Abstract
Thyroid hormone is essential for normal development in vertebrates. In amphibians, T3 controls metamorphosis by inducing tissue-specific gene regulation programs. A hallmark of T3 action is the modification of chromatin structure, which underlies changes in gene transcription. We found that mRNA for the de novo DNA methyltransferase (DNMT) dnmt3a, but not dnmt1, increased in the brain of Xenopus tadpoles during metamorphosis in parallel with plasma [T3]. Addition of T3 to the rearing water caused a time-dependent increase in dnmt3a mRNA in tadpole brain, tail, and hind limb. By analyzing data from a genome-wide analysis of T3 receptor (TR) binding in tadpole tail, we identified several putative T3 response elements (TREs) within the dnmt3a locus. Using in vitro DNA binding, transient transfection-reporter, and chromatin immunoprecipitation assays for TRs, we identified two functional TREs at −7.1 kb and +5.1 kb relative to the dnmt3a transcription start site. Sequence alignment showed that these TREs are conserved between two related frog species, X. laevis and X. tropicalis, but not with amniotes. Our previous findings showed that this gene is directly regulated by liganded TRs in mouse brain, and whereas the two mouse TREs are conserved among Eutherian mammals, they are not conserved in Xenopus species. Thus, although T3 regulation of dnmt3a may be an ancient pathway in vertebrates, the genomic sites responsible for hormone regulation may have diverged or arisen by convergent evolution. We hypothesize that direct T3 regulation of dnmt3a may be an important mechanism for modulating global changes in DNA methylation.
Thyroid hormone is well known to play important roles in vertebrate development, most notably its role in mammalian brain development where thyroid deficiency during late fetal and early postnatal life leads to severe mental retardation (cretinism) (1, 2). J. F. Gudernatsch (3) was the first to discover that the vertebrate thyroid gland contained a factor that could influence development in a vertebrate. He found that extracts of horse thyroid could induce precocious metamorphosis if fed to amphibian tadpoles. The active compound, later identified as T4, which is converted to T3 by monodeiodinases, is the primary hormone controlling amphibian metamorphosis (4). It orchestrates the entire suite of molecular, biochemical, and morphological changes that occur during metamorphosis by controlling gene expression programs in different tissues to promote cell proliferation, migration, differentiation, and death (5). The structure of T3 is identical in frogs and mammals, and its receptors are highly conserved among vertebrate taxa, as are the basic cellular and molecular processes initiated by the hormone during development (6).
The actions of T3 are mediated by T3 receptors (TRs) that regulate gene transcription, typically as heterodimers with retinoid X receptor (RXR). The TR-RXR complex binds to T3 response elements (TREs) in the genome that are comprised of two hexanucleotide half-sites (most commonly as a direct repeat plus four-base spacer: DR+4). The TRs modify local chromatin structure by recruiting histone-modifying enzymes (7). For genes that are activated by T3, unliganded TRs repress transcription through interaction with corepressors, whereas liganded TRs activate transcription through recruitment of coactivators (7).
Another important epigenetic modification that can influence chromatin structure is the methylation of cytosine residues in DNA. However, it is not known if T3 can modulate DNA methylation, or if it is important for T3 action. DNA methylation in vertebrate genomes occurs predominantly in the context of cytosine-guanine (CpG) dinucleotides. Cytosine residues in most CpG dinucleotides (70–80%) are methylated and are located in intergenic regions, within genes and transposable elements (8–10). The remaining approximately 20% are found near gene promoters and are referred to as CpG islands; these are mostly unmethylated, or they are differentially methylated depending on developmental stage or physiological state. The global pattern of DNA methylation is established by the de novo DNA methyltransferases DNMT3a and DNMT3b and is then preserved through cell division by the maintenance DNA methyltransferase, DNMT1 (11, 12). Current evidence supports that DNA methylation leads to long-term, stable transcriptional silencing through recruitment of methyl-CpG binding proteins that recruit histone-modifying enzymes to generate a transcriptionally silent state, and possibly also through physical blockade of transcription factor binding (11, 13). Modulation of DNA methylation has recently been shown to play critical roles in neurological development and plasticity (14, 15). Notably, DNMT3a is implicated in playing a critical role in establishing patterns of DNA methylation in the genomes of neural cells during early brain development (16).
Although the roles of liganded TR in controlling posttranslational modifications to histones have been extensively studied, it is not known whether T3 can influence DNA methylation. Here we investigated T3 regulation of dnmt genes in Xenopus tadpoles during metamorphosis, with the primary focus on the brain. The genomes of Xenopus species possess two dnmt genes, dnmt1 and dnmt3a. We found that dnmt3a, but not dnmt1, gene expression increased in parallel with the rise in plasma [T3] during metamorphosis, and that treatment of early prometamorphic tadpoles with T3 could induce a precocious elevation in dnmt3a mRNA in tadpole brain, tail, and hind limb. We identified and characterized two functional TREs associated with Xenopus dnmt3a genes. Our findings support the hypothesis that T3 directly modulates transcription of the dnmt3a gene and may thus modulate DNA methylation during metamorphosis.
