Abstract
Polymorphisms in the SLC30A8 gene, which encodes the ZnT8 zinc transporter, are associated with altered susceptibility to type 2 diabetes (T2D), and SLC30A8 haploinsufficiency is protective against the development of T2D in obese humans. SLC30A8 is predominantly expressed in pancreatic islet β-cells, but surprisingly, multiple knockout mouse studies have shown little effect of Slc30a8 deletion on glucose tolerance or glucose-stimulated insulin secretion (GSIS). Multiple other Slc30a isoforms are expressed at low levels in pancreatic islets. We hypothesized that functional compensation by the Slc30a7 isoform, which encodes ZnT7, limits the impact of Slc30a8 deletion on islet function. We therefore analyzed the effect of Slc30a7 deletion alone or in combination with Slc30a8 on in vivo glucose metabolism and GSIS in isolated islets. Deletion of Slc30a7 alone had complex effects in vivo, impairing glucose tolerance and reducing the glucose-stimulated increase in plasma insulin levels, hepatic glycogen levels, and pancreatic insulin content. Slc30a7 deletion also affected islet morphology and increased the ratio of islet α- to β-cells. However, deletion of Slc30a7 alone had no effect on GSIS in isolated islets, whereas combined deletion of Slc30a7 and Slc30a8 abolished GSIS. These data demonstrate that the function of ZnT8 in islets can be unmasked by removal of ZnT7 and imply that ZnT8 may affect T2D susceptibility through actions in other tissues where it is expressed at low levels rather than through effects on pancreatic islet function.
SLC30A8 encodes the zinc transporter ZnT8 and is highly expressed in pancreatic islets (1, 2). ZnT8 transports zinc into insulin secretory granules, which is thought to promote the proper maturation, storage, and secretion of insulin (1, 2). In humans a nonsynonymous SLC30A8 single nucleotide polymorphism (SNP) rs13266634 is associated with modest changes in proinsulin to insulin conversion (3), glucose tolerance (4), first phase insulin secretion (5), and susceptibility for the development of type 2 diabetes (T2D) (6). Similarly, studies in Slc30a8 knockout (KO) mice have generally shown modest effects of global Slc30a8 deletion on glucose tolerance and glucose-stimulated insulin secretion (GSIS) (7–10). Although Slc30a8 is the most highly expressed Slc30a isoform in human and mouse islets (8, 10), various studies have implicated a role for other Slc30a isoforms in islet function, including Slc30a3, which encodes ZnT3 (11), and Slc30a7, which encodes ZnT7 (12). We therefore hypothesized that functional compensation by one of these isoforms may be limiting the impact of Slc30a8 deletion on islet function. We show here that deletion of Slc30a7 alone has complex effects on glucose metabolism in vivo but no effect on GSIS in isolated islets, whereas combined deletion of Slc30a7 and Slc30a8 abolishes GSIS. These data demonstrate that the function of ZnT8 in islets can be unmasked by removal of another ZnT and imply that, because the absence of ZnT8 alone has limited effects on β-cell function, ZnT8 may affect T2D susceptibility through actions in other tissues.
Research Design and Materials
Animal care
The Vanderbilt University Medical Center Animal Care and Use Committee approved all protocols used. Mice were maintained on a standard rodent chow diet (LabDiet 5001; 23% protein and 4.5% fat; PMI Nutrition International). Food and water were provided ad libitum.
Generation of ZnT7 KO, ZnT8 KO, and combined ZnT7 KO and ZnT8 KO (DKO) mice
Details on the generation and genotyping of ZnT7 KO, ZnT8 KO, and DKO mice are presented in the Supplemental Materials and Methods.
Measurement of islet zinc content
The content of loosely bound zinc in isolated islets was measured as described previously (7, 8).
Insulin tolerance tests (ITTs), oral glucose tolerance tests (OGTTs), ip GTTs (IPGTTs), islet isolations, GSIS assays, and phenotypic analyses
These assays and phenotypic analyses of 6-hour fasted mice were all performed as previously described (7, 8).
Measurement of pancreatic insulin content and analysis of islet structure
Details on the methods used to quantitate pancreatic insulin content and analyze islet number, size, and cellular composition, as well as visualize β-cell structure using electron microscopy (EM), are presented in the Supplemental Materials and Methods.
