Abstract
Antiestrogen therapies targeting the function of estrogen receptor (ER) have been the cornerstone of therapy for ER+ breast cancer for decades. However, as long as these therapies have been in use, it has also been evident that response to antiestrogen therapy is not based solely on ER expression but that other factors modify breast cancer antiestrogen response. Such factors may include ER's relatives in the steroid hormone receptor (HR) family, androgen receptor (AR), progesterone receptor (PR), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR). A series of recent studies has demonstrated that these HRs are not bystanders in ER signaling but rather can alter ER genomic binding and subsequent control of target gene expression. For example, PR and GR may “reprogram” ER binding to DNA toward PR/GR sites; androgen receptor may reverse ER gene regulation functions or regulate ER DNA binding. Accordingly, modulation of HR function concurrently with antiestrogen therapy can either improve antiestrogen response or mediate antiestrogen resistance. This highlights the critical need to better understand how other HRs influence ER function, in particular in the context of antiestrogen therapy. This review discusses recent insights into the mechanisms by which HRs can modify ER function and antiestrogen response, as well as pharmacological implications for antiestrogen therapies and potential combined endocrine therapies.
The binding of estrogen receptor (ER)α (ESR1, also known as NR3A1) to DNA and subsequent regulation of gene expression is critical to its functions in driving both normal development and breast oncogenesis (1). Studies in breast cancer models have demonstrated that ER binds to DNA in concert with cofactors and protein complexes (2–4), including proteins that help locate ER to specific sites in the genome (5, 6). The critical nature of ER protein interactions is highlighted by recent observations that ER cofactors are differentially mutated in breast cancer subtypes (7, 8); as such, there have been a number of large-scale screening efforts to identify and characterize ER cofactors (4, 9–11). Additionally, the interaction of ER with other members of the nuclear receptor (NR) family of transcription factors has been investigated (discussed elsewhere, see Refs. 12, 13), because this family is linked by similar structure and mechanisms of activity.
The NR family of transcription factors consists of 48 genes in humans, including the closely related steroid hormone receptors (HRs) (14). Among steroid HRs, the NR3A family (ERα and ERβ) is ancestral to the NR3C family (androgen receptor [AR]; progesterone receptor [PR]; glucocorticoid receptor [GR]; mineralocorticoid receptor [MR]) (15). Recently, a number of high-profile studies have drawn renewed attention to interaction between ER and its “descendants” among the NR3C family. Potential interactions at the phenotypic level in cells and in the clinic have been investigated for decades (16–18), but new technology has allowed for detailed examination in to how HRs influence the binding of ER to chromatin and subsequent effects on gene expression and breast cancer cell phenotypes. Recent studies suggest that the ability of ER to regulate gene expression is strongly influenced by the concurrent expression and activity of its relatives in the HR family and that this “peer pressure” may have a substantial impact on the efficacy of antiestrogen therapies. This review will focus on recent mechanistic data on the interactions of ER (specifically ERα) with NR3C receptors in mammary gland and breast cancer models, effects on DNA binding and regulation of gene expression, and the related pharmacological implications for endocrine therapies.
Progesterone Receptor
Expression of PR (PGR, also known as NR3C3; isoforms PR-A and PR-B) has long been considered to be a marker of functional ER transcriptional activity and of active estrogenic signaling. Consistent with this, ER−/PR+ tumors are rare (19, 20), and PGR expression in ER+ breast cancer strongly correlates with serum estradiol (E2) (21). However, ER-independent functions of PR have become evident, particularly in normal mammary gland development, where P4 and PR signaling regulate pregnancy-induced mammary gland expansion (22, 23) and maintain the mammary progenitor cell niche (24, 25). These functions of PR in the normal breast, independent of ER, were recently corroborated by Hilton et al (26); expression of ER and PR were observed to be primarily in separate, distinct cell populations in the normal breast, and ER and PR each regulated unique transcriptional programs. However, this study demonstrated that in breast cancer, expression of ER and PR become tightly correlated and that their individual transcriptional programs converge on common targets and pathways. This convergence is also seen in breast cancer models, where E2 and P4 regulate largely overlapping gene sets (27). These observations, coupled with the evolving role of progestins in breast cancer (28, 29) and the ability of progestins to block estrogen-induced growth of patient-derived xenografts (30), strongly suggest that a functional interaction between ER and PR is critical to breast cancer biology. The mechanisms and functions of this interaction have been largely unclear until recently.
