Abstract
Exogenous glucocorticoid administration results in hyperglycemia, insulin resistance, hepatic dyslipidemia, and hypertension, a constellation of findings known as Cushing's syndrome. These effects are mediated by the glucocorticoid receptor (GR). Because GR activation in liver and adipose has been implicated in metabolic syndrome (MS), we wanted to determine the role of GR in these tissues in the development of MS. Because GR knockout (KO) mice (whole-body KO) exhibit perinatal lethality due to respiratory failure, we generated tissue-specific (liver or adipose) GRKO mice using cre-lox technology. Real-time PCR analysis of liver mRNA from dexamethasone-treated wildtype (WT) and liver GRKO mice indicated that hepatic GR regulates the expression of key genes involved in gluconeogenesis and glycogen metabolism. Interestingly, we have observed that liver-specific deletion of GR resulted in a significant increase in mRNA expression of key genes involved in gluconeogenesis and glycogen metabolism in kidney tissue, indicating a compensatory mechanism to maintain glucose homeostasis. We have also observed that GR plays an important role in regulating the mRNA expression of key genes involved in lipid metabolism. Liver GRKO mice demonstrated decreased fat mass and liver glycogen content compared with WT mice administered dexamethasone for 2 weeks. Adipose-specific deletion of GR did not alter glucose tolerance or insulin sensitivity of adipose GRKO mice compared with WT mice administrated dexamethasone. This indicates that liver GR might be more important in development of MS in dexamethasone-treated mice, whereas adipose GR plays a little role in these paradigms.
Metabolic syndrome (MS) is defined as the common constellation of metabolic abnormalities that accompany obesity, including insulin resistance, hypertension, and hyperlipidemia, all of which are risk factors for cardiovascular disease, the leading cause of death in the United States. Mechanisms linking obesity to MS are not clear. The prevalence of MS is more than 20% among United States adults adjusted for age (1) and increased cardiovascular and mortality risk are associated with MS (2).
Glucocorticoids (GCs) are stress-induced steroid hormones (3). The hypothalamic-pituitary-adrenal (HPA) axis mediates the endocrine stress response. When the body perceives stress, the HPA axis is activated, resulting in the release of the primary stress hormones, GCs, by the adrenal gland (4). The predominant GC in humans is cortisol (corticosterone in mice), which has both diverse and tissue-specific functions, including regulation of cell growth and development, metabolism, inflammation, and apoptosis (5–7). GCs mediate their effect via binding and activation of the GC receptor (GR), which belongs to the nuclear hormone receptor family. GR is a modular protein containing a ligand-binding domain, DNA-binding domain, and 2 distinct activation domains AF1 and AF2 that mediate interactions with transcriptional coregulators and the basic transcriptional machinery (8). In the absence of ligand, GR is sequestered in the cytoplasm by a heat shock protein 90-chaperone complex. GC binding induces a conformational change within GR, allowing its release from the heat shock protein 90 complex and translocation to the nucleus, where it modulates the expression of target genes (9). GCs demonstrate antiinflammatory and immunomodulatory properties, and they are used to treat common inflammatory diseases (10). However, GC excess leads to development of central obesity, hepatic hyperlipidemia, hypertension, and glucose intolerance, a constellation of metabolic abnormalities reminiscent of the MS (11). Two isozymes of 11β-hydroxysteroid dehydrogenase (11β-HSD) are responsible for the tissue-specific interconversion of cortisone (inactive form) and cortisol (active form). 11β-HSD1 is expressed primarily in liver, adipose, and brain and 11β-HSD2 is expressed mainly in kidney and salivary glands (12). 11β-HSD1 converts inactive cortisone to cortisol, whereas 11β-HSD2 catalyzes the opposite reaction. The dehydrogenase hypothesis states that 11β-HSD1 is activated in adipose tissue and that adipose-generated GCs act on adipocytes and/or the liver via the portal circulation (13). However, it is not clear what the relative contribution of adipose GR and hepatic GR are in the development of MS, a question we addressed by generating tissue-specific (liver or adipose) GR knockout (KO) mice (liver GRKO [LGRKO] and adipose GRKO [FGRKO]) using cre-lox technology.
Animal studies have shown that GC signaling is critical to the development of the MS phenotype. Leptin-deficient ob/ob mice are largely rescued from MS if they are adrenalectomized (14). In addition, the activity of GCs in the liver is especially important as small molecule liver-specific GC antagonists ameliorate MS in rodent models (15). Increased GC levels modulate genes involved in lipolysis and triglyceride (TG) synthesis (16). GC effects on the liver and adipose are thought to be responsible for hypertriglyceridemia and hepatic steatosis as seen in Cushing's patients (17). It has been shown that dexamethasone (a GR agonist)-induced insulin resistance in diet-induced obese mice is associated with a profound perturbation of lipid metabolism (18). GRKO mice (whole-body KO) exhibit perinatal lethality due to respiratory failure caused by defects in lung maturation (19). Liver-specific GRKO mice were generated previously (20). One group described perinatal mortality which was due to early inactivation of GR in the liver (21). These mice displayed reduced viability (50% of mice died in the first 48 h of life) and subsequent impairments in body growth. Instead, we have used a LGRKO mouse in which GR is deleted at age 6 weeks, and there were no viability or growth defects observed (22). To determine the role of GR in development of MS, we generated tissue-specific (liver or adipose) GRKO mice using cre-lox technology. Liver- and adipose-specific GRKO mice (LGRKO and FGRKO, respectively) were administered corticosterone or dexamethasone. To investigate the role of GR in development of diet-induced obesity, LGRKO and FGRKO mice were fed high-fat diet (HFD) for approximately 16 weeks. Thus, this is the first direct comparison with estimate the relative contribution of hepatic and adipose GR action in MS.
We have observed that KO of GR in liver tissue alters insulin sensitivity, reduces fat mass and glycogen content upon dexamethasone administration compared with WT mice. Interestingly, liver-specific deletion of GR stimulated mRNA expression of genes involved in gluconeogenesis and glycogen metabolism in kidney tissue. We also observed an increased glycogen content in kidney tissue of LGRKO mice administered dexamethasone compared with WT mice. This indicates that kidney might compensate for the absence of liver GR to maintain balance in glucose homeostasis.
Materials and Methods
Animals
Male mice (Mus musculus) on the C57BL6 background were used for experiments. LGRKO mice were generated by crossing albumin cre mice (23) with GRflox/flox mice (24, 25). FGRKO mice were generated by crossing GRflox/flox mice with adiponectin-cre mice (26). Initially we began studies with exon 2 floxed mice and observed full deletion of GR as expected. However, during the course of these studies, it was reported that in at least 1 cell type exon 3 floxed mice were superior to exon 2 floxed mice (27). We therefore switched to exon 3 floxed mice. Animals were maintained in a temperature-controlled room (22°C) on a 12-hour light, 12-hour dark cycle. All animal work was performed according to the policies of Animal Studies Committee at Washington University in St. Louis. All mice were under approved protocol and were provided appropriate care while undergoing research that complies with the standards in the Guide and the Animal Welfare Act. For corticosterone experiments, mice (∼6 mo age) were divided into 3 groups (WT, LGRKO, and FGRKO) and administered corticosterone in drinking water at a dose of 10 mg/kg · d for 4 weeks (28). For dexamethasone experiments, mice (∼6 mo age) were divided into 3 groups (WT, LGRKO, and FGRKO) and administered dexamethasone via ip injection or drinking water at a dose of 10 mg/kg · d for 2 weeks. For HFD experiments, mice (6 wk age) were divided into 3 groups (WT, LGRKO, and FGRKO) and fed HFD (TD.88137; Harlan diets) for 16 weeks. HFD contained 17.3% by weight protein, 48.5% by weight carbohydrate, and 21.2% by weight fat (42% calories from fat).
Body composition analysis
Body composition of mice was determined using EchoMRI-100H (EchoMRI LLC), which measures fat, lean, free water, and total water masses. Body compositions of mice were performed before starting corticosterone or dexamethasone water (wk 0) and after the end of the experiment (ie, wk 2 for dexamethasone experiment and wk 4 for corticosterone experiment). For HFD experiment, body compositions were measured at the end of HFD feeding (ie, wk 16).
Bone density scan (dual-energy x-ray absorptiometry [DEXA])
Bone mineral density (BMD) and bone mineral content (BMC) of mice were determined by DEXA (Lunar PIXImus; GE Medical Systems). Mice were anesthetized by using ketamine/xylazine at a dose of 100- and 10-mg/kg body weight, respectively (29). DEXA of mice was performed before starting corticosterone or dexamethasone water (wk 0) and after the end of the experiment (ie, wk 2 for dexamethasone experiment and wk 4 for corticosterone experiment). For HFD experiment, DEXA was performed at the end of HFD feeding (ie, wk 16).
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
For dexamethasone and corticosterone experiments, GTT and ITT were performed in mice after 2 and 4 weeks of dexamethasone and corticosterone administration, respectively. For HFD experiments, GTT and ITT were performed after 16 weeks of HFD feeding. GTT was performed by fasting mice for 4 hours (9 am to 1 pm) by transferring mice to clean cages with no food or feces in hopper or bottom of cage followed by an ip injection of glucose (1% volume/weight of 10% dextrose in water). Blood glucose levels were measured immediately before glucose injection and at 20, 40, 60, and 120 minutes after injection using a glucometer and tail bleed. ITT was performed by fasting mice for 4 hours (9 am to 1 pm) by transferring mice to clean cages with no food or feces in hopper or bottom of cage followed by an ip injection of insulin at a dose of 0.7- weight or 1-U/kg body weight for chow-fed and HFD-fed mice, respectively. HFD-fed mice received a higher dose of insulin, because these mice are more insulin resistant than chow-fed animals. Blood glucose was measured immediately before insulin injection and 20, 40, 60, and 120 minutes after insulin injection. Hypoglycemic mice (blood glucose <40 mg/dL) were rescued with glucose injection, given food, and were excluded from future data points.