Measurement of hepatic glycogen content, plasma C peptide, and plasma proinsulin
Hepatic glycogen content (13) and plasma proinsulin (8) were determined as previously described. Plasma C peptide was assayed by the Vanderbilt Diabetes Research and Training Center Hormone Assay Core using the MILLIPLEX Metabolic Hormone Multiplex Assay.
Assessment of ZnT7:ZnT8 dimer formation
Details on the generation of plasmids used to encode tagged variants of ZnT7 and ZnT8 and the assessment of ZnT7:ZnT8 dimer formation using pulldown assays are presented in the Supplemental Materials and Methods.
Arginine tolerance tests
Arginine tolerance tests using ip injection with 1-g/kg arginine were performed as described (14).
Statistical analyses
Mouse data were analyzed using a one- or two-way nonrepeated ANOVA assuming normal distribution and equal variance. Post hoc analyses were performed using the Bonferroni correction for multiple comparisons. P < .05 was considered significant.
Results
Combined deletion of Slc30a7 and Slc30a8 has mild effects on fasting metabolic parameters
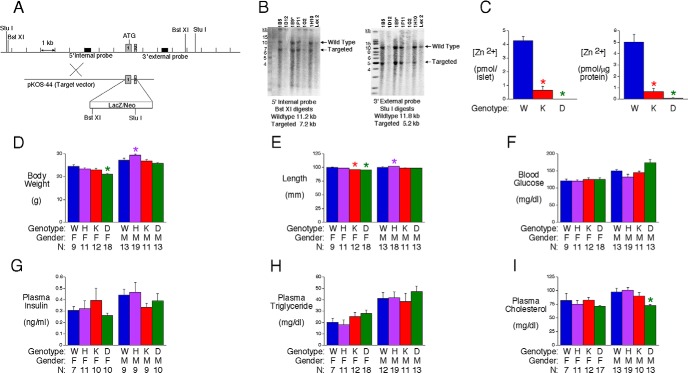
A modified mouse Slc30a7 allele, in which exons 1 and 2 (15) were replaced by a LacZ/Neo cassette, was generated by homologous recombination in ES cells (Figure 1A). This design abolishes Slc30a7 expression while leaving the transcription start site intact such that expression of LacZ mRNA is driven by the Slc30a7 promoter. Correct gene targeting was confirmed by Southern blot (Figure 1B) and PCR (data not shown) analyses before injection of ES cells into blastocysts and subsequent generation of Slc30a7 heterozygous (HET) mice on a mixed 129SvEvBrd X C57BL/6J genetic background. These mice were interbred to generate Slc30a7 KO mice, designated as ZnT7 KO.
Figure 1.
Generation and analysis of islet zinc content and fasting metabolic parameters in ZnT7 KO and DKO mice. A, Schematic representation of the WT murine Slc30a7 locus and the targeting construct used to generate ZnT7 KO mice by homologous recombination in ES cells. Exons 1 and 2 were replaced with a cassette containing the LacZ gene and a TK-neomycin selectable marker. B, Southern blot analysis of the Slc30a7 locus using genomic DNA extracted from the indicated targeted ES cell lines, or WT ES cell gDNA, designated Lex-2, as a control, using 5′ and 3′ diagnostic probes (A). The sizes of the WT locus, targeted allele, and DNA markers are indicated. Clone 1E9 was used to achieve germline transmission. C, Zinc content in isolated approximately 17-week-old ZnT7 KO and DKO male mouse islets. Results represent the mean ± SEM (n = 3–14); P < .0001, one-way ANOVA; *, differences with WT. D–I, Phenotypic parameters in 6-hour fasted 16-week-old ZnT7 KO and DKO mice. Results are the mean ± SEM of data with the genotype, gender, and number of animals indicated. W, WT; H, HET; K, KO; DKO; F, female; M, male. F, P < .0001 and M, P = .001 (D); F, P < .0001 and M, P = .0002 (E); M, P = .0010 (F); M, P = .0007 (I); one-way ANOVA; *, differences with matching WT are indicated.