Materials and Methods
Animal care and hormone treatment
We produced X. laevis and X. tropicalis tadpoles by in-house breeding, reared them in dechlorinated tap water (temperature, 21–23°C for X. laevis and 23–25°C for X. tropicalis) with a 12L:12D photoperiod, and fed them pulverized frog brittle powder (NASCO). We used the Nieuwkoop and Faber (NF) (17) staging table to assign developmental stage.
For gene expression analyses, we maintained early prometamorphic (NF stages 50–54; when plasma [T3] is low) (17) tadpoles in aquaria containing 2 L of water. We dissolved T3 (sodium salt; Sigma-Aldrich) at a high concentration in 0.01 N NaOH or dimethylsulfoxide (DMSO) and added it to the aquarium water to a final concentration of 5 nm; controls received vehicle (0.0003% DMSO or 0.000001 N NaOH, final concentration). The 5 nm concentration is similar to the plasma concentration of T3 obtained at metamorphic climax (NF stage 62) (17, 18). Tadpoles were treated for different times with T3; for the 48-hour treatment, we changed the water and replenished the hormone at 24 hours after initiating T3 treatment. Tadpoles were killed by rapid decapitation, and the brain, tail, and hind limbs were dissected and either snap-frozen in liquid nitrogen and stored at −80°C until RNA extraction or fixed (brain only) for in situ hybridization histochemistry (ISHH; described below).
All procedures involving animals were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Michigan.
RNA extraction and reverse transcriptase real-time quantitative PCR (RTqPCR)
We extracted total RNA from tadpole brain (middle brain region containing the preoptic area and diencephalon), tail, and hind limb using TRIZOL reagent (Invitrogen Life Technologies) and generated cDNA using the High Capacity cDNA Synthesis Kit (Applied Biosystems Inc). For brain, tail, and hind limb, we pooled tissues from five, two, or four tadpoles, respectively, to generate each biological replicate (four biological replicates per tissue were analyzed). For RTqPCR, we used ABsolute Blue qPCR SYBR Green Low ROX Mix (ABgene Thermo Scientific) and StepOne Real Time PCR Systems (Life Technologies). Primers were designed to span exon/exon boundaries for each gene (Table 1). We used a relative quantification method (19, 20) to compare gene expression levels across developmental stages or among treatments by generating standard curves using a pool of cDNAs. The levels of X. laevis dnmt1 and dnmt3a mRNAs (GenBank accession no. NM_001090552 and BJ059684, respectively) were normalized to α-actinin mRNA (NM_001090829.1), which did not change across development or after hormone treatment (data not shown). The level of X. tropicalis dnmt3a mRNA (GenBank accession no. XM_002942354.4) was normalized to ef1a mRNA (GenBank accession no. NM_001011418.1), which was unchanged after T3 treatment (data not shown). We also analyzed X. tropicalis dnmt3a heteronuclear RNA (hnRNA) as a proxy for gene transcription (20–22) using primers that targeted the intron between predicted exons 6 and 7 (Table 1). The dnmt3a hnRNA level was normalized to ef1a mRNA. For all analyses, controls without reverse transcriptase were conducted, which gave no amplification (data not shown).
Table 1.
Oligonucleotide Primers Used for RTqPCR Analysis of Gene Expression and ChIP Assays
| For SYBR Green RTqPCR |
| X. laevis |
| dnmt1 mRNA |
| Forward: 5′ CTG ATG CGG AAG AAG GTA AAA AG 3′ |
| Reverse: 5′ TGC AAT CCA TGC ATT TTG GT 3′ |
| dnmt3a mRNA |
| Forward: 5′ GGT CTA TGA AGT GAG GCA GAA GTG 3′ |
| Reverse: 5′ TTC AAA CTG CCG CAA GAA ATA C 3′ |
| α-actinin mRNA |
| Forward: 5′ GGA CAA TTA TCC TGC GTT TTG C 3′ |
| Reverse: 5′ CCT TCT TTG GCA GAT GTT TCT TC 3′ |
| X. tropicalis |
| dnmt3a mRNA |
| Forward: 5′ TCA ACC CTC TGG ACC CAA AG 3′ |
| Reverse: 5′ CCA CCC ACA TTT CTG TGT AAA CTT 3′ |
| dnmt3a hnRNA (targets the intron between predicted exons 6 and 7) |
| Forward: 5′ CAA ATT CTG GCT GCT TTG GTA G 3′ |
| Reverse: 5′ ACT GGG CAC TCC TCT AAT AGT 3′ |
| ef1a mRNA |
| Forward: 5′ CGG AAC TAC CCT GCT GGA AG 3′ |
| Reverse: 5′ GGC AAA GGT AAC CAC CAT GC 3′ |
| For ChIP assays |
| dnmt3a Region A |
| Forward: 5′ CCC CTC TCT CTG CCC AAC A 3′ |
| Reverse: 5′ GTG TCC TTA TTA ACC CCG TTA TCA CT 3′ |
| dnmt3a Region B |
| Forward: 5′ TGA CTC TGC GCT GCA GTG A 3′ |
| Reverse: 5′ AGG CTG CGT CCC CCT TAC 3′ |
| dnmt3a Region C |
| Forward: 5′ CTC CGT TTC CCC TGC AAA T 3′ |
| Reverse: 5′ ACA TAC ACA TTA ATC ACT CCA TTT CCC 3′ |
In situ hybridization histochemistry
We analyzed the spatial distribution of dnmt3a mRNA in X. laevis tadpole brain using ISHH as previously described (23). We isolated a partial cDNA for X. laevis dnmt3a (591 bp), subcloned it into the pGEM-T Easy (Promega), and verified the orientation and sequence by direct DNA sequencing. We synthesized sense and antisense riboprobes using the DIG RNA Labeling Kit (Sp6/T7; Roche) and hybridized 200 ng of each riboprobe to cryosections (12 μm) at 65°C overnight. RNA hybrids were detected using sheep antidigoxigenin antibody conjugated to alkaline phosphatase (1:500; Sigma-Aldrich). Primary immune complexes were detected by colorimetric reaction with NBT/BCIP (nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine) (Roche). The specificity of the riboprobe was determined by: 1) the absence of signal with sense probe on the adjacent sections; and/or 2) the absence of signal with antisense probe in cryosections of brains from vehicle-treated animals. All sections were carefully matched for anatomical level, and digital micrographic images were captured using an Olympus IX81 inverted microscope (Olympus). Brightness, contrast, and evenness of illumination were adjusted uniformly for images shown in the figures using Adobe Photoshop CS5 (Adobe Systems, Inc).