Western blot analysis
The protein expressions of GR in WT, LGRKO, and FGRKO mice were determined by Western blot analysis. Liver and brown adipose tissue (BAT) lysates were prepared by homogenizing a small portion (∼50 mg) of tissue in a tissue homogenizer (Glas-Col) using radioimmunoprecipitation assay buffer (Sigma) with protease/phosphatase inhibitor cocktail (Roche). Western blottings were performed using GR primary antibody (Cell Signaling) at a concentration of 1:1000. Actin levels were determined as a loading control using actin antibody (Cell Signaling). The Western band intensity data was quantified using Image Lab Software (Bio-Rad).
Real-time quantitative PCR analysis
mRNA levels of GR target genes were determined by real-time quantitative PCR analysis. WT, LGRKO, and FGRKO mice were injected with dexamethasone 10 mg/kg by ip injection and were killed after 6 hours, and tissues were harvested. RNA was extracted from liver and BAT tissues using TRIzol reagent (Thermo Fisher). BAT was used for gene expression validation given the high number of GR-expressing nonadipocyte cells in white adipose tissue. Any gDNA contamination was removed by TURBO DNA-free kit (Thermo Fisher). cDNA was prepared by iScript cDNA Synthesis kit (Bio Rad). Real-time PCR was performed by Power SYBR Green PCR Master Mix (Applied Biosystem) in a MicroAmp Optical 384-Well Reaction Plate (Applied Biosystem) using ViiA 7 Real-Time PCR System (Applied Biosystem). Relative gene expression was determined by the ΔΔCt method using cyclophilin A as a reference gene. Primers were designed with qPrimer Depot (https://mouseprimerdepot.nci.nih.gov). A list of forward and reverse primer sequences used is in the Supplemental Table 1.
TG assay
For determining liver TG levels, a small portion of liver tissue (∼50 mg) was homogenized in bullet blender (Biofrontier Technology, Next Advance) using chloroform:methanol (2:1). A small aliquot of supernatant (∼10 μL) in an Eppendorf tube was evaporated in a dry bath. Liver TG levels were determined by addition of Infinity Triglyceride reagent (Thermo Scientific) to the Eppendorf tube and reading absorbance at 630 and 540 nm. Absorbance at 630 nm was subtracted from 540 nm to determine TG levels using a standard curve.
Glycogen assay
For determining glycogen content in liver and kidney tissue, a small portion of tissue (∼20 mg) was boiled in 2N HCl (acid hydrolysis) for 1 hour with shaking, for determining total glucose concentration, then neutralized with NaOH. For free glucose determination, a similar piece of tissue was taken and treated with 2N NaOH, then neutralized with HCl. Glucose assay reagent (Sigma) was used to determine glucose content by reading absorbance at 340 nm. Glycogen levels were determined by subtracting free glucose from total glucose.
Corticosterone measurement
Corticosterone concentrations in plasma were measured via enzyme immunoassay kit (K014; Arbor Assays). Basal samples were collected at 9 am. For treated samples, animals were injected with either saline or 1-mg/kg dexamethasone at 9 am and then bled at 1 pm. Plasma samples were diluted 1:100 for assay.
Statistical analysis
All data are presented as mean ± SD and were analyzed by unpaired 2-tailed Student's t test for comparisons of 2 groups, one-way ANOVA or two-way ANOVA for comparisons of multiple groups. P < .05 was considered significant.
Results
Validation of GRKO in tissue-specific GRKO mice
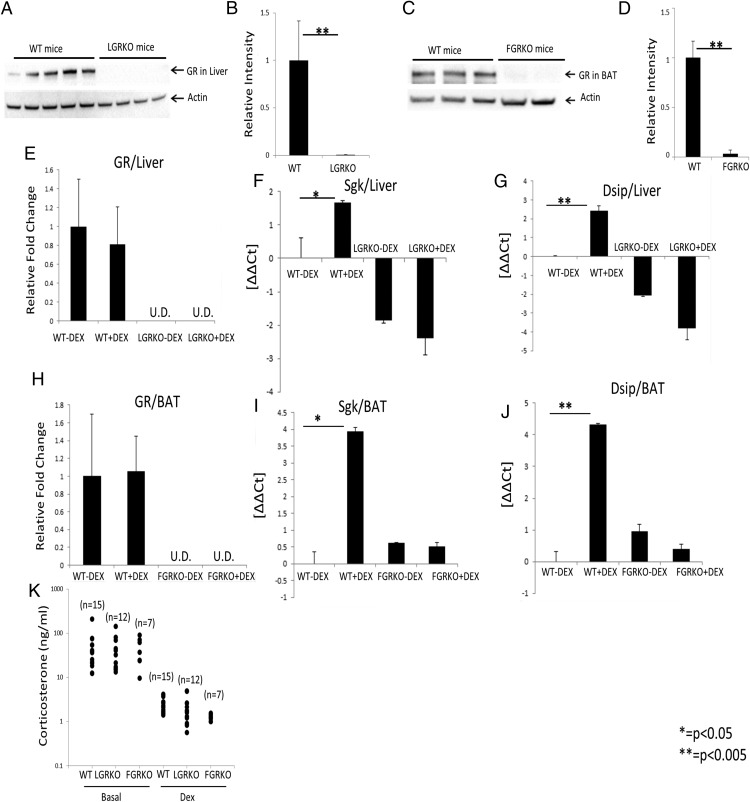
To confirm tissue-specific deletion of GR in LGRKO and FGRKO mice, we performed Western blot analysis. Western blotting demonstrated that the protein expression of GR was greatly reduced in the liver samples of LGRKO (GRflox/flox alb Cre+) mice compared with WT mice (P < .005) (Figure 1, A and B). GR protein expression level was greatly reduced in BAT tissue of FGRKO (GRflox/flox acdc Cre+) mice compared with WT mice (P < .005) (Figure 1, C and D). To make sure there is no nonspecific deletion of GR, we performed Western blotting for GR in liver samples of FGRKO mice. As expected, we did not see any nonspecific deletion of GR in liver tissue of FGRKO mice (data not shown), indicating that the deletion of GR was tissue specific. To determine the mRNA expression status of GR in LGRKO and FGRKO mice, we performed real-time PCR analysis. Mice from each group (WT, LGRKO, and FGRKO; n = 3 for each group) were used. We analyzed GR mRNA levels in both control and dexamethasone-treated (10 mg/kg for 6 h) mice. mRNA expression status of GR in liver tissue of LGRKO mice was undetectable by real-time PCR (Figure 1E). Similarly, mRNA expression status of GR in BAT tissue of FGRKO mice was undetectable (Figure 1H). These data indicated that GR expression was greatly reduced at both mRNA and protein level in the appropriate tissues. This experiment also revealed no change in GR mRNA levels in WT mice treated acutely with dexamethasone (Figure 1, E and H).
Figure 1.
Validation of KO of GR in tissue-specific GRKO mice. A, Western blot analysis for determining protein expression of GR in liver samples of WT and LGRKO mice. Each lane represents a separate mouse. Actin levels were determined as a loading control. B, Quantification of GR protein level in liver from Western blotting of A using Image Lab Software (Bio-Rad). C, Western blot analysis for determining protein expression of GR in BAT samples of WT and FGRKO mice. Each lane represents a separate mouse. Actin levels were determined as a loading control. D, Quantification of GR protein level in BAT from Western blotting of C using Image Lab Software (Bio-Rad). E–G, Real-time PCR analysis for determining the mRNA expression status of GR and GR target genes induced in most cell types (Sgk, Dsip1) in liver samples of WT and LGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 h); U.D., undetermined. H–J, Real-time PCR analysis for determining the mRNA expression status of GR and GR target genes induced in most cell types (Sgk, Dsip1), in BAT samples of WT and FGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 hours. K, Plasma concentration of corticosterone in WT, LGRKO, and FGRKO mice at basal state, injected with saline or 1-mg/kg dexamethasone for 4 hours. Values are mean ± SD. (real-time PCR data for E and H have been normalized with WT-Dex equal to 1 for ease of understanding, for all other real-time PCR data, we have represented the data as ΔΔCt).
To validate the functional KO of GR in liver, we performed real-time PCR analysis for determining the mRNA expression status of GR target genes induced in most cell types such as Sgk and Dsip1. Real-time PCR analysis in liver RNA demonstrated an up-regulation of GR target genes (Sgk, Dsip1) in WT mice injected with dexamethasone compared with WT mice injected with saline (Figure 1, F and G). Interestingly, for LGRKO mice, there was no significant up-regulation of GR target genes in mice injected with dexamethasone compared with mice injected with saline (Figure 1, F and G). This observation suggests that GR was knocked down functionally in liver of LGRKO mice. To validate the functional KO of GR in adipose, we performed real-time PCR analysis for determining the mRNA expression status of GR target genes induced in most cell types. Real-time PCR analysis in BAT RNA demonstrated an up-regulation of GR target genes induced in most cell types (Sgk, Dsip1) in WT mice injected with dexamethasone compared with WT mice injected with saline (Figure 1, I and J). Interestingly, for FGRKO mice, there was no significant up-regulation of GR target genes in mice injected with dexamethasone compared with mice injected with saline (Figure 1, I and J). This observation suggests that GR was knocked down functionally in BAT of FGRKO mice. Next, we examined the HPA axis in tissue-specific GRKO mice and WT mice by measuring basal corticosterone and performing a dexamethasone suppression test. Plasma concentration of corticosterone in WT (n = 15), LGRKO (n = 12), and FGRKO (n = 7) mice were measured via enzyme immunoassay kit (K014; Arbor Assays) at basal state, or 4 hours after a 1-mg/kg injection of dexamethasone. We observed that there was no significant difference in corticosterone levels of LGRKO and FGRKO mice compared with WT mice at the basal state (Figure 1K). Dexamethasone injection resulted in a significant decrease in corticosterone levels of WT, LGRKO, and FGRKO mice compared with the basal state (Figure 1K). These data indicate that HPA axis activation was not significantly altered due to tissue-specific KO of GR in either adipose or liver.