Mechanistic details of a physical ER:PR interaction were first observed in the context of nongenomic activation of kinase cascades (31, 32). However, this interaction did not regulate the activity of ER or PR as transcription factors. A direct ER:PR association at target gene promoters was reported by Giulianelli et al (33); using the progestin-responsive models C4-HD and T47D, this study demonstrated that 1) ER and PR physically interacted (via coimmunoprecipitation and nuclear colocalization) in the presence of the progestin MPA; 2) ER and PR were both required for MPA-induced expression of CCND1 and MYC via interaction at respective promoter regions; and 3) antiestrogens could block progestin-induced effects by preventing association of ER with PR. This showed that ER and PR interacted directly at chromatin to coregulate PR-target gene expression, and in conjunction, PR-mediated cancer cell phenotypes. A reciprocal relationship, ie, PR regulation of ER-mediated functions, was reported by Daniel et al (34). They observed that in a ligand-independent manner, PR-B formed a scaffold for the recruitment of activation complexes to ER at the CTSD promoter; ablation of PR (genetic or therapeutic) blocked E2-induced CTSD expression and breast cancer cell growth. Importantly, both of these studies demonstrated the interaction of ER and PR at chromatin in both breast cancer model systems and in human breast tumor tissue. Despite these reports, binding of ER with PR (or other HRs) was not observed in large scale efforts to map the ER “complexome” (4, 35); PR was found to bind ER using novel mass spectrometry methods but only as a low-confidence partner (9). The lack of detection of ER:PR binding raised 2 key questions. 1) Is the nuclear association of ER and PR limited to specific target genes, or more broad across the genome? 2) Are there specific factors required for ER and PR association?
Evidence that PR indeed regulates global chromatin binding and gene expression driven by ER, and that this function requires progestins, was presented by Mohammed et al (36). They demonstrated that in MCF-7 and T47D cells, upon treatment with P4 or synthetic progestin R5020, PR associated with ER complexes at chromatin. This association caused global reprogramming of ER binding, shifting ER away from estrogen-response elements (EREs) and onto progesterone-response elements. Furthermore, binding at progesterone-response elements was functional and associated with activate chromatin and gene expression. This reprogramming was consistent with signatures of good prognosis in patient cohorts, suggesting that progestins could induce more “favorable” biology in ER+/PR+ breast cancer cells. These observations were corroborated by the Greene laboratory; Singhal et al (27) used breast cancer cell lines and human tumor tissues to demonstrate that R5020 induced global ER reprogramming across ER+/PR+ tumor models. In these models, gene expression for estrogen + progestin was consistent with the progestin-only (rather than the estrogen-only) phenotype, effectively antagonizing estrogen-regulated gene expression. Both Mohammed et al (36) and Singhal et al (27) showed that treatment with progestins (P4 or PR antagonist CDB4124, respectively) blocked estrogen-induced proliferation in model systems. Notably, although both studies proposed use of progestins to reprogram ER function clinically, Need et al demonstrated using a different cell line model that progestin treatment in fact shifted cells toward a basal-like phenotype (37). This result, taken together with differing PR agonist (36) vs antagonist (27) use, suggests that both the pharmacology of the chosen progestin and tumor steroid receptor context should be carefully considered regarding clinical progestin use in breast cancer. These considerations are further discussed below.
Androgen Receptor
Although ER and PR currently serve as the standard steroid HR biomarkers for breast cancer, the most widely expressed HR is AR (AR, also known as NR3C4) (38, 39). AR agonists were historically efficacious in treating breast cancer; coupled with observations from mouse models and human genetic conditions, androgenic signaling via AR is generally considered antagonistic of estrogen activity (reviewed previously, see Ref. 39). However, the function of AR in breast cancer cells is in fact highly context dependent, including ER status, relative AR to ER protein ratio (40), menopausal status, associated hormonal milieu (ie, local estrogenic/androgenic steroid content), and antiestrogen treatment (these contexts are reviewed elsewhere, see Refs. 38, 39, 41). In particular, AR is oncogenic in triple-negative breast cancer and is the target of several ongoing trials (42, 43). In ER+ breast cancer, it is not yet fully clear whether AR is a tumor suppressor or oncogene, or whether there are additional contexts that define these functions. However, it does appear that the mechanisms of AR interactions with ER are distinct from the direct binding and genomic reprogramming driven by PR.
Two studies from the University of Adelaide have demonstrated that AR antagonizes the functions of ER. Peters et al (44) demonstrated that overexpression of AR could block the output of an ERE-driven luciferase reporter and could inhibit ER-mediated proliferation in T47D cells. Overexpression of the AR DNA binding domain alone was sufficient to block ER activity. This strongly suggested that AR occupancy of DNA regulates ER-mediated gene expression. Surprisingly, AR was found to bind at canonical ERE sequences (directly by electrophoretic mobility shift assay and via in silico modeling of AR:ERE interaction); both ER and AR were found to bind at E2-responsive CTSD and PGR promoter sites. ER:AR association at promoters did not appear to be driven by direct ER:AR binding, which could not be detected by immunoprecipitation. AR inhibition of ER transcription was confirmed at the genomic level by Need et al (13); in ZR-75–1 cells, cotreatment with 5α-dihydrotestosterone (DHT) (a testosterone metabolite and potent AR ligand) modified the expression of 26% of the E2-regulated transcriptome, and most of these changes were antagonistic. Additionally, AR genomic binding that was not at androgen-response element sites was enriched for half-ERE sites (consistent with previously observed binding at EREs). Further, AR binding was also enriched at or near ER-binding sites for E2-regulated genes that were modified by DHT treatment. Together, these studies demonstrated that AR antagonizes ER-mediated transcription by binding at EREs together with (but physically separate from) ER.