Plasmid constructs
We generated luciferase reporter constructs with PCR-generated DNA fragments corresponding to genomic sequences that contain predicted TREs for analysis in transient transfection assays. A chromatin interaction analysis (ChIA) by paired-end tag (PET) sequencing experiment with TR as the target protein was conducted on chromatin isolated from X. tropicalis tadpole tail fin to identify TR binding sites and long-range chromatin interactions (L. M. Sachs and N. Buisine, unpublished data). This identified three TR binding sites (regions A, B, and C) within the X. tropicalis dnmt3a locus, whose coordinates are provided in Table 2. We directionally cloned PCR-generated DNA corresponding to genomic regions A (281 bp) and B (322 bp) into the pGL4.23 luciferase vector (Promega) at the KpnI and SacI sites (Table 3). We did not subclone region C because we found no TR association here in tadpole brain chromatin by targeted chromatin immunoprecipitation (ChIP) analysis (see Results).
Table 2.
Genomic Coordinates for TR Binding Sites Associated With the Xenopus tropicalis dnmt3a Locus Identified by ChIA-PET Analysis
| TR ChIA-PET conducted on premetamorphic Xenopus tropicalis tail fina |
| Region A: Scaffold_971: 107805–108924 |
| Region B: Scaffold_971: 120574–120823 |
| Region C: Scaffold_971: 124856–125536 |
L. M. Sachs and N. Buisine, unpublished data. Coordinates are based on the XenTro2 genome build.
Table 3.
Oligonucleotides Used to Generate DNA Fragments by PCR for Plasmid Constructs Used in Transfection-Reporter Assays, for ISHH, and for EMSA
| Oligonucleotide primers used to amplify Xenopus laevis dnmt3a cDNA by PCR for subcloning into the pGEM-Teasy cloning vector for generation of ISHH probe |
| Forward: 5′ TTT CCC ATC ATG CCC AGA GAA CGA 3′ |
| Reverse: 5′ GCC GTC GTC ATC GTA TTG GT 3′ |
| Oligonucleotide primers used to amplify Xenopus tropicalis genomic fragments by PCR for subcloning into the pGL4.23 luciferase vector (restriction sites are underlined) |
| dnmt3a region A (281 bp) |
| Forward: 5′ TAT GGT ACC GGG CCC TAC AGA GCA TAT AGT 3′ |
| Reverse: 5′ CAA TGA GCT CCA TTT CTG TGA GAC TGC CTA TCC 3′ |
| dnmt3a region B (322 bp) |
| Forward: 5′ AAG GTA CCA CAG TCA GTT CTG CTC ATG TTG GG 3′ |
| Reverse: 5′ TTC TCG AGC TGC ACA TAC CCT TCC CTG TGT AT 3′ |
| Oligonucleotides used for site-directed mutagenesis of TREs within DNA fragments encompassing regions A or B (X. tropicalis) subcloned into the pGL4.23 luciferase vector (lowercase letters indicate mutated nucleotides; underlining indicates TRE half-sites) |
| dnmt3a TRE-A1 |
| Forward: 5′ CAG AGC GGG GGc TAG CTG AGa ACT GGC CTG CA 3′ |
| Reverse: 5′ TGC AGG CCA GTt CTC AGC TAg CCC CCG CTC TG 3′ |
| dnmt3a TRE-B2 |
| Forward: 5′ CCC CAG TGG GTT AtC TGG TTT GgC TCT GCG CTG CA 3′ |
| Reverse: 5′ TGC AGC GCA GAG cCA AAC CAG aTA ACC CAC TGG GG 3′ |
| Oligonucleotides used for EMSA probes (lowercase letters indicate non-native nucleotides added to the 5′ends for [32P] labeling by Klenow fill-in). The TRE sequences are from X. tropicalis |
| dnmt3a TRE-A1 |
| Forward: 5′ gat cAC AGA GCG GGG GTT AGC TGA GGA CTG GCC TGC AC 3′ |
| Reverse: 5′ gat cGT GCA GGC CAG TCC TCA GCT AAC CCC CGC TCT GT 3′ |
| dnmt3a TRE-B2 |
| Forward: 5′ gat cTG CAG CGC AGA GTC AAA CCA GGT AAC CCA CTG GG 3′ |
| Reverse: 5′ gat cCC CAG TGG GTT ACC TGG TTT GAC TCT GCG CTG CA 3′ |
| Oligonucleotide primers used to generate double-stranded DNA fragments by PCR for competitive EMSA Xenopus tropicalis |
| dnmt3a region A with TRE-A1 (wild-type and mutant) (180 bp) |
| Forward: 5′ GCC CTA CAG AGT AAT GTT GCT TAT 3′ |