Liver-specific KO of GR inhibits dexamethasone-mediated stimulation of genes involved in carbohydrate metabolism
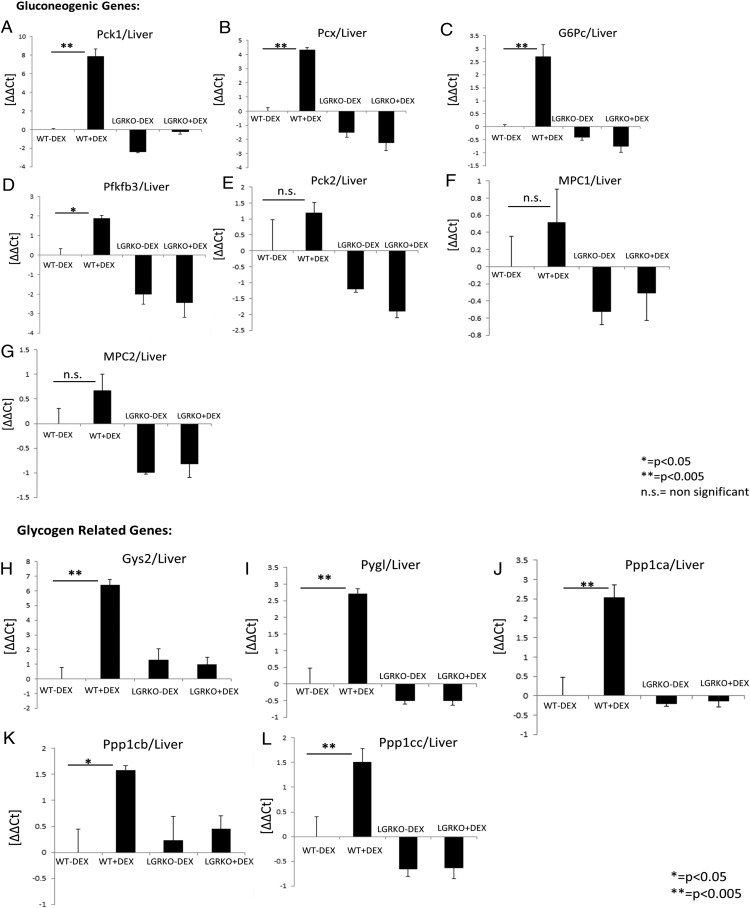
GR is known to be involved in carbohydrate metabolism (30, 31). GCs increase the transcription of genes involved in de novo gluconeogenesis. Increased endogenous or exogenous GCs, result in hyperglycemia and insulin resistance. Phosphoenolpyruvate carboxykinase (Pck) (Pck1, Pck2) and glucose-6-phosphatase (G6Pc) enzymes catalyze, respectively, the rate-limiting step and final step in hepatic glucose production (see figure 7 below). During gluconeogenesis, pyruvate carboxylase (Pcx) is involved in the synthesis of phosphoenolpyruvate from pyruvate. Some other genes playing key roles in gluconeogenesis include 6-phosphofructo-2-kinase (Pfkfb3) and mitochondrial pyruvate carriers (MPCs) (Mpc1, Mpc2). Pfkfb3 is involved in synthesis and degradation of fructose-2,6-bisphosphate, a key modulator of gluconeogenesis. MPCs play a key role in pyruvate entry into the mitochondrial matrix during gluconeogenesis. Real-time PCR analysis of liver RNA demonstrated that acute dexamethasone injection (10 mg/kg for 6 h) in WT mice leads to a significant increase in mRNA expression of key genes involved in gluconeogenesis (Pck1, Pcx, G6pc, and Pfkfb3) compared with WT mice injected with saline (Figure 2, A–D). Interestingly, we did not observe up-regulation in mRNA expression of key genes involved in gluconeogenesis (Pck1, Pcx, G6pc, and Pfkfb3) in LGRKO mice injected with dexamethasone compared with LGRKO mice injected with saline (Figure 2, A–D). Although there was a trend, mRNA expression of Pck2, Mpc1, and Mpc2 was not significantly induced in WT mice injected with dexamethasone (Figure 2, E–G). Two-way ANOVA showed P < .05 for interaction between genotypes and dexamethasone treatment for all genes except Pck2, Mpc1, and Mpc2. These data indicate that dexamethasone stimulates the mRNA expression of several key genes involved in gluconeogenesis in WT mice and liver GR is required for dexamethasone-mediated stimulation of these genes involved in gluconeogenesis. This highlights the importance of GR in the regulation of gluconeogenesis. Next, we wanted to determine the mRNA expression status of key genes involved in glycogen metabolism. Glycogen synthase (Gys) catalyzes the rate-limiting step in the synthesis of glycogen. Glycogen phosphorylase (Pygl) is involved in breakdown of glycogen. Protein phosphatase (Ppp)1 (Ppp1ca, Ppp1cb, Ppp1cc) is a serine/threonine-specific Ppp, which inactivates phosphorylase kinase and accelerates glycogen synthesis (see figure 7 below). We have observed that acute dexamethasone injection in WT mice leads to a significant increase in mRNA expression of key genes involved in glycogen metabolism (Gys2, Pygl, Ppp1ca, Ppp1cb, and Ppp1cc) compared with WT mice injected with saline (Figure 2, H–L). Interestingly, we did not observe a significant up-regulation in mRNA expression of key genes involved in glycogen metabolism (Gys2, Pygl, Ppp1ca, Ppp1cb, and Ppp1cc) in LGRKO mice injected with dexamethasone compared with LGRKO mice injected with saline (Figure 2, H–L). Two-way ANOVA showed P for interaction <.05 for all genes. This indicates that liver GR is required for dexamethasone-mediated stimulation of genes involved in glycogen metabolism. This also highlights the importance of GR in glycogen metabolism.
Figure 2.
Liver-specific KO of GR inhibits dexamethasone-mediated stimulation of genes involved in carbohydrate metabolism. A–G, Real-time PCR analysis for determining the mRNA expression status of genes involved in gluconeogenesis (Pck1, Pcx, G6Pc, Pfkfb3, Pck2, Mpc1, Mpc2) in liver tissue of WT and LGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 hours. H–L, Real-time PCR analysis for determining the mRNA expression status of genes involved in glycogen metabolism (Gys2, Pygl, Ppp1ca, Ppp1cb, Ppp1cc) in liver tissue of WT and LGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 hours.
Adipose-specific KO of GR inhibits dexamethasone-mediated stimulation of genes involved in lipid metabolism
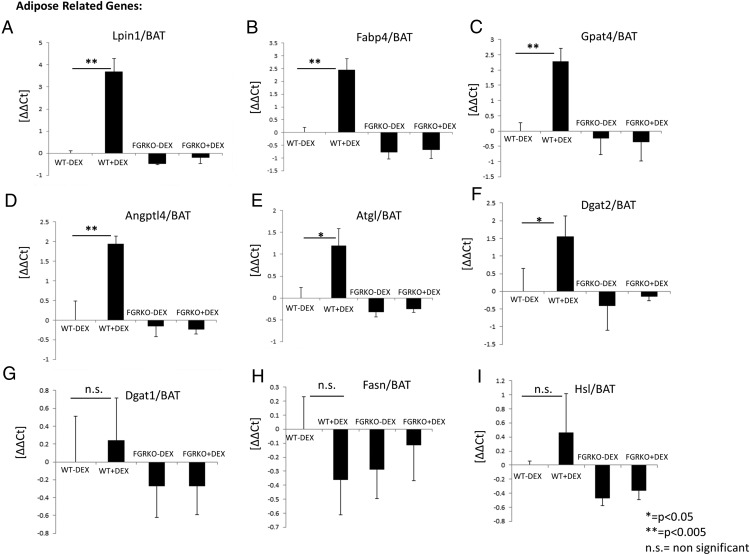
GR is known to play an important role in lipid metabolism (16, 32). Although GCs are essential for the regulation of lipid homeostasis, excess, and/or chronic exposure to GCs can cause disorders, including central obesity, dyslipidemia, and fatty liver (33, 34). We wanted to determine the mRNA expression status of key genes involved in lipid metabolism. Lipin1 (Lpin1) is involved in catalyzing the penultimate step in TG synthesis. Fatty acid-binding protein 4 (Fabp4) binds long-chain fatty acids and is involved in fatty acid uptake, transport, and metabolism. Glycerol-3-phosphate acyltransferase 4 (Gpat4) and angiopoietin-like 4 (Angptl4) are involved in lipid biosynthesis and metabolism. Patatin-like phospholipase domain containing 2 (Pnpla2) (also known as adipose triglyceride lipase) is involved in the first step in the hydrolysis of TGs. Dgat2 is involved in TG synthesis. Real-time PCR analysis of BAT RNA demonstrated that acute dexamethasone injection (10 mg/kg for 6 h) in WT mice leads to a significant increase in mRNA expression of key genes involved in lipid metabolism (Lpin1, Fabp4, Gpat4, Angptl4, Pnpla2, and Dgat2) compared with WT mice injected with saline (Figure 3, A–F). We did not observe up-regulation in mRNA expression of some genes involved in lipid metabolism (Lpin1, Fabp4, Gpat4, Angptl4, Pnpla2, and Dgat2) in FGRKO mice injected with dexamethasone compared with FGRKO mice injected with saline (Figure 3, A–F). mRNA expression of Dgat1, Fasn, and Hsl was not significantly induced in WT mice injected with dexamethasone (Figure 3, G–I). Two-way ANOVA showed P < .05 for interaction between genotype and dexamethasone treatment for all genes except Dgat1, Fasn, Hsl. Real-time PCR analysis of inguinal white adipose tissue RNA also demonstrated that acute dexamethasone injection (10 mg/kg for 6 h) in WT mice leads to a significant increase in mRNA expression of key genes involved in lipid metabolism (Lpin1, Fabp4, Gpat4, Angptl4, Dgat1, and Dgat2) compared with WT mice injected with saline (Supplemental Figure 1, A–F). We did not observe up-regulation in mRNA expression of these genes in FGRKO mice injected with dexamethasone compared with FGRKO mice injected with saline (Supplemental Figure 1, A–F). These data indicate that dexamethasone stimulates the mRNA expression of key genes involved in lipid metabolism in WT mice and adipose-specific KO of GR inhibits dexamethasone-mediated stimulation of genes involved in lipid metabolism. This highlights the importance of GR in lipid metabolism.