Using an assay that detects free or loosely bound zinc, Figure 1C shows that zinc content was markedly reduced in isolated ZnT7 KO mouse islets relative to those isolated from wild-type (WT) mice. ZnT7 KO mice were interbred with ZnT8 KO mice (8) to generate mice lacking both ZnT7 and ZnT8, designated as DKO mice. Figure 1C shows that, as expected, zinc content was markedly reduced in isolated DKO mouse islets.
Female, but not male, DKO mice showed a modest reduction in body weight (Figure 1D) and length (Figure 1E), whereas male ZnT7 HET mice showed a modest increase in body weight (Figure 1D) and length (Figure 1E) relative to WT mice. The explanation for these differences is unclear. Despite the marked reduction in islet zinc content, in 16-week-old mice after a 6-hour fast, deletion of ZnT7 alone or in combination with ZnT8 had no effect on fasting glucose (Figure 1F), insulin (Figure 1G), or triglycerides (Figure 1H), although cholesterol was slightly reduced in male DKO mice (Figure 1I). These results suggest that ZnT7 and ZnT8 both have a limited effect on whole-body glucose metabolism under fasting conditions, at least in 16-week-old mice.
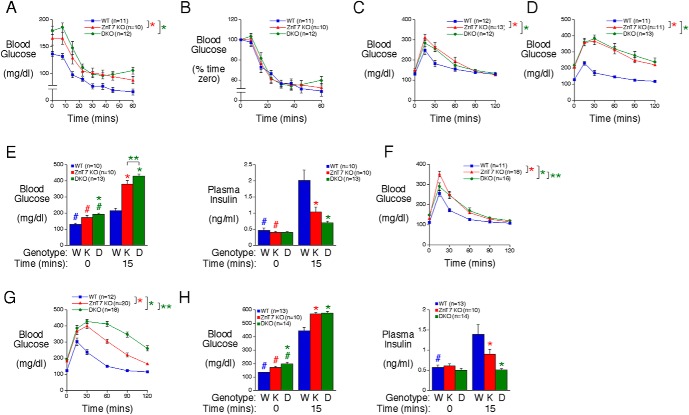
The combined absence of ZnT7 and ZnT8 markedly impairs glucose tolerance in male mice
Because some metabolic disturbances only become readily apparent under stimulatory rather than basal conditions we next investigated whether the absence of ZnT7 alone or in combination with ZnT8 affected the responses to the physiological challenges associated with injections of insulin or glucose. We observed a consistent elevation in blood glucose levels at t = 0 in ZnT7 KO mice relative to WT mice after tailing before ITTs, OGTTs, and IPGTTs (Figure 2). This contrasts with no difference after retroorbital bleeding (Figures 1 and 2) and suggests that ZnT7 KO mice are sensitive to the rise in corticosterone that is associated with the stress of tail (16) but not retroorbital bleeding (17).
Figure 2.
Analysis of insulin sensitivity, glucose tolerance, and plasma insulin in ZnT7 KO and DKO mice. A and B, ITTs were performed on 21- to 24-week-old 5-hour fasted conscious male mice. Results show the mean glucose concentrations ± SEM expressed as mg/dL (A) or as a percentage of blood glucose at t = 0 (B). A, P = .0141, two-way ANOVA. C and D, OGTTs were performed on 6-hour fasted 20-week-old female (C) and male (D) conscious mice. Results show the mean glucose concentrations in tail blood ± SEM. C and D, P < .0001, two-way ANOVA. E, OGTTs were performed on 6-hour fasted 29- to 30-week-old male conscious mice. Results show the mean glucose and insulin concentrations in blood isolated from the retroorbital plexus ± SEM. Glucose and insulin data: P < .0001, two-way ANOVA; *, differences with WT; **, differences between ZnT7 KO and DKO; #, differences between t = 0 and t = 15. F and G, IPGTTs were performed on 6-hour fasted 10-week-old female (F) and male (G) conscious mice. Results show the mean glucose concentrations in tail blood ± SEM. F and G, P < .0001, two-way ANOVA; *, differences with WT; **, differences between ZnT7 KO and DKO. H, IPGTTs were performed on 6-hour fasted 22- to 25-week-old male conscious mice. Results show the mean glucose and insulin concentrations in blood isolated from the retroorbital plexus ± SEM. Glucose data, P < .0099, two-way ANOVA. Insulin data: P < .0017, two-way ANOVA; *, differences with WT; #, differences between t = 0 and t = 15.