| Reverse: 5′ CCT CAC TCT GCC GTC ATC TA 3′ |
| dnmt3a region A with TRE-A2 (199 bp) |
| Forward: 5′ ACT TCC CAG TTT GCC CTC T 3′ |
| Reverse: 5′ AAA GCT GCT TCC CAC AAT CC 3′ |
| dnmt3a region B with TRE-B1 (143 bp) |
| Forward: 5′ GGG CGC AGC CAA GAA TGA TT 3′ |
| Reverse: 5′ CCT GCA CAT ACC CTT CCC TGT GTA T 3′ |
| dnmt3a region B with TRE-B2 (wild-type and mutant) (118 bp) |
| Forward: 5′ AAG TGC AGC GAT GGG AGG G 3′ |
| Reverse: 5′ AAT CAT TCT TGG CTG CGC CC 3′ |
| dnmt3a region B with TRE-B3 (97 bp) |
| Forward: 5′ AAG GTA CCA CAG TCA GTT CTG CTC ATG TTG GG 3′ |
| Reverse: 5′ CCC TCC CAT CGC TGC ACT T 3′ |
| dnmt3a region B with no TRE (124 bp) |
| Forward: 5′ ATA CAC AGG GAA GGG TAT GTG CAG G 3′ |
| Reverse: 5′ TTA CCA AAC TGC AAC TCC CAG CAG 3′ |
We generated point mutations in the predicted TREs by site-directed mutagenesis using the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies; primer sequences are in Table 3).
ChIP assay
We conducted ChIP assays as described previously (24). We extracted chromatin from whole brains of NF stages 50–52 X. laevis tadpoles treated with vehicle (0.0003% DMSO) or T3 (5 nm) for 48 hours (18 brains pooled per sample; n = 4), and from untreated tadpoles at metamorphic climax (NF stage 62; five brains pooled per sample, n = 4). We sonicated chromatin to approximately 500 bp using a Sonic Dismembrator 100 (Fisher) for a total of 200 seconds per sample (20 cycles of 10-second sonication with 1 minute between each cycle) at an output rating of 5–6 W. All chromatin samples were snap-frozen in liquid nitrogen and stored at −80°C until analysis.
We used the following primary antibodies for ChIP assays: rabbit polyclonal antiserum raised against full-length X. laevis TRβ (PB antiserum, which recognizes both TRα and TRβ; 5 μL) (24), anti-histone 3 (H3) (5 μL; Millipore, no. 07-670), or anti-acetylated H3 (acH3) (5 μL; Millipore, no. 06-599). Negative controls for the assays included the replacement of primary antibody with normal rabbit serum (NRS; for the PB antiserum) or IgG purified from NRS (21). We analyzed ChIP DNA by real-time qPCR using SYBR Green assays (oligonucleotide primers in Table 1).
Electrophoretic mobility shift assay (EMSA)
We conducted EMSA as described previously (25). We synthesized X. laevis TRβ and RXRα proteins in vitro using pSp64a-TRβ and pSp64a-RXRα plasmids (gift of Yun-Bo Shi) and the TnT SP6 Quick-Coupled Translation System (Promega). We used radioinert duplex oligonucleotides (1 μm) as competitors (see Table 3).
Cell culture and transient transfection assays
To test for the T3 responsiveness of cloned DNA sequences in transient transfection reporter assays, we used Neuro2a cells engineered to express the TRβ1 isoform (Neuro2a[TRβ1]) (26) because these cells transfect well and produce strong transcriptional responses to T3 (22, 24, 26). We cultured the cells in DMEM/F12 supplemented with hygromycin B (500 μg/mL; Invitrogen) and 10% fetal bovine serum that had been stripped of T3 and T4 (24, 27). We plated cells at a density of 0.15 × 106 per well in 24-well plates and transfected cells at 60–70% confluency with luciferase reporter constructs (200 ng per well plus 10 ng pRenilla-tk) using FuGene 6 transfection reagent (Promega). Twenty-four hours after transfection, we treated cells with vehicle (0.1% DMSO) or T3 (30 nm) for 5 hours before harvest for dual luciferase assay (Promega). Firefly luciferase activity was quantified using a luminometer (Femtometer FB 12; Zylux Corp.) and normalized to Renilla luciferase activity. Each transfection experiment was conducted at least three times with four to five wells per treatment.