Figure 3.
Adipose-specific KO of GR inhibits dexamethasone-mediated stimulation of genes involved in lipid metabolism. A–I, Real-time PCR analysis for determining the mRNA expression status of adipose-specific genes (Lpin1, Fabp4, Gpat4, Angptl4, Pnpla2, Dgat2, Dgat1, Fasn, Hsl) in BAT tissue of WT and FGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 hours.
Liver-specific KO of GR results in increased dexamethasone-mediated expression of genes involved in carbohydrate metabolism in kidney tissue
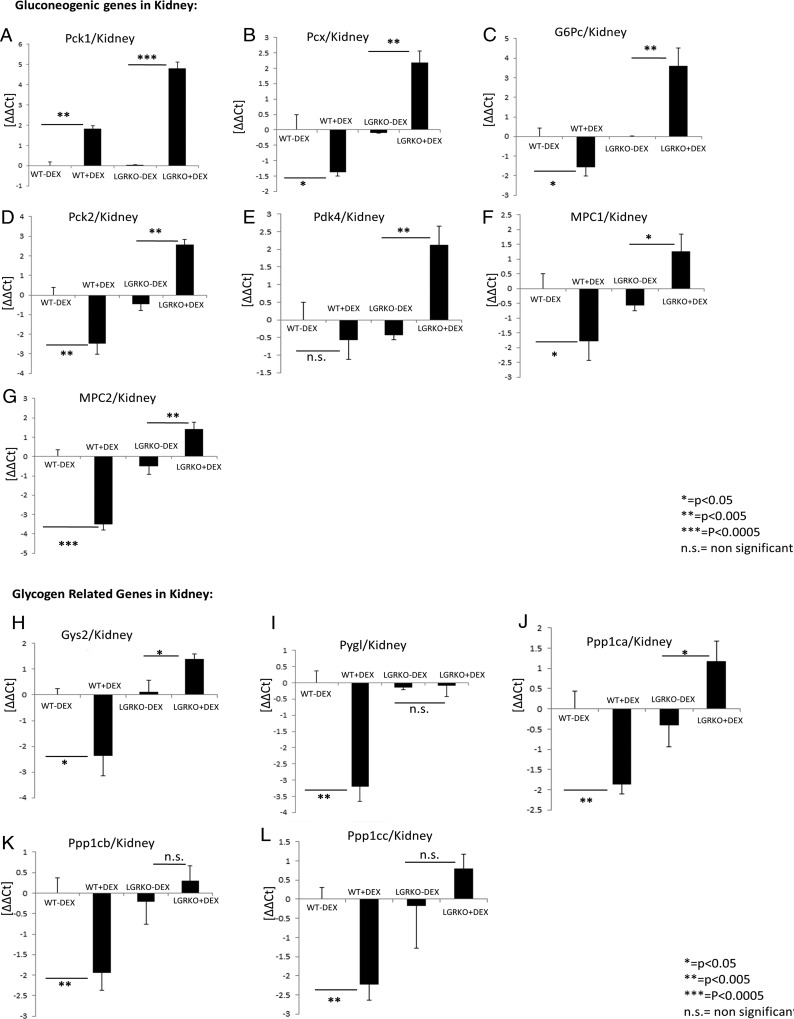
Apart from liver, kidney is known to play a role in carbohydrate metabolism accounting for approximately 20% of gluconeogenesis (35, 36). We have previously observed that liver-specific KO of GR inhibits dexamethasone-mediated stimulation of genes involved in gluconeogenesis in liver. Because kidney is also a site of gluconeogenesis, we wanted to determine the mRNA expression status of key genes involved in gluconeogenesis in kidney tissue of LGRKO mice. Real-time PCR analysis of kidney RNA demonstrated that acute dexamethasone injection (10 mg/kg for 6 h) in WT mice decreased the mRNA expression status of key genes involved in gluconeogenesis (Pcx, G6pc, Pck2, Mpc1, and Mpc2) compared with WT mice injected with saline (Figure 4, B–D and F and G). We observed up-regulation of Pck1 gene in kidney tissue of WT mice injected with dexamethasone compared with WT mice injected with saline (Figure 4A). Interestingly, we observed that the expression of gluconeogenic genes (Pck1, Pcx, G6pc, Pck2, Pdk4, Mpc1, and Mpc2) were significantly increased in kidney tissue of LGRKO mice injected with dexamethasone compared with LGRKO mice injected with saline (Figure 4, A–G). Next, we wanted to determine the mRNA expression status of some key genes involved in glycogen metabolism in kidney tissue of LGRKO mice. We have observed that acute dexamethasone injection in WT mice leads to a significant decrease in mRNA expression status of key genes involved in glycogen metabolism (Gys2, Pygl, Ppp1ca, Ppp1cb, and Ppp1cc) in kidney compared with WT mice injected with saline (Figure 4, H–L). Interestingly, we observed up-regulation in mRNA expression of some specific genes involved in glycogen metabolism (Gys2, Ppp1ca, and Ppp1cc) in kidney tissue of LGRKO mice injected with dexamethasone compared with LGRKO mice injected with saline (Figure 4, H, J, and L). We did not observe a significant change in mRNA expression of Pygl and Ppp1cb genes in kidney tissue of LGRKO mice injected with dexamethasone compared with LGRKO mice injected with saline (Figure 4, I and K). KO of GR in liver of LGRKO mice impaired the dexamethasone-mediated activation of liver glycogen metabolism-related genes. This impairment in activation of glycogen metabolism-related genes in liver of LGRKO mice is compensated by an increased expression of glycogen-related genes in the kidney tissue of LGRKO mice injected with dexamethasone.
Figure 4.
Liver-specific KO of GR results in increased dexamethasone-mediated expression of genes involved in carbohydrate metabolism in kidney tissue. A–G, Real-time PCR analysis for determining the mRNA expression status of genes involved in gluconeogenesis (Pck1, Pcx, G6Pc, Pck2, Pdk4, Mpc1, Mpc2) in kidney tissue of WT and LGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 hours. H–L, Real-time PCR analysis for determining the mRNA expression status of genes involved in glycogen metabolism (Gys2, Pygl, Ppp1ca, Ppp1cb, Ppp1cc) in kidney tissue of WT and LGRKO mice injected with saline or dexamethasone (10 mg/kg) for 6 hours.