The difference in blood glucose levels between WT, ZnT7 KO, and DKO mice at t = 0 makes the interpretation of the results of ITTs complex. When expressed relative to starting glucose values, insulin sensitivity appears impaired in both ZnT7 KO and DKO mice relative to WT mice (Figure 2A). However, when expressed as a percentage of starting glucose values, insulin sensitivity appears unchanged between WT, ZnT7 KO, and DKO mice (Figure 2B).
In contrast, in approximately 20-week-old mice, OGTTs using 2-g/kg glucose showed a clear impairment in glucose clearance between female (Figure 2C) and male (Figure 2D) WT and ZnT7 KO mice. This impairment was not further enhanced in DKO mice (Figure 2, C and D). In slightly older approximately 30-week-old mice, OGTTs showed a marked reduction in glucose-stimulated plasma insulin levels in both male ZnT7 KO and DKO mice relative to WT mice (Figure 2E). Huang et al previously analyzed a ZnT7 KO mouse model generated by gene trap insertional mutagenesis (12, 18). The insertion in intron 6 of the Slc30a7 gene resulted in the generation of a ZnT7 protein truncated at amino acid residue 221 (18). In contrast to our data, Huang et al (12) reported a selective impairment in glucose tolerance in OGTTs in male mice but not females.
IPGTTs also showed an impairment in glucose tolerance between female (Figure 2F) and male (Figure 2G) WT and ZnT7 KO mice, but in contrast to OGTTs, this impairment was clearly enhanced in male (Figure 2G), although not female (Figure 2F), DKO mice. This impaired ip glucose tolerance was associated with a marked reduction in glucose-stimulated plasma insulin levels in male ZnT7 KO mice relative to WT mice, whereas in DKO mice, the normal increase in plasma insulin levels in response to glucose injection was abolished (Figure 2H). The difference in the response of male DKO mice to OGTTs (Figure 2, D and E) and IPGTTs (Figure 2, G and H) suggests that incretin-stimulated insulin secretion remains at least partially intact, consistent with a trend towards increased plasma insulin in DKO mice 15 minutes after glucose gavage (Figure 2E).
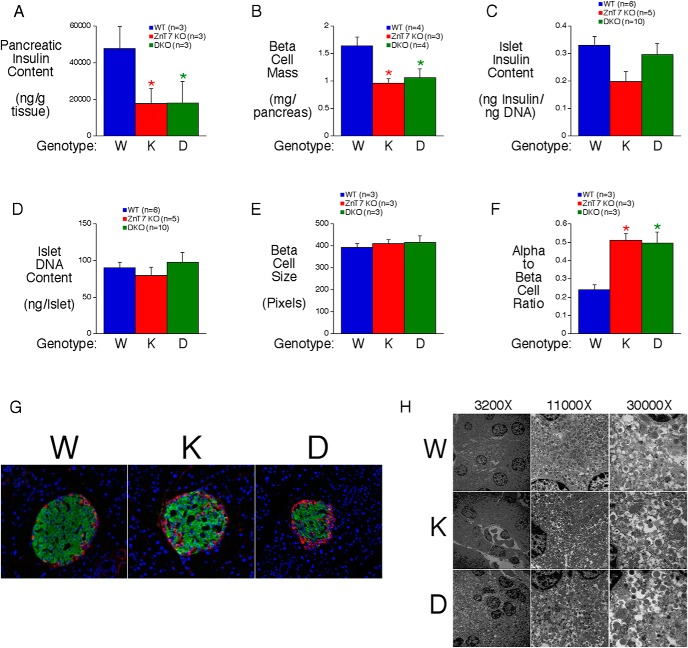
The combined absence of ZnT7 and ZnT8 affects islet morphology
We next investigated the basis for the impaired glucose-stimulated increase in plasma insulin in ZnT7 KO and DKO mice. We first considered the possibility that islet abundance or morphology had been affected. Figure 3A shows that pancreatic insulin content is reduced in both ZnT7 KO and DKO mice. This is associated with reduced β-cell mass (Figure 3B) but not insulin (Figure 3C) or DNA content (Figure 3D) in isolated islets or with a change in β-cell size (Figure 3E). These data suggest that islet size is unchanged in ZnT7 KO and DKO mice but pancreatic insulin content is reduced due to reduced β-cell mass. We also observed an increase in α- to β-cell ratio in both ZnT7 KO and DKO mice (Figure 3F). In ZnT7 KO mice we observed an increase in α-cells within the islet core, whereas in DKO mice, peripheral α-cells appeared to be increased (Figure 3G). EM revealed no major structural differences in β-cells (Figure 3H).