Data analysis and statistics
We analyzed data using SPSS statistical software (version 19; SPSS Inc). We conducted one-way ANOVA followed by Fisher's least significant difference post hoc test and Student's independent sample t test (α = 0.05). Data were log10-transformed before statistical analysis when variances were found to be heterogeneous.
We searched for putative DR+4 TREs using the sequence analysis program Vector NTI (version 10; Invitrogen) with “TGWCCYnnnnTGWCCY” as a search term with 3 base-pair mismatches allowed. We also used TRANSFAC 6.0 (www.gene-regulation.com) based on 0.80 or higher match to the vertebrate transcription factor matrix to search for putative TREs in TR ChIP-enriched regions and potentially orthologous regions in other vertebrate genomes. The conservation of the identified TREs and flanking sequences in other vertebrates was assessed using BLAT (http://genome.ucsc.edu/).
Results
Developmental and T3-dependent changes in dnmt3a mRNA
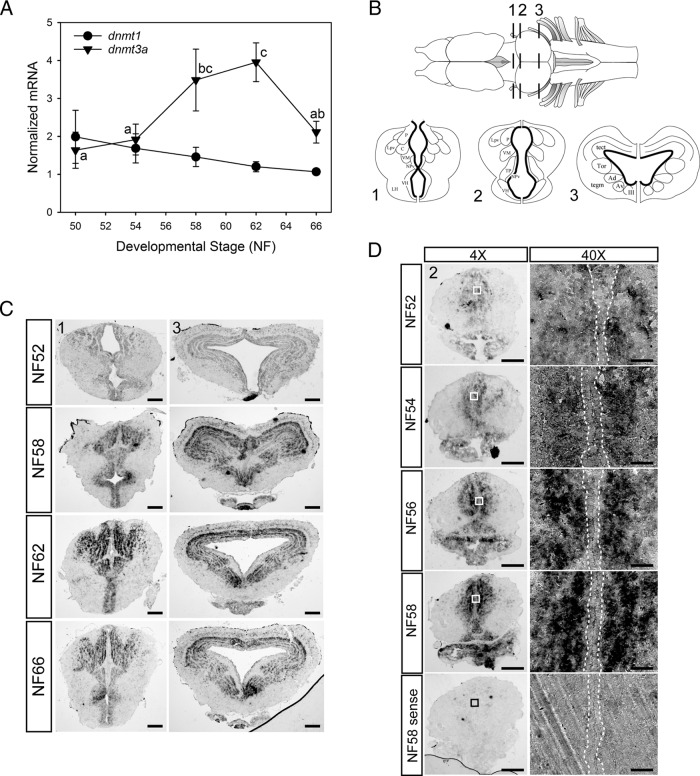
We measured mRNA levels for dnmt genes by RTqPCR in X. laevis tadpole brain (middle brain region, including the preoptic area and diencephalon) during metamorphosis. The mRNA for dnmt3a, but not dnmt1, exhibited developmental changes (Figure 1A), increasing during midprometamorphosis (NF stage 58) and metamorphic climax (NF stage 62), then declining in the newly metamorphosed frog (NF stage 66). This period of development lasts about 40 days and corresponds to a dramatic increase in T3 production (4, 17). The dnmt3a mRNA level showed an identical developmental profile in X. tropicalis brain during metamorphosis (Y. Kyono and R.J. Denver, unpublished data). This quantitative analysis was corroborated by ISHH for dnmt3a mRNA in X. laevis tadpole brain (Figure 1, B–D), the signal for which was low at NF stage 52, but then increased throughout metamorphosis and peaked at metamorphic climax (NF stage 62). We detected a strong ISHH signal for dnmt3a mRNA in different regions of the tadpole brain during metamorphosis, including the anterior preoptic area, thalamic nuclei, suprachiasmatic nucleus, ventral hypothalamus, and optic tectum (Figure 1, B–D; shown are: 1, two transverse planes, the region of the ventral hypothalamus and thalamic nuclei; and 2, the optic tectum and tegmentum). We did not detect dnmt3a mRNA in neuropil, and it was low or absent in cells located in ventricular/subventricular zones (VZ/SVZ) where neural stem cells proliferate and then migrate out to differentiate into neurons and glia (23) (see Figure 1D, 40× magnification panel).
Figure 1.