In contrast to this antagonism of ER by AR, the Richer laboratory has reported that ER and AR are cooperative and that AR is required for maximal binding of target DNA by ER (40, 45). These studies found either E2 or DHT treatment induced AR nuclear translocation in MCF-7 cells and that E2-induced AR genomic binding was enriched at EREs together with ER (similar to observed above). Treatment with E2 alone was sufficient to induce both ER and AR binding at canonical ER target genes, including GREB1 and PGR. However, AR blockade not only reduced AR translocation but also reduced nuclear ER, overall genomic ER binding, and ER:AR binding at targets including GREB1 and PGR. This occurred without inducing “reprogramming” as seen with PR. Importantly, these studies used the AR antagonist enzalutamide (MDV3100), which, unlike the selective AR modulator bicalutamide, has limited agonist activity and blocks AR nuclear translocation (46). Enzalutamide, but not bicalutamide, reduced E2-induced ER chromatin binding; E2-induced breast cancer cell line and xenograft growth were also inhibited specifically by enzalutamide. Taken together, ER and AR do have cooperative functions in mediating estrogen-regulated phenotypes, and inhibiting AR function with second generation AR antagonists is a viable strategy for the treatment of ER+ breast cancer.
On the surface, these studies make conflicting conclusions; however, they highlight the potentially complex relationship between ER and AR. Concurrent activation of AR and ER may cause AR to antagonize some ER-mediated regulatory effects, but complete AR inhibition (ie, enzalutamide) may have a similar global effect by ablating all cooperativity between ER and AR. Clearly, cellular HR context is of critical importance in the function of ER:AR interactions. AR appears to have distinct activity in regulating gene expression in ZR-75–1 (13) vs MCF-7 (45). In these studies, AR DNA binding in ZR-75–1 had minimal overlap vs androgen-responsive cell lines LNCaP and MDA MB 453 (6.5% overall), whereas MCF-7 strongly overlaps these models (46% vs LNCaP, 74% vs MDA MB 453). This major difference suggests that AR function is modified by other contexts in the cell. One potential context is the intracrine environment (41), because the AR ligand used in each of these studies was DHT. Although DHT is a specific, potent AR ligand and is not an aromatase substrate, DHT can be metabolized to the ER ligand 3βAdiol; this has been reported in MCF-7 cells (47) but may not be functional in ZR-75–1 (DHT- and E2-regulated gene expression had minimal overlap) (13). Thus, DHT may be specifically activating AR in ZR-75–1 but, through metabolism, may be activating both ER and AR in MCF-7 cells. In support of this, Hu et al recently reported on cooperative regulation of UGT2B15 and UGT2B17 by ER and AR in MCF-7 cells (and breast tumor explants) (48). DHT-mediated induction of these genes was in fact driven by DHT itself and 3βAdiol production and subsequent activation of both AR and ER. This also suggests that modulation of ER activity via antiestrogen therapy may also alter AR function; this may be of particular importance in the context of relative ER vs AR expression (40), and has significant implications regarding the pharmacology of antiandrogens applied in these settings.
Glucocorticoid Receptor
The interactions of GR (NR3C1) with ER and estrogen signaling have been an area of active research outside of breast cancer, owing in part to the roles of GR in other malignancies and in processes including inflammation (49, 50; and discussed elsewhere, see Ref. 51). In breast cancer, the action of GR appears to parallel AR, in that the tumor suppressor vs oncogenic potential of GR is dependent upon ER expression. In ER− breast cancer, GR expression is associated with poor outcome (52) and GR antagonists are in clinical trials (53, 54). Conversely, GR expression is associated with improved outcomes in ER+ breast cancer (55). Mechanistic studies in ER+ mammary and breast cancer models suggest that the ER:GR interaction may mirror that observed for ER:PR, with activation of both ER and GR inducing reprogramming of the other's global DNA binding.