Figure 3.
Analysis of pancreatic insulin content and islet structure in ZnT7 KO and DKO mice. A, Insulin content in whole pancreas from 20- to 24-week-old 6-hour fasted male mice. Results represent the mean ± SEM (n = 3); P = .0148, one-way ANOVA; *, differences with WT. B–D, β-Cell mass (B), isolated islet insulin content (C), isolated islet DNA content (D), β-cell size (E), and α- to β-cell ratio (F) in male mice. Pancreas and islet results represent the mean ± SEM; P = .0174, one-way ANOVA (B); P = .0029, one-way ANOVA (F); *, differences with WT. G, Immunofluorescent staining of 18-month-old male mouse pancreata with antisera raised to insulin and glucagon. Representative pictures (×15 magnifications) are shown. H, Analysis of insulin secretory granule structure in 12-week-old male mice using EM. Representative micrographs are shown.
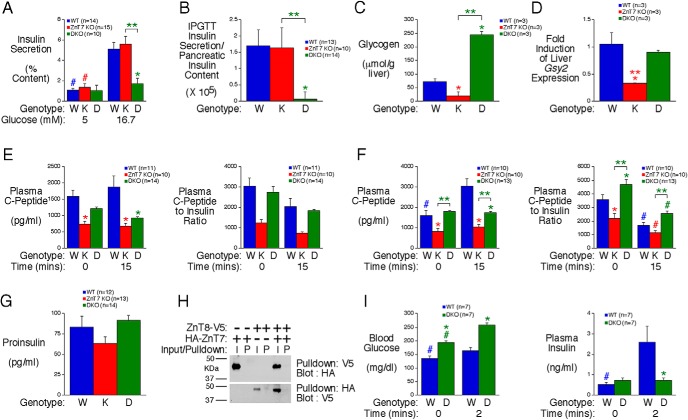
GSIS is abolished in islets lacking both ZnT7 and ZnT8
We next considered the possibility that the absence of ZnT7 alone or in combination with ZnT8 also directly affects islet cell function. After overnight culture in 11mM glucose followed by culture in 5mM glucose for 1-hour GSIS was assessed after static islet incubations in either 5mM or 16.7mM glucose for 30 minutes. Strikingly, GSIS was unaffected in ZnT7 KO islets but was abolished in DKO islets (Figure 4A). These results imply a key role for ZnT7 and ZnT8 in GSIS and demonstrate that deletion of ZnT7 unmasks the function of ZnT8.
Figure 4.