Elevation of dnmt3a (but not dnmt1) mRNA levels, and localization of dnmt3a mRNA in Xenopus tadpole brain during metamorphosis. A, Changes in dnmt3a and dnmt1 mRNAs in X. laevis tadpole brain (middle brain region containing the preoptic area and diencephalon) analyzed by RTqPCR. We normalized dnmt3a and dnmt1 mRNAs to the reference gene α-actinin, which did not change across development. Points represent the means ± SEM (n = 5/developmental stage), and means for dnmt3a mRNA with the same letter are not significantly different (F(4,20) = 3.963; P = .017; ANOVA). The dnmt1 mRNA level was not significantly different across metamorphosis, so no statistical symbols are provided for this gene. B, Dorsal view of Xenopus brain (top) and three transverse sections (bottom) at the region of the ventral hypothalamus and thalamic nuclei (region 1) and the optic tectum and tegmentum (regions 2 and 3). The ventricular and subventricular zones are indicated by the bold lines on the transverse sections. P, posterior thalamic nucleus; C, central thalamic nucleus; Lpv, lateral thalamic nucleus, pars posteroventralis; VM, nucleus motorius nervi trigemini; NPv, nucleus of the paraventricular organ; VH, ventral hypothalamic nucleus; LH, lateral hypothalamus; tect, optic tectum; Tor, torus semicircularis; tegm, mesencephalic tegmentum; Ad, dorsal anterior thalamic nucleus; Av, ventral anterior thalamic nucleus; III, nucleus nervi oculomotorii; TP, posterior tuberculum. C, Distribution of dnmt3a mRNA in X. laevis tadpole brain during metamorphosis analyzed by ISHH. Shown are representative photomicrographs of transverse sections at the two brain regions shown in panel B. For region 1, scale bars = 160 μm for NF stages 52 and 59 and 200 μm for NF stages 62 and 66; for region 3, scale bars = 160 μm for NF stage 52 and 200 μm for NF stages 59, 62, and 66. D, The increase in dnmt3a mRNA in tadpole brain during metamorphosis occurs outside of neurogenic zones. We conducted ISHH for dnmt3a mRNA on X. laevis tadpole brain between premetamorphosis and late prometamorphosis. Shown are representative micrographs of transverse sections at the region of the ventral hypothalamus and thalamus (region 2 in panel B). Right panels show a magnified view (40×) of regions indicated by the boxes in the left panels. Areas within dotted lines indicate the VZ/SVZ. Note that dnmt3a mRNA is absent from the VZ/SVZ. Scale bars = 200 μm (4×) and 20 μm (40×). Hybridization with sense probe gave no signal at any developmental stage; only NF stage 58 is shown at the bottom of the figure.
Addition of T3 to the aquarium water (to 5 nm) of early prometamorphic X. tropicalis tadpoles (NF stages 53–54) caused a time-dependent increase in dnmt3a mRNA and hnRNA in brain (middle region containing the preoptic area and diencephalon), tail, and hind limb, analyzed by RTqPCR (Figure 2). The mean dnmt3a mRNA level was significantly higher at 8 hours of T3 treatment in all tissues and continued to increase through 16 hours. The level of dnmt3a hnRNA (a proxy for the rate of gene transcription) was significantly higher at 2 hours after T3 treatment in brain, continued to increase at 8 hours, and remained elevated at 16 hours. In tail and hind limb, dnmt3a hnRNA was significantly higher at 8 hours and remained elevated at 16 hours. The magnitudes of dnmt3a mRNA and hnRNA responses to T3 at 16 hours were, respectively: brain, 1.9- and 2.3-fold; tail, 5.7- and 4.7-fold; and hind limb, 2.8- and 2.2-fold.
Figure 2.
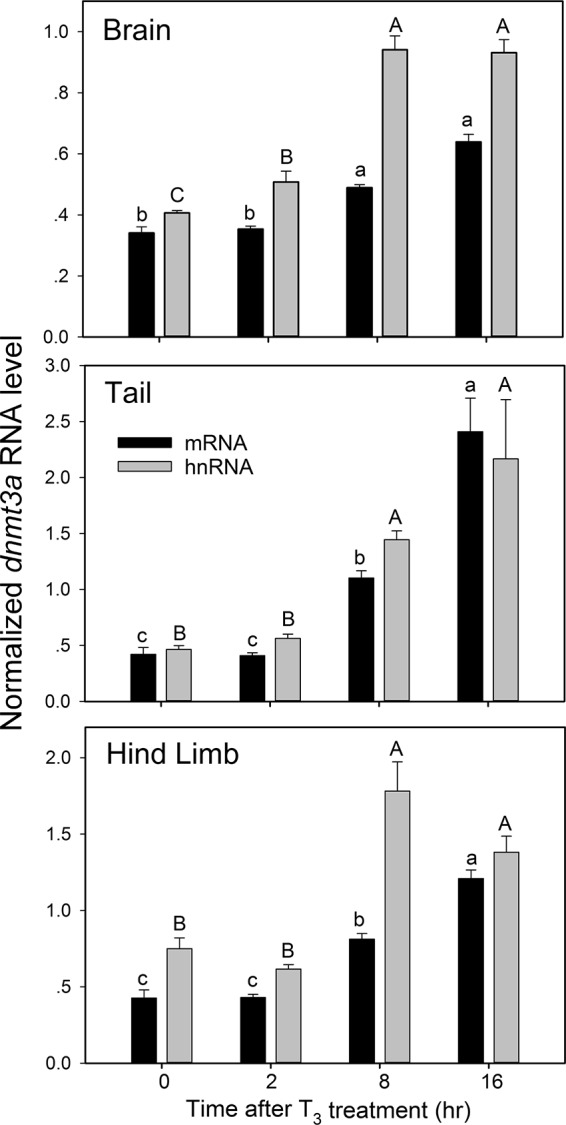
Exogenous T3 induces dnmt3a mRNA and hnRNA in early prometamorphic X. tropicalis tadpole brain (middle brain region containing the preoptic area and diencephalon), tail, and hind limb. We treated early prometamorphic (NF stages 53–54) tadpoles with T3 (5 nm) for 0, 2, 8, or 16 hours and measured dnmt3a mRNA and hnRNA by RTqPCR. We normalized dnmt3a mRNA and hnRNA to ef1a mRNA, which did not change with T3 treatment (data not shown). Control reactions without reverse transcriptase were conducted on each RNA sample and produced no amplification. Bars represent the mean ± SEM (n = 4/time point/treatment), and means with the same letter (a, b, c for mRNA; A, B, C for hnRNA) are not significantly different (brain mRNA: F(3,12) = 101.997, P < .0001; brain hnRNA: F(3,12) = 76.795, P < .0001; tail mRNA: F(3,12) = 67.891, P < .0001; tail hnRNA: F(3,12) = 36.235, P < .0001; hind limb mRNA: F(3,12) = 41.84, P < .0001; hind limb hnRNA: F(3,12) = 32.83, P < .0001; ANOVA).