Deletion of GR in liver tissue alters insulin sensitivity, body composition, and glycogen content upon dexamethasone administration
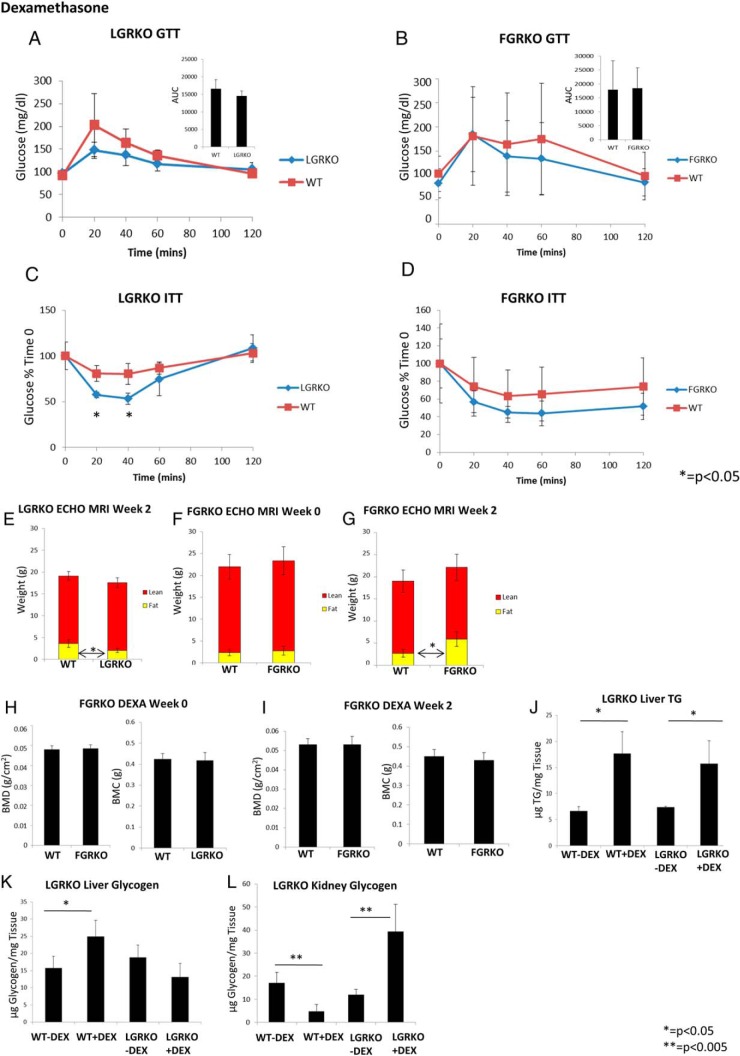
We wanted to determine the effect of dexamethasone on liver-specific GRKO mice. To determine the role of liver GR in development of MS in response to dexamethasone administration, male mice (∼6 mo age) were divided into 2 groups WT (n = 5) and LGRKO (n = 5) and administered dexamethasone via ip injection (10 mg/kg · d) for 2 weeks. Then, we performed GTT, ITT, and body composition analysis using EchoMRI. Finally, mice were killed, and liver TG and glycogen levels were determined. There was no difference in baseline glucose levels (92 ± 8 vs 96 ± 8 mg/dL for WT and LGRKO mice, respectively). There was a nonsignificant trend for higher glucose level at 20 minutes after start of GTT in WT mice (203 ± 69 mg/dL) compared with LGRKO mice (147 ± 18 mg/dL) (Figure 5A). The area under the curve (AUC) for LGRKO mice was similar to WT mice (Figure 5A). ITT demonstrated that LGRKO mice were more sensitive to insulin compared with WT mice. We observed a significantly higher glucose level in WT mice (81 ± 9% time 0) at 20 minutes after start of ITT compared with LGRKO mice (57 ± 3% time 0) indicating that deletion of GR significantly increases insulin sensitivity in dexamethasone-treated mice. After 40 minutes of start of ITT, there was a significantly higher glucose level in WT mice (80 ± 11% time 0) compared with LGRKO mice (53 ± 6% time 0). Thus, deletion of liver-specific GR improves insulin sensitivity in mice administered dexamethasone compared with WT mice (Figure 5C). Body composition analysis demonstrated that there was no difference in overall body weight (20.22 ± 1.67 vs 19.14 ± 1.53 g for WT and LGRKO mice, respectively). WT mice had a slightly higher fat mass (3.62 ± 0.89 g) compared with LGRKO mice (2.00 ± 0.46 g) (Figure 5E). There was no difference in lean mass (15.49 ± 0.98 vs 15.52 ± 1.15 g for WT and LGRKO mice, respectively). Because we have observed that acute dexamethasone injection to WT and LGRKO mice stimulates glycogen-related genes only in liver tissue of WT mice (Figure 2, H–L), we wanted to determine liver glycogen levels in WT and LGRKO mice after 2 weeks of dexamethasone treatment. We observed that dexamethasone treatment significantly increased liver glycogen level in WT mice (25.0 ± 4.7-μg glycogen/mg tissue) compared with WT mice injected with saline (15.8 ± 3.4-μg glycogen/mg tissue) (Figure 5K). Interestingly, dexamethasone injection did not increase liver glycogen levels in LGRKO mice (13.1 ± 4.0-μg glycogen/mg tissue) compared with LGRKO mice injected with saline (18.8 ± 3.6-μg glycogen/mg tissue) (Figure 5K). We have previously observed that acute dexamethasone injection to WT and LGRKO mice stimulates glycogen-related genes in kidney tissue of LGRKO mice (Figure 4, H–L). Thus, we determined kidney glycogen levels in WT and LGRKO mice treated with dexamethasone for 2 weeks. We observed that dexamethasone treatment significantly reduced kidney glycogen level in WT mice (4.7 ± 3.0-μg glycogen/mg tissue) compared with WT mice injected with saline (17.1 ± 4.6-μg glycogen/mg tissue) (Figure 5L). Interestingly, we observed that dexamethasone injection significantly increased kidney glycogen level in LGRKO mice (39.3 ± 11.8-μg glycogen/mg tissue) compared with LGRKO mice injected with saline (12.03 ± 2.19-μg glycogen/mg tissue) (Figure 5L). These data support our real-time PCR data, where we have seen up-regulation of genes involved in glycogen metabolism in kidney LGRKO mice injected with dexamethasone (Figure 4, H–L). Dexamethasone injection increased liver TG levels equally in WT and LGRKO mice (17.7 ± 4.2- vs 15.8 ± 4.3-μg TG/mg tissue for WT and LGRKO mice injected with dexamethasone, respectively) (Figure 5J).
Figure 5.
Deletion of GR in liver tissue alters insulin sensitivity, body composition, and glycogen content upon dexamethasone administration. A and B, GTT in WT, LGRKO, and FGRKO mice administered dexamethasone for 2 weeks, respectively. C and D, ITT in LGRKO and FGRKO mice administered dexamethasone for 2 weeks, respectively. Blue line indicates tissue-specific GRKO mice (LGRKO/FGRKO). Red line indicates WT mice. E, Body composition analysis by EchoMRI in WT, LGRKO mice at week 2 after dexamethasone administration. F and G, Body composition analysis by EchoMRI in WT, FGRKO mice at week 0 (before dexamethasone administration) and week 2 after dexamethasone administration, respectively. H and I, BMD, BMC analysis by DEXA in WT, FGRKO mice at week 0 and week 4 after dexamethasone administration, respectively. J, Liver TG content in WT, LGRKO mice administered saline or dexamethasone for 2 weeks. K, Liver glycogen content in WT, LGRKO mice administered saline or dexamethasone for 2 weeks. L, Kidney glycogen content in WT, LGRKO mice administered saline or dexamethasone for 2 weeks.
To determine the effect of dexamethasone on adipose-specific GRKO mice, mice (∼6 mo age) were divided into 2 groups, WT (n = 5) and FGRKO (n = 8), and administered dexamethasone via drinking water at a dose of 10 mg/kg · d for 2 weeks. FGRKO mice did not have significant differences in body composition from WT mice before dexamethasone administration. Specifically, there was no difference in body weight (23.54 ± 3.84 vs 24.18 ± 3.01 g for WT and FGRKO mice, respectively). There was no difference in fat mass (2.34 ± 0.68 vs 2.78 ± 1.02 g for WT and FGRKO mice, respectively), lean mass (19.62 ± 2.81 vs 20.54 ± 3.23 g for WT and FGRKO mice, respectively) (Figure 5F), or percent fat (12.06 ± 1.10 vs 13.46 ± 2.38 for WT and FGRKO mice, respectively). There was no difference in BMD (0.0480 ± 0.0019 vs 0.0480 ± 0.0017 g/cm2 for WT and FGRKO mice, respectively) and BMC (0.421 ± 0.026 vs 0.410 ± 0.038 g for WT and FGRKO mice, respectively) (Figure 5H). Mice were then administered dexamethasone via drinking water at a dose of 10 mg/kg · d for 2 weeks. At the end of dexamethasone administration, we did not observe significant difference in baseline glucose levels (103 ± 37 vs 84 ± 30 mg/dL for WT and FGRKO mice, respectively) or glucose levels during GTT (Figure 5B). The AUC for WT and FGRKO mice were similar (Figure 5B, inset). There was no difference in glucose levels between WT and FGRKO mice during ITT (Figure 5D). Body composition analysis demonstrated that there was no difference in overall body weight (21.70 ± 2.62 vs 24.90 ± 3.89 g for WT and FGRKO mice, respectively). Interestingly, we observed that FGRKO mice had a significantly higher fat mass (5.89 ± 1.62 g) compared with WT mice (2.66 ± 0.86 g) (Figure 5G).There was no difference in lean mass (16.31 ± 2.59 vs 16.24 ± 2.92 g for WT and FGRKO mice, respectively) (Figure 5G). We also observed a significant increase in percent fat of FGRKO mice (23.36 ± 3.98) compared with WT mice (13.52 ± 2.08). There was no difference in BMD (0.0530 ± 0.0032 vs 0.0531 ± 0.0041 g/cm2 for WT and FGRKO mice, respectively) and BMC (0.450 ± 0.030 vs 0.430 ± 0.030 g for WT and FGRKO mice, respectively) (Figure 5I).
Tissue-specific deletion of GR does not alter the metabolic response of mice administered corticosterone
We wanted to determine the effect of corticosterone on tissue-specific GRKO mice. To determine the role of liver GR in development of MS in response to corticosterone administration, male mice (∼6 mo age) were divided into 2 groups WT (n = 8) and LGRKO (n = 5). Similarly, to determine the role of adipose GR in development of MS in response to corticosterone administration, male mice (∼6 mo age) were divided into 2 groups WT (n = 4) and FGRKO (n = 4). Before starting corticosterone water, initial body composition analysis, and BMD, BMC of mice was determined by using EchoMRI and DEXA, respectively. LGRKO mice did not have significant differences in body composition from WT mice before corticosterone treatment. Specifically, there was no difference in body weight (27.28 ± 2.11 vs 26.65 ± 2.88 g for WT and LGRKO mice, respectively). There was no difference in fat mass (4.18 ± 0.80 vs 5.03 ± 1.46 g for WT and LGRKO mice, respectively), lean mass (21.08 ± 1.73 vs 22.12 ± 1.74 g for WT and LGRKO mice, respectively) (Supplemental Figure 2E), or percent fat (17.03 ± 4.07 vs 14.30 ± 1.77 for WT and LGRKO mice, respectively). There was no difference in BMD (0.0480 ± 0.0029 vs 0.0490 ± 0.0017 g/cm2 for WT and LGRKO mice, respectively) and BMC (0.420 ± 0.043 vs 0.440 ± 0.033 g for WT and LGRKO mice, respectively) (Supplemental Figure 2I). Mice were then administered corticosterone via drinking water at a dose of 10 mg/kg · d for 4 weeks. Then, we performed GTT, ITT, body composition, and DEXA. Finally, mice were killed, and liver TG levels were determined. There was no difference in baseline glucose levels (118.38 ± 54.28 vs 118.40 ± 16.89 mg/dL for WT and LGRKO mice, respectively) or glucose levels during GTT (Supplemental Figure 2A). There was no difference in glucose levels between WT and LGRKO mice during ITT (Supplemental Figure 2C). Body composition analysis demonstrated that there was no difference in body weight (28.08 ± 3.60 vs 28.82 ± 3.87 g for WT and LGRKO mice, respectively). There was no difference in fat mass (8.35 ± 1.93 vs 8.69 ± 1.73 g for WT and LGRKO mice, respectively), lean mass (18.51 ± 1.78 vs 18.74 ± 1.93 g for WT and LGRKO mice, respectively) (Supplemental Figure 2F), or percent fat (27.06 ± 2.90 vs 27.23 ± 2.23 for WT and LGRKO mice, respectively). There was no difference in BMD (0.0490 ± 0.0010 vs 0.0503 ± 0.0020 g/cm2 for WT and LGRKO mice, respectively) and BMC (0.450 ± 0.048 vs 0.450 ± 0.068 g for WT and LGRKO mice, respectively) (Supplemental Figure 2J). There was no significant difference in liver TG levels (14.2 ± 7.5- vs 21.9 ± 8.3-μg TG/mg tissue for WT and LGRKO mice, respectively) (Supplemental Figure 2M).