Analysis of insulin secretion in vitro in islets isolated from ZnT7 KO and DKO mice and the molecular basis for altered plasma insulin levels in vivo. A, GSIS in islets isolated from approximately 34-week-old male ZnT7 KO and DKO mice. Results show the mean data ± SEM from 5–10 islet preparations. P = .0046, two-way ANOVA; *, differences between WT and DKO; **, differences between ZnT7 KO and DKO; #, differences between 5mM and 16.7mM glucose. B, Ratio of plasma glucose-stimulated insulin at t = 15 during IPGTTs in 6-hour fasted 20- to 25-week-old male conscious mice relative to pancreatic insulin content. Results represent the mean ± SEM; P = .0125, one-way ANOVA; *, difference between WT and DKO; **, difference between ZnT7 KO and DKO. C and D, Hepatic glycogen content (C) and Gsy2 expression (D) in 6-hour fasted 28- to 36-week-old male mice. Results represent the mean ± SEM; P < .0001, one-way ANOVA (C); P = .0140, one-way ANOVA (D); *, differences with WT; **, differences between ZnT7 KO and DKO. E and F, IPGTTs (E) or OGTTs (F) were performed on 6-hour fasted 22- to 30-week-old conscious male mice. Results show the mean C peptide concentration in blood isolated from the retroorbital plexus ± SEM. Matching insulin data are from Figure 2, E and H. C peptide data: P = .0362 (E); C peptide data: P < .0001 (F); C peptide:insulin data: P = .0105 (F), two-way ANOVA; *, differences with WT; **, differences between ZnT7 KO and DKO; #, differences between t = 0 and t = 15. G, Plasma proinsulin levels in 6-hour fasted 29- to 30-week-old conscious male mice. Results represent the mean ± SEM. H, Absence of ZnT7:ZnT8 dimer formation as assessed using pulldown assays. Representative blots are shown. I, Arginine tolerance tests were performed on 6-hour fasted 28- to 34-week-old conscious male mice. Results show the mean glucose and insulin concentrations in blood isolated from the retroorbital plexus ± SEM. Glucose data: P = .0005; insulin data: P = .0161, two-way ANOVA; *, differences with WT; #, differences between t = 0 and t = 2.
The apparent difference between the marked impairment in glucose-stimulated plasma insulin levels in ZnT7 KO mice in vivo (Figure 2, E and H) compared with the lack of effect on GSIS in isolated ZnT7 KO mouse islets in vitro (Figure 4A) presumably largely reflects a deficit in pancreatic insulin content (Figure 3A). Indeed, when glucose-stimulated plasma insulin levels in the IPGTT are corrected for pancreatic insulin content a difference is observed between WT and DKO but not ZnT7 KO mice consistent with the isolated islet data (Figure 4B). In contrast, in vitro isolated islet studies compare GSIS between equivalent numbers of islets, which reveals the inherent secretory capacity of the ZnT7 KO and DKO islets.
Because ZnT7 is expressed in multiple tissues other than islets including liver, kidney, and brain (15), we investigated whether the absence of ZnT7 in other tissues affected other aspects of metabolism in vivo. In liver, both glycogen accumulation (Figure 4C) and expression of Gsy2 (Figure 4D), which encodes glycogen synthase, are reduced in ZnT7 KO mice. In addition insulin clearance actually appears reduced in ZnT7 KO mice in both IPGTTs (Figure 4E) and OGTTs (Figure 4F), although the interpretation of these data is complex, because ZnT7 expression in kidney may have affected clearance of C peptide (19). Interestingly, in DKO mice hepatic glycogen accumulation was markedly increased (Figure 4C), although Gsy2 expression (Figure 4D) and insulin clearance in both IPGTTs (Figure 4E) and OGTTs (Figure 4F) were unaffected.
We next explored the molecular basis for the impaired GSIS in isolated DKO islets in vitro (Figure 4A). Because zinc is important for proinsulin to insulin conversion (1) and, in humans, SLC30A8 SNPs are associated with impaired proinsulin conversion (3), we hypothesized that insulin processing might be altered in DKO islets. However, no difference in plasma proinsulin was observed in ZnT7 KO or DKO mice (Figure 4G), suggesting the absence of a defect in insulin processing, which is consistent with normal fasting insulin levels (Figure 1G). Although ZnT7 is predominantly located in the Golgi (15) and ZnT8 in secretory granules (20), because ZnTs form dimers (1, 10), we considered the possibility that ZnT7:ZnT8 dimers may be functionally important in β-cells. However, pulldown assays showed no evidence for dimer formation (Figure 4H). Finally, arginine tolerance tests were performed to begin to determine the location of the defect in GSIS in DKO islets. Although arginine enters β-cells, it is not metabolized and causes depolarization (21). Figure 4I demonstrates that ip injection with 1-g/kg arginine stimulated an increase in plasma insulin in WT but not DKO mice, suggesting that the defect in GSIS in DKO islets is distal to the activation of voltage-sensitive calcium channels.