Treatment of early prometamorphic (NF stages 53–54) X. laevis tadpoles with T3 (to 5 nm) similarly induced dnmt3a mRNA in the brain, as analyzed by RTqPCR (Supplemental Figure 1A) and by ISHH (Supplemental Figure 1B). The mean dnmt3a mRNA level (analyzed by RTqPCR) was higher at 8 and 12 hours after T3 treatment (approached statistical significance at 12 hours; P = .06) and was statistically significant at 24 and 48 hours (Supplemental Figure 1A). There was no change in dnmt1 mRNA levels after T3 treatment (data not shown). In situ hybridization analysis further supports that T3 can induce dnmt3a mRNA in tadpole brain (Supplemental Figure 1B). The precocious induction by T3 caused the dnmt3a mRNA signal to appear in cells of the VZ/SVZ where it is normally low or absent during spontaneous metamorphosis.
Identification of TREs at the frog dnmt3a locus
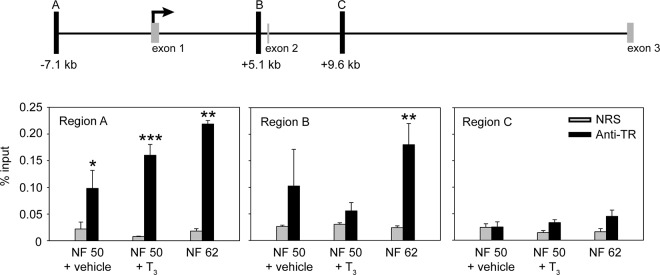
By analyzing data from a ChIA-PET experiment conducted on tadpole tail fin chromatin with TR as the target protein (28), we identified three putative TR binding sites within the X. tropicalis dnmt3a locus. They are located 7.1 kb upstream (region A), 5.1 kb downstream (region B), and 9.6 kb downstream (region C) of the transcription start site (TSS) (Figure 3) (see Table 2 for genomic coordinates of these regions; the TSS was determined by RNA-PET analysis) (28). Using BLAST search, we also identified loci in the X. laevis genome that are homologous to regions A, B, and C in the X. tropicalis genome.
Figure 3.
Thyroid hormone receptors associate in chromatin with the dnmt3a locus in Xenopus tadpole brain. The schematic diagram (top) shows the location of the putative TR binding sites (black vertical bars; regions A, B, and C) identified by a ChIA-PET experiment conducted on X. tropicalis tail fin chromatin (L. M. Sachs and N. Buisine, unpublished data). Numbers below the bars indicate the distance from the TSS. The TSS was determined by an RNA-PET experiment (30), and the relative positions of the first two coding exons (CDS 1 and 2) are shown. We conducted targeted ChIP assays for TR (bottom) at regions A, B, and C using chromatin isolated from the brains of NF stages 50–52 X. laevis tadpoles treated with vehicle (0.0003% DMSO) or T3 (5 nm) for 48 hours, and chromatin from the brain of tadpoles at metamorphic climax (NF stage 62). Bars represent the mean ± SEM of the ChIP signal expressed as a percentage of input for anti-TR serum or NRS (negative control) (n = 4/group). Asterisks indicate statistically significant differences between the TR ChIP signal and NRS (*, P < .05; **, P < .01; ***, P < .001; Student's independent sample t test).
We conducted ChIP assays to analyze TR association in chromatin with dnmt3a genomic regions A, B, and C. We isolated chromatin from brain of NF stages 50–52 X. laevis tadpoles treated with or without T3 (5 nm) for 48 hours and at metamorphic climax (NF stage 62) when endogenous plasma [T3] reaches a maximum (18). The TR ChIP signal was significantly enriched at region A in all treatments; the mean value was higher at NF stage 62 compared with NF stages 50–52, but this was not statistically significant (Figure 3). The TR ChIP signal at region A in NF stages 50–52 tadpole brain was unaffected by hormone treatment, consistent with TRs being associated with chromatin in the unliganded state. We also found TR ChIP signal enriched at region B, but only in the brain of metamorphic climax (NF stage 62) animals. For region C, the TR ChIP signal was at background level in all treatments (Figure 3).