Using GR as a model, Voss et al described the “assisted loading” mechanism of DNA binding (56). In this model, a steroid receptor serves as a pioneer factor to render chromatin accessible to a second receptor; this mechanism appears to be used in a reciprocal manner by ER and GR. This was first observed using an engineered “super-enhancer” with ERE/steroid-response element (SRE) repeats (bound by AR, PR, GR, or MR) derived from the PRL promoter (12). Using this system, ER bound the superenhancer after E2 treatment, but dexamethasone (dexa) (GR agonist) alone was insufficient to recruit GR. However, combined E2+dexa treatment allowed for recruitment of both ER and GR but ER binding led to sequential recruitment of specific coregulators, which then allowed for GR binding. This assisted loading was observed to affect GREB1 expression in MCF-7 cells; dexa alone was insufficient to recruit GR to the GREB1 enhancer, but E2+dexa allowed ER and GR cobinding and induced a synergistic increase in GREB1 expression. The ability of ER:GR to alter chromatin accessibility was examined at the genomic level by Miranda et al (51) using murine mammary cell lines. In these models, E2+dexa (vs either alone) reprogrammed approximately 25% of ER- and GR-binding sites, with new ER-binding sites (ie, those induced by cotreatment only) enriched at SREs and new GR-binding sites enriched at EREs. This reprogramming was connected to increased chromatin accessibility, because new ER-binding sites at SREs were associated with DNase-hypersensitivity upon dexa treatment alone, and the reciprocal was also true. Further, new E2+dexa-binding sites were enriched for AP1 motifs, and AP1 itself was required for recruitment of the reciprocal HR. This requirement for AP1 at new cotreatment ER:GR-binding sites was confirmed in MCF-7 cells at the TFF1, PGR, and CCND1 promoters in a concurrent manuscript from Karmakar et al (57). Collectively, these findings were reproduced in MCF-7 cells via ER and GR chromatin-immunoprecipitation sequencing by West et al (55); they also identified putative downstream mediators of the improved outcomes driven by active GR. Notably, direct ER:GR association by immunoprecipitation was reported in 2 of these studies (55, 57).
These studies together demonstrate that chromatin access driven by either ER or GR can render the local chromatin accessible for the other HR, which then modulates the expression of target genes. The prevalence of GR-induced ER remodeling in patient tumors in vivo is an important future area of research, as GR activity is likely maintained or induced in patients. Circulating cortisol in breast cancer patients is roughly 100nM–1μM (58, 59), and the GR agonist prednisone is commonly administered to patients to treat chemotherapy-related side effects, either of which may maintain GR in a chronically active state. Further, GR can interact with other HRs (60), which may further alter other ER:HR interactions discussed herein.
Mineralocorticoid Receptor
Despite the recent interest in HR interactions, relatively few studies on the function of MR (NR3C2) in breast cancer have been reported. MR has been shown to partially compensate for GR-loss during mammary gland development (61) and can interact with PR similarly to GR (60) and, thus, may have similar functions to GR in breast cancer. Importantly, although aldosterone is considered the primary ligand for MR, glucocorticoids (eg, cortisol) also bind MR with high affinity. MR is normally “protected” from cortisol via clearance by 11β-hydroxysteroid dehydrogenase II (HSD11B2), which eliminates cortisol but not aldosterone, but this enzyme is not broadly expressed in breast cancer (62, 63). However, MR may indeed be expressed in most breast cancers (62). Thus, MR should be equally considered as GR in potentially interacting with ER in breast cancer.
Although ER:MR interaction has not been directly examined in breast cancer models, Barrett Mueller et al (64) used HEK293 cells to demonstrate that ER strongly suppresses MR-induced mouse mammary tumor virus promoter-driven-luciferase reporter output. The reciprocal was not true; MR could not inhibit ER activation of ERE-driven luciferase output. Further, ER and MR were found to associate via coimmunoprecipitation, and the domain of ER necessary to bind MR and inhibit MR-mediated transcription was mapped to the ER N terminus. The ability of ER to inhibit MR-mediated gene expression was also confirmed in endothelial cell models. Although this study is limited based on primarily using overexpression in HEK293 to model ER:MR interaction, in light of recent findings with other ER:HR interactions, the functions of ER:MR interaction in breast cancer merit further study.
Pharmacological Implications for Endocrine Therapies
Most the ER:HR interactions described to date are dependent upon ligand-mediated activation of respective HRs. However, the mechanisms of currently used endocrine therapies are either to 1) activate or inactivate a target HR directly with a ligand/drug or 2) deplete endogenous ligand by blocking components of steroid synthesis. In either case, application of endocrine therapy may secondarily modulate ER:HR interactions or create a new context for HR activity. Although the application of novel endocrine therapies may be considered to reprogram ER to improve outcomes (discussed in Ref. 65), it is feasible that current or novel endocrine therapies may potentially eliminate a beneficial ER:HR interaction or switch the context for HR function from tumor suppressive to oncogenic.