FGRKO mice did not have significant differences in body composition from WT mice before corticosterone administration. Specifically, there was no difference in body weight (20.16 ± 2.84 vs 18.94 ± 2.51 g for WT and FGRKO mice, respectively). There was no difference in fat mass (2.76 ± 0.51 vs 2.71 ± 0.29 g for WT and FGRKO mice, respectively), lean mass (16.17 ± 2.04 vs 16.17 ± 1.91 g for WT and FGRKO mice, respectively) (Supplemental Figure 1G), or percent fat (11.36 ± 0.98 vs 11.32 ± 1.15 for WT and FGRKO mice, respectively). There was no difference in BMD (0.0371 ± 0.0041 vs 0.0361 ± 0.0041 g/cm2 for WT and FGRKO mice, respectively) and BMC (0.280 ± 0.061 vs 0.257 ± 0.051 g for WT and FGRKO mice, respectively) (Supplemental Figure 2K). Mice were then administered corticosterone via drinking water at a dose of 10 mg/kg · d for 4 weeks. At the end of corticosterone administration, we did not observe significant difference in baseline glucose levels (79.00 ± 8.54 vs 85.00 ± 3.16 mg/dL for WT and FGRKO mice, respectively) or glucose levels during GTT (Supplemental Figure 2B). There was no difference in glucose levels between WT and FGRKO mice during ITT (Supplemental Figure 2D). Body composition analysis demonstrated that there was no difference in body weight (20.40 ± 3.90 vs 19.22 ± 1.83 g for WT and FGRKO mice, respectively). There was no difference in fat mass (5.82 ± 2.03 vs 5.29 ± 0.57 g for WT and FGRKO mice, respectively), lean mass (13.71 ± 2.71 vs 12.87 ± 1.84 g for WT and FGRKO mice, respectively) (Supplemental Figure 2H) or percent fat (22.96 ± 5.25 vs 23.07 ± 2.73 for WT and FGRKO mice, respectively). There was no difference in BMD (0.0420 ± 0.0030 vs 0.0420 ± 0.0020 g/cm2 for WT and FGRKO mice, respectively) and BMC (0.280 ± 0.040 vs 0.270 ± 0.021 g for WT and FGRKO mice, respectively) (Supplemental Figure 2L). There was no significant difference in liver TG levels (7.3 ± 3.5- vs 6.1 ± 2.8-μg TG/mg tissue for WT and FGRKO mice, respectively) (Supplemental Figure 2N). LGRKO and FGRKO mice administered corticosterone demonstrated similar glucose tolerance, insulin sensitivity, body composition analysis, BMD, BMC, and liver TG levels compared with WT mice. These data indicate that tissue-specific deletion of GR did not alter the metabolic response of mice administered corticosterone.
Tissue-specific deletion of GR does not alter the metabolic response of mice HFD
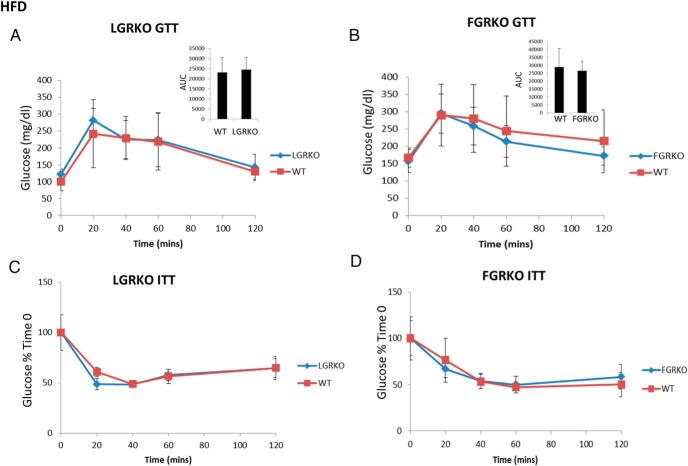
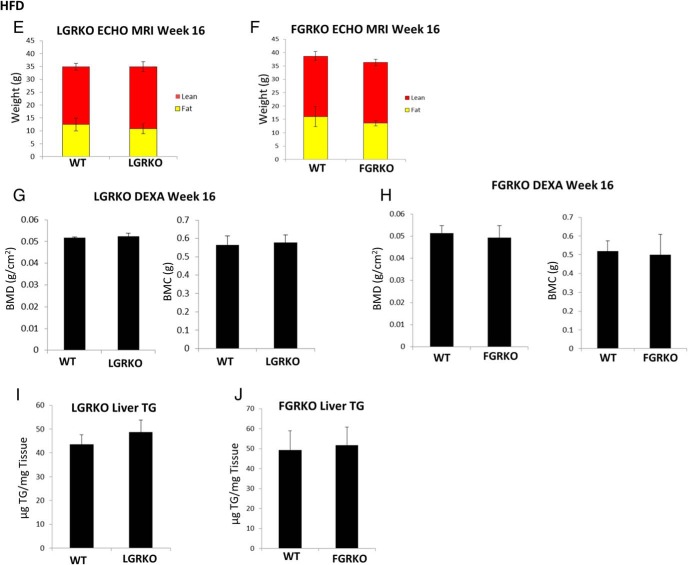
We wanted to determine whether there is any role of GR in development of diet-induced obesity. To determine the role of liver-specific GR in diet-induced obesity, male mice (6 wk age) were divided into 2 groups, WT (n = 4) and LGRKO (n = 4). Similarly, to determine the role of adipose GR in diet-induced obesity, male mice (∼6 mo age) were divided into 2 groups, WT (n = 7) and FGRKO (n = 8). Mice were fed HFD (TD.88137; Harlan diets) for 16 weeks. At the end of 16 weeks of HFD feeding, we performed GTT and ITT experiments. Body composition analysis and BMD, BMC of mice were determined by using EchoMRI and DEXA, respectively. Finally, mice were killed, and liver TG levels were determined. For LGRKO mice, there was no difference in baseline glucose levels (101 ± 28 vs 122 ± 15 mg/dL for WT and LGRKO mice, respectively) or glucose levels during GTT (Figure 6A). The AUC was similar for WT and LGRKO mice were similar (Figure 6A). There was no difference in glucose levels between WT and LGRKO mice during ITT (Figure 6C). Body composition analysis demonstrated that there was no difference in body weight (37.03 ± 1.77 vs 35.22 ± 1.58 g for WT and LGRKO mice, respectively). There was no difference in fat mass (12.47 ± 2.48 vs 10.81 ± 1.89 g for WT and LGRKO mice, respectively), lean mass (22.39 ± 1.36 vs 24.11 ± 1.95 g for WT and LGRKO mice, respectively) (Figure 6E), or percent fat (38.70 ± 7.66 vs 35.60 ± 3.43 for WT and LGRKO mice, respectively). There was no difference in BMD (0.0528 ± 0.0003 vs 0.0521 ± 0.0023 g/cm2 for WT and LGRKO mice, respectively) and BMC (0.564 ± 0.040 vs 0.577 ± 0.041 g for WT and LGRKO mice, respectively) (Figure 6G). There was no significant difference in liver TG levels (43.5 ± 4.1- vs 48.8 ± 5.0-μg TG/mg tissue for WT and LGRKO mice, respectively) (Figure 6I).
Figure 6.
Tissue-specific deletion of GR does not alter the metabolic response of mice fed HFD. A and B, GTT in LGRKO and FGRKO mice fed HFD for 16 weeks, respectively. C and D, ITT in LGRKO and FGRKO mice fed HFD for 16 weeks, respectively. E, Body composition analysis by EchoMRI in WT, LGRKO mice after 16 weeks of HFD feeding, respectively. F, Body composition analysis by EchoMRI in WT, FGRKO mice after 16 weeks of HFD feeding, respectively. G, BMD, BMC analysis by DEXA in WT, LGRKO mice after 16 weeks of HFD feeding. H, BMD, BMC analysis by DEXA in WT, FGRKO mice after 16 weeks of HFD feeding. I, Liver TG content in WT, LGRKO mice after 16 weeks of HFD feeding. J, Liver TG content in WT, FGRKO mice after 16 weeks of HFD feeding. All values are given as mean ± SD.
For FGRKO mice, there was no difference in baseline glucose levels (168 ± 28 vs 158 ± 33 mg/dL for WT and FGRKO mice, respectively) or glucose levels during GTT (Figure 6B). The AUC was similar for WT and FGRKO mice were similar (Figure 6B). There was no difference in glucose levels between WT and FGRKO mice during ITT (Figure 6D). Body composition analysis demonstrated that there was no difference in body weight (40.27 ± 5.65 vs 36.93 ± 3.22 g for WT and FGRKO mice, respectively). There was no significant difference in fat mass (16.00 ± 3.70 vs 13.55 ± 0.97 g for WT and FGRKO mice, respectively), lean mass (22.62 ± 1.71 vs 22.70 ± 1.30 g for WT and FGRKO mice, respectively) (Figure 6F) or percent fat (43.25 ± 4.11 vs 40.96 ± 3.23 for WT and FGRKO mice, respectively). There was no difference in BMD (0.0511 ± 0.0033 vs 0.0490 ± 0.0054 g/cm2 for WT and FGRKO mice, respectively) and BMC (0.520 ± 0.051 vs 0.510 ± 0.108 g for WT and FGRKO mice, respectively) (Figure 6H). There was no significant difference in liver TG levels (49.3 ± 9.6- vs 51.8 ± 9.0-μg TG/mg tissue for WT and FGRKO mice, respectively) (Figure 6J). LGRKO and FGRKO mice fed HFD demonstrated similar glucose tolerance, insulin sensitivity, body composition analysis, BMD, BMC, and liver TG levels compared with WT mice.