Discussion
Although the global absence of ZnT8 has little effect on glucose tolerance or GSIS (7–10), the absence of ZnT7 markedly impairs glucose tolerance and the rise in plasma insulin in IPGTTs (Figure 2). However, strikingly GSIS is not impaired in isolated ZnT7 KO islets (Figure 4). In contrast, the rise in plasma insulin in IPGTTs in DKO mice in vivo (Figure 2) and GSIS in isolated DKO islets in vitro (Figure 4) are both abolished. These observations appear to dissociate islet zinc levels and GSIS because a marked reduction in zinc is observed in both ZnT7 KO (Figure 1) and ZnT8 KO (7–10) islets with little or no effect on GSIS. Future studies will use this mouse model to uncover the molecular mechanism whereby these ZnTs regulate GSIS (Figure 4) and islet development (Figure 3).
The observation that insulin clearance is not altered in DKO mice (Figure 4) contrasts with a recent study using RIP-Cre mice to specifically remove ZnT8 from β-cells. This study suggested that the absence of ZnT8 increased hepatic insulin clearance thereby impairing insulin secretion in vivo and glucose tolerance (22). However, this study used Slc30a8 floxed mice rather than RIP-Cre mice as matching controls (22) and the reported impairments in insulin secretion in vivo and glucose tolerance were very similar to those associated with RIP-Cre alone (23). Indeed, another study looking at β-cell-specific Slc30a8 deletion using RIP-Cre mice as controls reported little effect of Slc30a8 deletion on glucose tolerance or glucose-stimulated plasma insulin in vivo (24).
Genome-wide association studies have linked the SLC30A8 SNP rs13266634 with altered susceptibility for the development of T2D (6). These data were originally interpreted to suggest that the major rs13266634 C allele was associated with increased risk of T2D (6). However, Flannick et al (25) have strikingly shown that SLC30A8 haploinsufficiency is protective against the development of T2D in obese humans. To reconcile the observations of Flannick et al (25) with previous human genome-wide association study data, we suggested in a recent review (1) that the genome-wide association study data should be reinterpreted in terms of the minor rs13266634 T allele being associated with protection against T2D, although other interpretations have been suggested (2). We hypothesized that the protective mechanism might relate to regulating oxidative stress in β-cells (1), but the conclusion that ZnT7 can functionally compensate for ZnT8 in β-cells may indicate that this protective effect is mediated by altered ZnT8 action in other tissues, where it is expressed at much lower levels than islets.
Acknowledgments
We thank Susan Hajizadeh, Suzan Vaughan, and Anastasia Coldren (Vanderbilt University) for performing insulin assays, C peptide assays, and islet isolations, respectively. We also thank Mary Dawes, Janice Williams, and Jay Jerome for performing the EM. Finally, we thank Masakazu Shiota and Tracy O'Brien for performing glycogen assays and Maureen Gannon, Peter Kropp, Bethany Carboneau and Ray Pasek for assistance with the measurement of beta cell mass.
Author contributions: K.E.S., K.A.B., and J.K.O. performed the metabolic analyses and wrote parts of the manuscript. A.U. performed the zinc analyses. K.A.P. and M.K.S. contributed to the generation of KO mice. O.P.M. and D.W.P. provided advice on the metabolic analyses and zinc analyses, respectively. D.R.P. was the PI for the generation of the KO mice and wrote parts of the manuscript. R.M.O. was the PI for the metabolic analyses and wrote parts of the manuscript.
Research in the laboratory of R.M.O. was supported by the National Institutes of Health (NIH) Grant DK92589. Research in the laboratory of O.P.M. was supported by NIH Grants DK043748 and DK078188. Research in the laboratory of D.W.P. was supported by the NIH Grant DK085064. The Vanderbilt Hormone Assay and Analytical Services Core, Islet Procurement and Analysis Core and Cell Imaging Shared Resource are all supported by the NIH Grant P60 DK20593 (to the Vanderbilt Diabetes Research Training Center) and the NIH Grant DK59637 (to the Vanderbilt Mouse Metabolic Phenotyping Center). K.E.S. was supported by the Vanderbilt Molecular Endocrinology Training Program Grant 5T32 DK07563.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 4542
- DKO
- Double ZnT7 and ZnT8 Knockout
- EM
- electron microscopy
- GSIS
- glucose-stimulated insulin secretion
- HET
- heterozygous
- IPGTT
- intraperitoneal glucose tolerance test
- ITT
- insulin tolerance test
- KO
- knockout
- OGTT
- oral glucose tolerance test
- SNP
- single nucleotide polymorphism
- T2D
- type 2 diabetes
- WT
- wild type.
