Google Scholar]
- 48. Yamashita S, Tai P, Charron J, Ko C, Ascoli M. The Leydig cell MEK/ERK pathway is critical for maintaining a functional population of adult Leydig cells and for fertility. Mol Endocrinol. 2011;25(7):1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sheean ME, McShane E, Cheret C, et al. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28(3):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abid SN, Richardson TE, Powell HM, et al. A single spermatogonia heterogeneity and cell cycles synchronize with rat seminiferous epithelium stages VIII-IX. Biol Reprod. 2014;90(2):32–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes: persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273(17):10261–10269. [DOI] [PubMed] [Google Scholar]
- 52. Flores AI, Mallon BS, Matsui T, et al. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20(20):7622–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55(4):895–901. [DOI] [PubMed] [Google Scholar]
- 54. Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131(2):459–467. [DOI] [PubMed] [Google Scholar]
- 55. Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10(1):40–53. [DOI] [PubMed] [Google Scholar]
- 56. Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiol Behav. 2003;79(4–5):633–641. [DOI] [PubMed] [Google Scholar]
- 57. Harding SM, Velotta JP. Comparing the relative amount of testosterone required to restore sexual arousal, motivation, and performance in male rats. Horm Behav. 2011;59(5):666–673. [DOI] [PubMed] [Google Scholar]
- 58. Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–703. [DOI] [PubMed] [Google Scholar]