Discussion
GCs are stress hormones with antiinflammatory and immunomodulatory properties, whereas excess GCs have been implicated in the pathophysiology of the MS (37). It has been reported that in Cushing's syndrome, increased secretion of GCs leads to central obesity, hypertension, hyperlipidemia, and glucose intolerance (11, 37). Administration of GCs for the treatment of chronic inflammatory diseases has been associated with development of adverse metabolic effects such as hypertension, obesity, hyperlipidemia, and insulin resistance (38–40). GCs acting through the GR regulate key enzymes of glucose and lipid metabolism in the liver and adipose tissue. It has been reported that genetic polymorphisms of the GR can directly or indirectly affect the pathophysiology and development of MS (41).
Because GRKO mice (whole-body GRKO) exhibit postnatal lethality due to respiratory failure (19), we have generated tissue-specific GRKO mice using cre-lox technology. Based on the dehydrogenase hypothesis, we focused on GR action in adipose and liver to determine which is the critical player in development of MS. GR KO was validated by Western blot analysis of GR in liver and BAT tissues of WT, LGRKO, and FGRKO mice. Because GR is known to be involved in carbohydrate and lipid metabolism (30–32), we determined the mRNA expression status of key genes involved in carbohydrate and lipid metabolism in WT and KO mice. We have observed that dexamethasone injection to WT mice resulted in increased mRNA expression of genes involved in carbohydrate and lipid metabolism, whereas in the tissue-specific GRKO mice, the induction of these genes with GCs were significantly reduced. These results indicate that GR is involved in regulation of key genes in carbohydrate and lipid metabolism and that we have successfully generated tissue-specific GRKO mice.
We have used 2 different GCs (corticosterone and dexamethasone) because of the observation that dexamethasone, while wholly specific for GR elicits more catabolic effects, whereas corticosterone elicits more anabolic effects of GR, including obesity at the expense of specificity, because corticosterone also binds mineralocorticoid receptor. We have observed that administration of corticosterone (via drinking water) to WT, LGRKO, and FGRKO mice did not significantly alter their metabolic responses as had been described previously for WT mice (28). LGRKO and FGRKO mice displayed similar glucose tolerance as compared with WT mice. Tissue-specific deletion of GR did not significantly alter insulin sensitivity in the LGRKO and FGRKO mice as compared with WT mice. Body composition analysis by EchoMRI revealed that corticosterone administration to LGRKO and FGRKO mice altered fat and lean mass to an extent similar to WT mice. These tissue-specific GRKO mice also displayed similar bone density and BMC as compared with WT mice. Liver TG levels in LGRKO and FGRKO mice were similar to WT mice. Dexamethasone administration (ip injection) to WT mice resulted in decreased insulin sensitivity, which was not observed in LGRKO mice administered dexamethasone. Fat mass and liver glycogen levels were also significantly reduced in LGRKO mice administered dexamethasone as compared with WT mice. These results indicate that liver-specific GR is an important player in the development of MS, when administered a potent GR agonist (dexamethasone). Dexamethasone injection increased liver TG levels equally in WT and LGRKO mice. One possible explanation is that dexamethasone acts as a xenobiotic and activates targets other than GR like the pregnane X receptor, resulting in increased TG accumulation (42, 43). Dexamethasone administration to FGRKO mice did not alter their glucose tolerance, insulin sensitivity, lean mass, BMD, and BMC. This indicates that liver GR might be more important in development of MS than adipose GR (Figure 7A). To determine the role of GR in diet-induced obesity, WT, LGRKO, and FGRKO mice were fed HFD for 16 weeks. Interestingly, there was no significant difference in insulin sensitivity, body composition (fat and lean mass), BMD, BMC, and liver TG levels in either KO mice as compared with WT mice. Our data are consistent with a recent finding (44) showing minimal effects of FGRKO in diet-induced obesity. These data suggest that either the dehydrogenase hypothesis is incorrect or that the combined action of GR in liver and adipose is required for full development of diet-induced obesity and MS. Studies using 2′-O-methoxyethyl antisense oligonucleotides targeting both liver and adipose GR in db/db mice support the latter hypothesis (45).
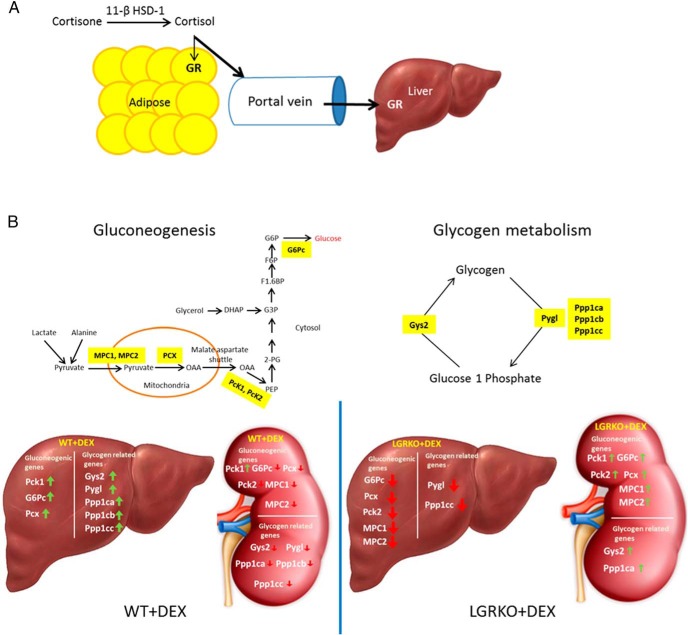
Figure 7.
Schematic diagram showing the importance of liver GR in MS and renal compensation. A, 11β-HSD1 activates GC precursors (cortisone) into active hormone (cortisol) that acts locally in adipose tissue and also on the liver due to the portal venous drainage of visceral adipose tissue. Our data indicate that liver GR plays a greater role in development of MS as compared with adipose GR. B, Simplified schematic of gluconeogenesis and glycogen metabolism with the key genes highlighted in yellow. Our data indicate that in WT mice, dexamethasone activates gluconeogenic and glycogen metabolism-related genes in the liver and suppresses their expression in the kidney. On the other hand, we have observed that in LGRKO mice, dexamethasone represses gluconeogenic and glycogen metabolism-related genes in the liver and activates their expression in the kidney.
A previous finding suggests that adipocyte-specific GC inactivation protects against diet-induced obesity (46). In this study, the authors used a transgenic mouse model in which human 11β-HSD2 was expressed under the control of the murine adipocyte fatty acid binding protein (also known as adipocyte protein 2) promoter. Transgenic mice had increased 11β-HSD2 expression and activity exclusively in adipose tissue. The authors observed that transgenic mice resist weight gain on HFD due to reduced fat mass accumulation. They also observed improved glucose tolerance and insulin sensitivity in the transgenic mice. Our data are different from the study by Kershaw (46), because in their study, the authors have modulated ligand (GCs) levels (by modulating prereceptor activation), and we have directly altered receptor levels. Therefore, this apparent discrepancy could be explained either by action of unliganded GR or by GC actionon mineralocorticoid receptor to induce MS. In our study, we have observed that liver-specific deletion of GR leads to improved insulin sensitivity in dexamethasone-treated mice. We also observed that liver-specific deletion of GR reduced fat mass in dexamethasone-treated mice as compared with WT mice administered dexamethasone. Interestingly, deletion of GR in adipose tissue did not result in improved insulin sensitivity and alter fat mass of mice administered dexamethasone as compared with WT mice. It might be possible that a double GR KO (liver and adipose simultaneously) would result in a greater response towards metabolic disorders as compared with GR KO in a single tissue.
Apart from liver, kidney is known to play a role in carbohydrate metabolism, accounting for approximately 20% of gluconeogenesis (35, 36). Because we observed that deletion of GR in liver (LGRKO mice) inhibited dexamethasone-mediated induction of hepatic mRNA expression of genes involved in gluconeogenesis and glycogen metabolism as compared with WT mice, we wanted to determine whether any compensatory mechanism is at work in the kidney. Interestingly, we have observed that acute dexamethasone injection (10 mg/kg for 6 h) to LGRKO mice resulted in a significant increase in kidney mRNA expression of key genes involved in gluconeogenesis (Pck1, Pck2, Pcx, G6pc, Pdk4, etc) and glycogen metabolism (Gys2, Ppp1ca, Ppp1cc) as compared with WT mice. KO of GR in liver of LGRKO mice impaired the dexamethasone-mediated activation of liver gluconeogenic genes. This impairment in activation of gluconeogenic genes in liver of LGRKO mice is compensated by an increased expression of gluconeogenic genes in the kidney tissue of LGRKO mice injected with dexamethasone (Figure 7B). We observed that administration of dexamethasone to WT mice resulted in a significant increase in glycogen levels, which was reduced in LGRKO mice. Interestingly, dexamethasone resulted in a decrease in glycogen levels in the kidney of WT mice and an increase in kidney glycogen levels in LGRKO mice. This is the inverse response to that seen in the liver (increase in WT glycogen levels and decrease in glycogen levels in the LGRKO mice). Using inhibitors of liver gluconeogenesis (47, 48) or gene targeting techniques (49), it has been shown that kidney can compensate for loss of hepatic gluconeogenesis. A novel finding of ours was the dual regulated nature of some genes in WT vs LGRKO mice, ie, genes that are repressed in WT and induced in LGRKO by dexamethasone. So called dual regulated genes have been described in different cell types (50). Such dual regulation could be explained by 1) compensatory changes in transcriptional coregulator expression; 2) differences in signaling in WT and LGRKO kidney that result in altered posttranslational modification and activity of GR; and 3) differential regulation of composite glucocorticoid response elements governing GR regulation of target genes. Overall, our results indicate that liver GR is more important than adipose GR in development of MS in dexamethasone-treated mice. Also, our results indicate a compensatory mechanism by the kidney to stimulate genes involved in gluconeogenesis and glycogen metabolism in the absence of liver GR.