References
- 1. Davidson HW, Wenzlau JM, O'Brien RM. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol Metab. 2014;25(8):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rutter GA, Chimienti F. SLC30A8 mutations in type 2 diabetes. Diabetologia. 2015;58(1):31–36. [DOI] [PubMed] [Google Scholar]
- 3. Kirchhoff K, Machicao F, Haupt A, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51(4):597–601. [DOI] [PubMed] [Google Scholar]
- 4. Xu K, Zha M, Wu X, et al. Association between rs13266634 C/T polymorphisms of solute carrier family 30 member 8 (SLC30A8) and type 2 diabetes, impaired glucose tolerance, type 1 diabetes–a meta-analysis. Diabetes Res Clin Pract. 2011;91(2):195–202. [DOI] [PubMed] [Google Scholar]
- 5. Boesgaard TW, Zilinskaite J, Vänttinen M, et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients–the EUGENE2 study. Diabetologia. 2008;51(5):816–820. [DOI] [PubMed] [Google Scholar]
- 6. Yaghootkar H, Frayling TM. Recent progress in the use of genetics to understand links between type 2 diabetes and related metabolic traits. Genome Biol. 2013;14(3):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pound LD, Sarkar SA, Benninger RK, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421(3):371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pound LD, Sarkar SA, Ustione A, et al. The physiological effects of deleting the mouse slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS One. 2012;7(7):e40972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lemaire K, Ravier MA, Schraenen A, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009;106(35):14872–14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58(9):2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smidt K, Brønden A, Sørensen KS, et al. The zinc transporter ZNT3 co-localizes with insulin in INS-1E pancreatic β cells and influences cell survival, insulin secretion capacity, and ZNT8 expression. Biometals. 2016;29(2):287–298. [DOI] [PubMed] [Google Scholar]
- 12. Huang L, Kirschke CP, Lay YA, Levy LB, Lamirande DE, Zhang PH. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J Biol Chem. 2012;287(40):33883–33896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Oeser JK, Yang C, et al. Deletion of the gene encoding the ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein (UGRP)/glucose-6-phosphatase catalytic subunit-β results in lowered plasma cholesterol and elevated glucagon. J Biol Chem. 2006;281(52):39982–39989. [DOI] [PubMed] [Google Scholar]
- 14. Zhang C, Moriguchi T, Kajihara M, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25(12):4969–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirschke CP, Huang L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J Biol Chem. 2003;278(6):4096–4102. [DOI] [PubMed] [Google Scholar]
- 16. Tuli JS, Smith JA, Morton DB. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim. 1995;29(1):90–95. [DOI] [PubMed] [Google Scholar]
- 17. Teilmann AC, Nygaard Madsen A, Holst B, Hau J, Rozell B, Abelson KS. Physiological and pathological impact of blood sampling by retro-bulbar sinus puncture and facial vein phlebotomy in laboratory mice. PLoS One. 2014;9(11):e113225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang L, Yu YY, Kirschke CP, Gertz ER, Lloyd KK. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J Biol Chem. 2007;282(51):37053–37063. [DOI] [PubMed] [Google Scholar]
- 19. Zavaroni I, Deferrari G, Lugari R, et al. Renal metabolism of C-peptide in man. J Clin Endocrinol Metab. 1987;65(3):494–498. [DOI] [PubMed] [Google Scholar]
- 20. Chimienti F. Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev. 2013;26(1):1–11. [DOI] [PubMed] [Google Scholar]
- 21. Henquin JC. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res Clin Pract. 2011;93(suppl 1):S27–S31. [DOI] [PubMed] [Google Scholar]
- 22. Tamaki M, Fujitani Y, Hara A, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123(10):4513–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic β-cell function. J Biol Chem. 2006;281(5):2649–2653. [DOI] [PubMed] [Google Scholar]
- 24. Wijesekara N, Dai FF, Hardy AB, et al. β Cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53(8):1656–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flannick J, Thorleifsson G, Beer NL, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014;46(4):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]