The latter scenario may be of chief concern, given that laboratory and clinical data strongly support that in ER-positive tumors, AR and GR are tumor suppressive, but in ER− tumors, AR and GR are oncogenic and associated with poor outcome. This raises the question of whether ER functionally positive or negative regarding the functions of AR or GR in tamoxifen- or aromatase inhibitor (AI)-treated patients. Currently available data suggest that ER may in fact be functionally negative in these settings, and AR and/or GR may thus switch to oncogenic functions. For instance, clinical and laboratory data suggest that castrate-resistant prostate cancer may use GR signaling specifically to escape AR blockade (discussed in Ref. 54). Similarly, in breast cancer cell lines, acquired AI and tamoxifen resistance have been linked to activation of AR signaling (66, 67), and an AR-associated gene expression network was also found to be activated in breast tumors during neoadjuvant fulvestrant treatment (68). The role of AR is further complicated by the presence of AR splice variants in breast cancer (69), which have as of yet unexplored function in interacting with ER. Taken together, this potential for an activation of AR or GR oncogenic functions during antiestrogen therapy complicates the choice of an AR or GR agonist vs antagonist to combine with antiestrogen therapy. For example, activating GR with dexa may be chosen to reprogram ER favorably, but if combined with an AI, dexa may instead fuel tumor growth via now oncogenic GR signaling. Alternatively, AR or GR therapies with pure antagonist functions and independent of ER reprogramming, may be “safer” approaches to limit the likelihood of inadvertently activating an oncogene, as suggested by the relative efficacy of enzalutamide in both ER− and ER+ settings (40, 45, 65, 70).
Unique issues exist with the use of progestins in combination with antiestrogens. PGR expression is controlled by ER in breast cancer, and PR levels may subsequently be altered by antiestrogens. Tamoxifen and AIs (eg, letrozole, anastrozole) have also been shown to have differential effects on PR levels. In the IMPACT neoadjuvant trial, tumor PR decreased by 82% after 12 weeks in anastrozole-treated patients, whereas in tamoxifen-treated patients, PR increased at 2 weeks and returned to baseline at 12 weeks (71). Similar findings were reported for letrozole vs tamoxifen (72). Because AI treatment depletes PR protein, this may affect the efficacy of PR-targeted therapy. Perhaps based on this, both Mohammed et al (36) and Singhal et al (27) combined tamoxifen with a PR agonist or antagonist, respectively, and saw reduced breast cancer cell line xenograft growth. Additional studies are needed to confirm whether regulation of PGR affects efficacy of progestin therapy and whether tamoxifen specifically should be used in combination with progestins. Further, the similar effects of PR agonist P4 (36) and PR antagonist CDB4124 (27) highlight the complicated pharmacology of progestins. First, a definition of “agonist” or “antagonist” may be insufficient to describe an HR ligand's capacity to regulate ER:HR interactions. For example, neither GR nor ER antagonists precluded receptor binding to DNA, and either were sufficient to drive assisted loading, in the super-enhancer system (12). Second, P4 and synthetic progestins have widely variable abilities to agonize or antagonize other HRs (29, 73). Lack of response to the progestin MPA, which can bind AR, has been linked to loss of AR rather than PR (74); CDB4124 may also target GR and ER (75). Care should be taken in the choice of antiestrogen + PR ligand combinations and to expand biomarker analysis beyond ER and PR in planning or evaluating laboratory studies and clinical trials. PGR copy number has been investigated as a biomarker of ER:PR remodeling (36), but remodeling was observed in cells both with PGR deletion (MCF-7) and PGR amplification (T47D). Thus, PR may also have additional novel capabilities to antagonize the effects of ER.
Newer agents targeting steroid hormone synthesis present with additional concerns in modifying the hormonal milieu. CYP17A1 inhibitors such as abiraterone acetate (42) completely inhibit estrogen and androgen synthesis by blocking conversion of pregnenolone to sex steroid precursors. This also blocks production of glucocorticoids (which requires CYP17A1); the loss of negative feedback causes increased production of steroid precursors and mineralocorticoids (76). This leads to mineralocorticoid excess, which can be managed with prednisone or dexa (GR agonists, to restore negative feedback). Loss of both estrogens and androgens, and subsequent increase in MR and GR activity (from changes in metabolism or the added treatments), may lead to novel consequences on HR interaction (also discussed in Ref. 42). Further, decrease in estrogens and androgens may allow for the activity of weak or partial ER ligands, including abiraterone itself (47, 77, 78), potentially activating ER in a new HR context.
Conclusions
Understanding the mechanisms and consequences of interactions between ER and other HRs has rapidly progressed, and it has become clear that ER does not function in isolation in breast cancer. Rather, ER is in fact substantially affected by the activity of PR, AR, GR, and MR (summarized in Figure 1). Recent studies have begun to understand individual interactions (eg, ER:PR or ER:AR), but future studies must move toward evaluating breast cancer in an environment where the full family of steroid HRs is active to varying degrees and is modified by endocrine therapies. Understanding the effects of this hormone signaling milieu has the promise to improve our ability to predict, prevent, and reverse antiestrogen resistance driven by hormone signaling.