Acknowledgments
This work was funded by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) Grant K08DK081680.
Disclosure Summary: The authors have nothing to disclose.
Appendix
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | RRID | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|---|
| GR protein | GR (D8H2) XP | Cell Signaling, 3660S | AB_11179215 | Rabbit mAb | 1:1000 | |
| Actin | b-Actin (13E5) rabbit mAb | Cell Signaling, 5125S | AB_1903890 | Rabbit mAb | 1:10 000 |
Footnotes
- Angptl4
- angiopoietin-like 4
- AUC
- area under the curve
- BAT
- brown adipose tissue
- BMC
- bone mineral content
- BMD
- bone mineral density
- DEXA
- dual-energy x-ray absorptiometry
- Fabp4
- fatty acid-binding protein 4
- FGRKO
- adipose GRKO
- GC
- glucocorticoid
- Gpat4
- glycerol-3-phosphate acyltransferase 4
- G6Pc
- glucose-6-phosphatase
- GR
- GC receptor
- GTT
- glucose tolerance test
- Gys
- glycogen synthase
- HFD
- high-fat diet
- HPA
- hypothalamic-pituitary-adrenal
- 11β-HSD
- 11β-hydroxysteroid dehydrogenase
- ITT
- insulin tolerance test
- KO
- knockout
- LGRKO
- liver GRKO
- Lpin1
- Lipin1
- MPC
- mitochondrial pyruvate carrier
- MS
- metabolic syndrome
- Pck
- phosphoenolpyruvate carboxykinase
- Pcx
- pyruvate carboxylase
- Pfkfb3
- 6-phosphofructo-2-kinase
- Pnpla2
- patatin-like phospholipase domain containing 2
- Ppp
- protein phosphatase
- Pygl
- glycogen phosphorylase
- TG
- triglyceride
- WT
- wildtype.
References
- 1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. [DOI] [PubMed] [Google Scholar]
- 2. Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. [DOI] [PubMed] [Google Scholar]
- 3. Ramamoorthy S, Cidlowski JA. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr Dev. 2013;24:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–219. [DOI] [PubMed] [Google Scholar]
- 5. Greenberg AK, Hu J, Basu S, et al. Glucocorticoids inhibit lung cancer cell growth through both the extracellular signal-related kinase pathway and cell cycle regulators. Am J Respir Cell Mol Biol. 2002;27(3):320–328. [DOI] [PubMed] [Google Scholar]
- 6. Rose AJ, Herzig S. Metabolic control through glucocorticoid hormones: an update. Mol Cell Endocrinol. 2013;380(1–2):65–78. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11(suppl 1):S45–S55. [DOI] [PubMed] [Google Scholar]
- 8. Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70(5–7):407–417. [DOI] [PubMed] [Google Scholar]
- 9. Galliher-Beckley AJ, Cidlowski JA. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61(10):979–986. [DOI] [PubMed] [Google Scholar]
- 10. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. [DOI] [PubMed] [Google Scholar]
- 11. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593–5602. [DOI] [PubMed] [Google Scholar]
- 12. Walker EA, Stewart PM. 11β-hydroxysteroid dehydrogenase: unexpected connections. Trends Endocrinol Metab. 2003;14(7):334–339. [DOI] [PubMed] [Google Scholar]
- 13. Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? Lancet. 1997;349(9060):1210–1213. [DOI] [PubMed] [Google Scholar]
- 14. Naeser P. Effects of adrenalectomy on the obese-hyperglycemic syndrome in mice (gene symbol ob). Diabetologia. 19(5)73;9:376–379. [DOI] [PubMed] [Google Scholar]
- 15. Zinker B, Mika A, Nguyen P, et al. Liver-selective glucocorticoid receptor antagonism decreases glucose production and increases glucose disposal, ameliorating insulin resistance. Metabolism. 2007;56(3):380–387. [DOI] [PubMed] [Google Scholar]
- 16. Wang JC, Gray NE, Kuo T, Harris CA. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2(1)012;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taskinen MR, Nikkilä EA, Pelkonen R, Sane T. Plasma lipoproteins, lipolytic enzymes, and very low density lipoprotein triglyceride turnover in Cushing's syndrome. J Clin Endocrinol Metab. 1983;57(3):619–626. [DOI] [PubMed] [Google Scholar]
- 18. Gounarides JS, Korach-André M, Killary K, Argentieri G, Turner O, Laurent D. Effect of dexamethasone on glucose tolerance and fat metabolism in a diet-induced obesity mouse model. Endocrinology. 2008;149(2):758–766. [DOI] [PubMed] [Google Scholar]
- 19. Cole TJ, Blendy JA, Monaghan AP, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 19(13)95;9:1608–1621. [DOI] [PubMed] [Google Scholar]
- 20. Kellendonk C, Opherk C, Anlag K, Schütz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26(2):151–153. [DOI] [PubMed] [Google Scholar]
- 21. Opherk C, Tronche F, Kellendonk C, et al. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004;18(6):1346–1353. [DOI] [PubMed] [Google Scholar]
- 22. Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40(6):1322–1332. [DOI] [PubMed] [Google Scholar]
- 23. Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–315. [DOI] [PubMed] [Google Scholar]
- 24. Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122(7):2384–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brewer JA, Khor B, Vogt SK, et al. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med. 2003;9(10):1318–1322. [DOI] [PubMed] [Google Scholar]
- 26. Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laryea G, Schütz G, Muglia LJ. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol Endocrinol. 2013;27(10):1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151(5):2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith W. Responses of laboratory animals to some injectable anaesthetics. Lab Anim. 1993;27(1):30–39. [DOI] [PubMed] [Google Scholar]
- 30. Jitrapakdee S. Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis. Int J Biochem Cell Biol. 2012;44(1):33–45. [DOI] [PubMed] [Google Scholar]
- 31. Patel R, Williams-Dautovich J, Cummins CL. Minireview: new molecular mediators of glucocorticoid receptor activity in metabolic tissues. Mol Endocrinol. 2014;28(7):999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. John K, Marino JS, Sanchez ER, Hinds TD., Jr The glucocorticoid receptor: cause of or cure for obesity? Am J Physiol Endocrinol Metab. 2016;310(4):E249–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing's syndrome. Neuroendocrinology. 2010;92(suppl 1):86–90. [DOI] [PubMed] [Google Scholar]
- 34. Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197(2):189–204. [DOI] [PubMed] [Google Scholar]
- 35. Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24(2):382–391. [DOI] [PubMed] [Google Scholar]
- 36. Stumvoll M, Meyer C, Mitrakou A, Gerich JE. Important role of the kidney in human carbohydrate metabolism. Med Hypotheses. 1999;52(5):363–366. [DOI] [PubMed] [Google Scholar]
- 37. Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr Metab (Lond). 2005;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Covar RA, Leung DY, McCormick D, Steelman J, Zeitler P, Spahn JD. Risk factors associated with glucocorticoid-induced adverse effects in children with severe asthma. J Allergy Clin Immunol. 2000;106(4):651–659. [DOI] [PubMed] [Google Scholar]
- 39. Davis GF. Adverse effects of corticosteroids: II. Systemic. Clin Dermatol. 1986;4(1):161–169. [DOI] [PubMed] [Google Scholar]
- 40. Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. [DOI] [PubMed] [Google Scholar]
- 41. Moraitis AG, Block T, Nguyen D, Belanoff JK. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness [published online ahead of print March 18, 2016]. J Steroid Biochem Mol Biol. 10.1016/j.jsbmb.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 42. Bitter A, Rümmele P, Klein K, et al. Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Arch Toxicol. 2015;89(11):2089–2103. [DOI] [PubMed] [Google Scholar]
- 43. Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone induces pregnane X receptor and retinoid X receptor-α expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol. 2000;58(2):361–372. [DOI] [PubMed] [Google Scholar]
- 44. Desarzens S, Faresse N. Adipocyte glucocorticoid receptor has a minor contribution in adipose tissue growth. J Endocrinol. 2016;230(1):1–11. [DOI] [PubMed] [Google Scholar]
- 45. Watts LM, Manchem VP, Leedom TA, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54(6):1846–1853. [DOI] [PubMed] [Google Scholar]
- 46. Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 2005;54(4):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lupiáñez JA, Faus MJ, Muñoz-Clares R, Sánchez-Medina F. Stimulation of rat kidney gluconeogenic ability by inhibition of liver gluconeogenesis. FEBS Lett. 1976;61(2):277–281. [DOI] [PubMed] [Google Scholar]
- 48. Sanchez-Medina F, Garcia-Ruiz JP, Lupiañez JA, Faus MJ, Hortelano P. Induction of rat kidney gluconeogenic ability after impairment of liver gluconeogenesis. Curr Probl Clin Biochem. 1977;8:310–317. [PubMed] [Google Scholar]
- 49. She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol. 20(17)00;20:6508–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen SH, Masuno K, Cooper SB, Yamamoto KR. Incoherent feed-forward regulatory logic underpinning glucocorticoid receptor action. Proc Natl Acad Sci USA. 2013;110(5):1964–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]