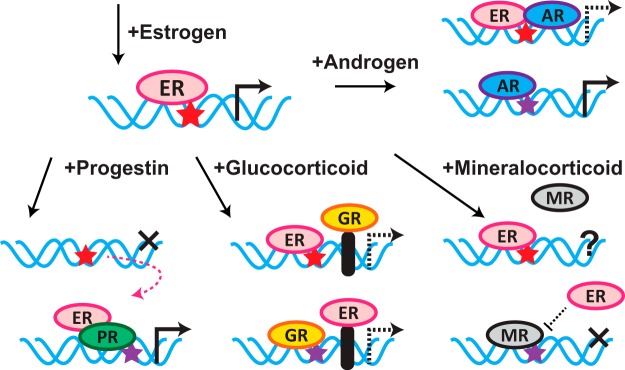
Figure 1.
HR activation by agonists modify ER genomic binding and regulation of target genes. Mechanisms described for the interaction of HRs with ER upon activation by agonists are described in detail in the text. Red star, ERE; purple star, SRE (bound by any of PR, AR, GR, or MR). PR activation moves ER off EREs, to SREs, and causes ER to differentially regulate new target genes. AR activation modulates ER function by binding at EREs with ER, but this may not be via direct interaction with ER; AR may also be critical for the nuclear localization of ER (see text). GR activation increases chromatin accessibility at SREs and allows for cofactor-mediated ER binding; the reciprocal is also true. The effects of MR activation on ER function are not well understood, but ER may modify MR regulation of target genes.
Acknowledgments
The author thanks Dr Britta Jacobsen, Dr Carol Sartorius, Dr Jennifer Richer, Dr Nicholas D'Amato, and Dr Rebecca Ferguson for their critical reading of this manuscript.
This work is supported by the School of Medicine and Department of Pathology of the University of Colorado Denver Anschutz Medical Campus and by a K99/R00 Pathway to Independence Award from the National Cancer Institute.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- AI
- aromatase inhibitor
- AR
- androgen receptor
- dexa
- dexamethasone
- DHT
- 5α-dihydrotestosterone
- E2
- estradiol
- ER
- estrogen receptor
- ERE
- estrogen-response element
- GR
- glucocorticoid receptor
- HR
- hormone receptor
- MR
- mineralocorticoid receptor
- NR
- nuclear receptor
- P4
- progesterone
- PR
- P4 receptor
- SRE
- steroid-response element.
References
- 1. Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69(4):1243–1254. [DOI] [PubMed] [Google Scholar]
- 2. Johnson AB, O'Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer. 2007;7(9):713–722. [DOI] [PubMed] [Google Scholar]
- 4. Jung SY, Malovannaya A, Wei J, O'Malley BW, Qin J. Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Mol Endocrinol. 2005;19(10):2451–2465. [DOI] [PubMed] [Google Scholar]
- 5. Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12(6):381–385. [DOI] [PubMed] [Google Scholar]
- 6. Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. [DOI] [PubMed] [Google Scholar]
- 7. Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desmedt C, Zoppoli G, Gundem G, et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34(16):1872–1881. [DOI] [PubMed] [Google Scholar]
- 9. Mohammed H, D'Santos C, Serandour AA, et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013;3(2):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foulds CE, Feng Q, Ding C, et al. Proteomic analysis of coregulators bound to ERα on DNA and nucleosomes reveals coregulator dynamics. Mol Cell. 2013;51(2):185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cirillo F, Nassa G, Tarallo R, et al. Molecular mechanisms of selective estrogen receptor modulator activity in human breast cancer cells: identification of novel nuclear cofactors of antiestrogen-ERα complexes by interaction proteomics. J Proteome Res. 2013;12(1):421–431. [DOI] [PubMed] [Google Scholar]
- 12. Bolt MJ, Stossi F, Newberg JY, Orjalo A, Johansson HE, Mancini MA. Coactivators enable glucocorticoid receptor recruitment to fine-tune estrogen receptor transcriptional responses. Nucleic Acids Res. 2013;41(7):4036–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Need EF, Selth LA, Harris TJ, Birrell SN, Tilley WD, Buchanan G. Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor α in luminal breast cancer cells. Mol Endocrinol. 2012;26(11):1941–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58(4):685–704. [DOI] [PubMed] [Google Scholar]
- 15. Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. [DOI] [PubMed] [Google Scholar]
- 16. Horwitz KB, Freidenberg GR. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985;45(1):167–173. [PubMed] [Google Scholar]
- 17. Horwitz KB, Zava DT, Thilagar AK, Jensen EM, McGuire WL. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978;38(8):2434–2437. [PubMed] [Google Scholar]
- 18. Allegra JC, Lippman ME, Thompson EB, et al. Relationship between the progesterone, androgen, and glucocorticoid receptor and response rate to endocrine therapy in metastatic breast cancer. Cancer Res. 1979;39(6 pt 1):1973–1979. [PubMed] [Google Scholar]
- 19. Itoh M, Iwamoto T, Matsuoka J, et al. Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Res Treat. 2014;143(2):403–409. [DOI] [PubMed] [Google Scholar]
- 20. Hefti MM, Hu R, Knoblauch NW, et al. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013;15(4):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunbier AK, Anderson H, Ghazoui Z, et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J Clin Oncol. 2010;28(7):1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajaram RD, Buric D, Caikovski M, et al. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34(5):641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brisken C, Heineman A, Chavarria T, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- 24. Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 25. Roarty K, Rosen JM. Wnt and mammary stem cells: hormones cannot fly wingless. Curr Opin Pharmacol. 2010;10(6):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilton HN, Doan TB, Graham JD, et al. Acquired convergence of hormone signaling in breast cancer: ER and PR transition from functionally distinct in normal breast to predictors of metastatic disease. Oncotarget. 2014;5(18):8651–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singhal H, Greene ME, Tarulli G, et al. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv. 2016;2(6):e1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol. 2015;54(2):R31–R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34(2):171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kabos P, Finlay-Schultz J, Li C, et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat. 2012;135(2):415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ballaré C, Uhrig M, Bechtold T, et al. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23(6):1994–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Migliaccio A, Piccolo D, Castoria G, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17(7):2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giulianelli S, Vaqué JP, Soldati R, et al. Estrogen receptor α mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res. 2012;72(9):2416–2427. [DOI] [PubMed] [Google Scholar]
- 34. Daniel AR, Gaviglio AL, Knutson TP, et al. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene. 2015;34(4):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malovannaya A, Lanz RB, Jung SY, et al. Analysis of the human endogenous coregulator complexome. Cell. 2011;145(5):787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;523(7560):313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Need EF, Selth LA, Trotta AP, et al. The unique transcriptional response produced by concurrent estrogen and progesterone treatment in breast cancer cells results in upregulation of growth factor pathways and switching from a luminal A to a basal-like subtype. BMC Cancer. 2015;15:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lim E, Ni M, Cao S, Hazra A, Tamimi RM, Brown M. Importance of breast cancer subtype in the development of androgen receptor directed therapy. Curr Breast Cancer Rep. 2014;6(2):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hickey TE, Robinson JL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol. 2012;26(8):1252–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cochrane DR, Bernales S, Jacobsen BM, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. 2014;21(4):T161–T181. [DOI] [PubMed] [Google Scholar]
- 42. Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, Traina TA. Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr Relat Cancer. 2015;22(3):R87–R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barton VN, D'Amato NC, Gordon MA, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14(3):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peters AA, Buchanan G, Ricciardelli C, et al. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131–6140. [DOI] [PubMed] [Google Scholar]
- 45. D'Amato NC, Gordon MA, Babbs B, et al. Cooperative dynamics of AR and ER activity in breast cancer [published online ahead of print August 26, 2016]. Mol Cancer Res. 10.1158/1541-7786.MCR-16-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5α-androstane-3β,17β-diol (3βAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115(2):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu DG, Selth LA, Tarulli GA, et al. Androgen and estrogen receptors in breast cancer co-regulate human UDP-glucuronosyltransferases 2B15 and 2B17. Cancer Res. 2016;76(19):5881–5893. [DOI] [PubMed] [Google Scholar]
- 49. Cvoro A, Yuan C, Paruthiyil S, Miller OH, Yamamoto KR, Leitman DC. Cross talk between glucocorticoid and estrogen receptors occurs at a subset of proinflammatory genes. J Immunol. 2011;186(7):4354–4360. [DOI] [PubMed] [Google Scholar]
- 50. Gong H, Jarzynka MJ, Cole TJ, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68(18):7386–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miranda TB, Voss TC, Sung MH, et al. Reprogramming the chromatin landscape: interplay of the estrogen and glucocorticoid receptors at the genomic level. Cancer Res. 2013;73(16):5130–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pan D, Kocherginsky M, Conzen SD. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71(20):6360–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skor MN, Wonder EL, Kocherginsky M, et al. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin Cancer Res. 2013;19(22):6163–6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kach J, Conzen SD, Szmulewitz RZ. Targeting the glucocorticoid receptor in breast and prostate cancers. Sci Transl Med. 2015;7(305):305ps19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. West DC, Pan D, Tonsing-Carter EY, et al. GR and ER coactivation alters the expression of differentiation genes and associates with improved ER+ breast cancer outcome. Mol Cancer Res. 2016;14(8):707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Voss TC, Schiltz RL, Sung MH, et al. Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell. 2011;146(4):544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karmakar S, Jin Y, Nagaich AK. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) α and activator protein 1 (AP1) in dexamethasone-mediated interference of ERα activity. J Biol Chem. 2013;288(33):24020–24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rossi E, Morabito A, Di Rella F, et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009;27(19):3192–3197. [DOI] [PubMed] [Google Scholar]
- 59. Wu SM, Yang HC, Thayer JF, Andersen BL. Association of the physiological stress response with depressive symptoms in patients with breast cancer. Psychosom Med. 2014;76(4):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leo JC, Guo C, Woon CT, Aw SE, Lin VC. Glucocorticoid and mineralocorticoid cross-talk with progesterone receptor to induce focal adhesion and growth inhibition in breast cancer cells. Endocrinology. 2004;145(3):1314–1321. [DOI] [PubMed] [Google Scholar]
- 61. Kingsley-Kallesen M, Mukhopadhyay SS, Wyszomierski SL, Schanler S, Schütz G, Rosen JM. The mineralocorticoid receptor may compensate for the loss of the glucocorticoid receptor at specific stages of mammary gland development. Mol Endocrinol. 2002;16(9):2008–2018. [DOI] [PubMed] [Google Scholar]
- 62. Sasano H, Frost AR, Saitoh R, et al. Localization of mineralocorticoid receptor and 11 β-hydroxysteroid dehydrogenase type II in human breast and its disorders. Anticancer Res. 17(3C):2001–2007. [PubMed] [Google Scholar]
- 63. Koyama K, Myles K, Smith R, Krozowski Z. Expression of the 11β-hydroxysteroid dehydrogenase type II enzyme in breast tumors and modulation of activity and cell growth in PMC42 cells. J Steroid Biochem Mol Biol. 76(1–5):153–159. [DOI] [PubMed] [Google Scholar]
- 64. Barrett Mueller K, Lu Q, Mohammad NN, et al. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155(11):4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lim E, Tarulli G, Portman N, Hickey T, Tilley WD, Palmieri C. Pushing estrogen receptor around in breast cancer. Endocr Relat Cancer. 2016;pii:ERC-16–0427. [DOI] [PubMed] [Google Scholar]
- 66. Ciupek A, Rechoum Y, Gu G, et al. Androgen receptor promotes tamoxifen agonist activity by activation of EGFR in ERα-positive breast cancer. Breast Cancer Res Treat. 2015;154(2):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rechoum Y, Rovito D, Iacopetta D, et al. AR collaborates with ERα in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat. 2014;147(3):473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Patani N, Dunbier AK, Anderson H, et al. Differences in the transcriptional response to fulvestrant and estrogen deprivation in ER-positive breast cancer. Clin Cancer Res. 2014;20(15):3962–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hickey TE, Irvine CM, Dvinge H, et al. Expression of androgen receptor splice variants in clinical breast cancers. Oncotarget. 2015;6(42):44728–44744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Traina TA, Miller K, Yardley DA, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). ASCO Meet Abstr. 2015;33(15 suppl):1003. [Google Scholar]
- 71. Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer–a study from the IMPACT trialists. J Clin Oncol. 2005;23(11):2477–2492. [DOI] [PubMed] [Google Scholar]
- 72. Miller WR, Dixon JM, Macfarlane L, Cameron D, Anderson TJ. Pathological features of breast cancer response following neoadjuvant treatment with either letrozole or tamoxifen. Eur J Cancer. 2003;39(4):462–468. [DOI] [PubMed] [Google Scholar]
- 73. Moore NL, Hickey TE, Butler LM, Tilley WD. Multiple nuclear receptor signaling pathways mediate the actions of synthetic progestins in target cells. Mol Cell Endocrinol. 2012;357(1–2):60–70. [DOI] [PubMed] [Google Scholar]
- 74. Buchanan G, Birrell SN, Peters AA, et al. Decreased androgen receptor levels and receptor function in breast cancer contribute to the failure of response to medroxyprogesterone acetate. Cancer Res. 2005;65(18):8487–8496. [DOI] [PubMed] [Google Scholar]
- 75. Wiehle R, Lantvit D, Yamada T, Christov K. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila). 2011;4(3):414–424. [DOI] [PubMed] [Google Scholar]
- 76. Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97(2):507–516. [DOI] [PubMed] [Google Scholar]
- 77. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Capper CP, Larios JM, Sikora MJ, Johnson MD, Rae JM. The CYP17A1 inhibitor abiraterone exhibits estrogen receptor agonist activity in breast cancer. Breast Cancer Res Treat. 2016;157(1):23–30. [DOI] [PubMed] [Google Scholar]