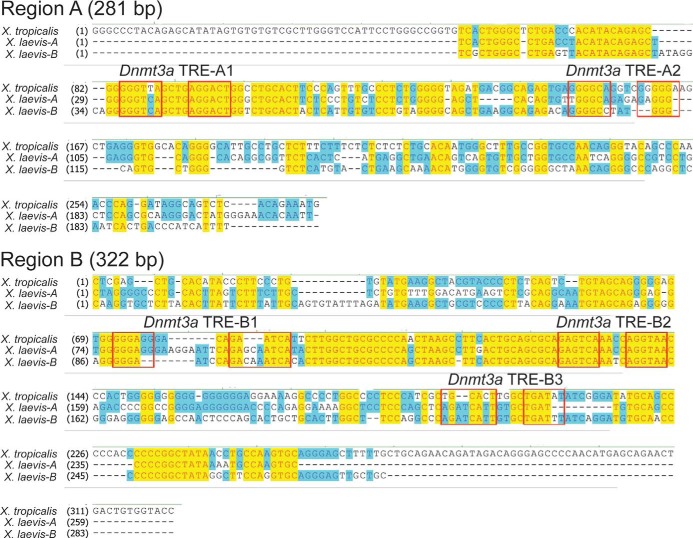
Using computer analysis, we searched for the DR+4 TRE sequence within genomic regions A and B of the X. tropicalis dnmt3a locus, and we identified two putative DR+4 TREs within region A (TRE-A1 and -A2) and three putative DR+4 TREs within region B (TRE-B1, -B2, and -B3; Figure 4). Sequence alignment of the homologous regions of the X. tropicalis and X. laevis genomes showed that only one of the TREs within each of these regions (TRE-A1 and TRE-B2) was conserved between the two frog species (Figure 4). We therefore focused on TRE-A1 and -B2 for further analysis.
Figure 4.
Sequence alignment of regions A and B of X. tropicalis and X. laevis dnmt3a genes. Alignments were done using Clustal W. For X. tropicalis, we used genome build version 4.1, and for X. laevis we used genome build version 5.0 (there are two X. laevis genes owing to its pseudotetraploidy); region C is not shown because we found no evidence for TR association in brain chromatin by ChIP assay (Figure 2). Boxes indicate the locations of predicted DR+4 TRE half-sites numbered in the 5′ to 3′ direction based on the X. tropicalis reference genome. Note that the TRE-A1 and TRE-B2 sequences are conserved between the two frog species.
EMSA using oligonucleotide probes encompassing the two conserved TREs showed that TRβ-RXRα heterodimers bound to both sequences in vitro (Figure 5A). Competitive EMSA confirmed that radioinert duplex oligonucleotides corresponding to the conserved TRE sequences could displace TRβ-RXRα from the radiolabeled probe, which was eliminated after mutation of the predicted half-sites (Figure 5B and Table 3). Competitive EMSA with PCR-generated DNA fragments corresponding to the genomic regions containing the predicted TREs also displaced TRβ-RXRα from the radiolabeled probe, which was reduced (TRE-A1) or eliminated (TRE-B2) after mutation of the predicted half-sites within the DNA fragment (Supplemental Figure 2). The failure of the mutation of TRE-A1 to eliminate the ability of this DNA fragment to displace the radiolabeled probe may indicate the presence of another TRE-like sequence capable of binding TR-RXR in an in vitro assay (but see next paragraph; mutation of TRE-A1 eliminates the ability of this fragment to support T3-dependent transactivation in promoter-reporter assays).
Figure 5.
Predicted TREs at the Xenopus dnmt3a locus bind TR-RXR heterodimers in vitro and support T3-dependent transactivation. A, We conducted EMSA using [32P]-labeled oligonucleotide probes corresponding to X. tropicalis dnmt3a TRE-A1 and TRE-B2 sequences (Table 3). We used in vitro synthesized X. laevis TRβ plus RXRα, and in vitro synthesized luciferase as a negative control. The arrow indicates the location of shifted bands due to TR binding to the probes. B, We conducted competitive EMSAs using a [32P]-labeled duplex oligonucleotide probe corresponding to the T3RE-B2 sequence from the X. tropicalis dnmt3a locus (Table 3). Before gel electrophoresis we incubated the probe with in vitro synthesized Xenopus TRβ and RXRα with or without the indicated radioinert oligonucleotides (1 μm). Duplex oligonucleotides with wild-type sequence (wt) or containing single base pair mutations at each of the two half-sites of the indicated TREs (mut; see Table 3) were used as competitors. The arrow indicates the location of shifted bands due to TRβ-RXRα binding to the probe. C, We tested for transactivation activity of DNA fragments corresponding to X. tropicalis dnmt3a regions A (281 bp) and B (322 bp) in transient transfection-reporter assays. After transfection, we treated cells with vehicle (0.1% DMSO) or T3 (30 nm) for 24 hours before conducting dual luciferase assay. These DNA fragments supported T3-dependent transactivation, which was abolished by single base mutations introduced into each of the two half-sites of TRE-A1 and TRE-B2 (Table 3). Bars represent the mean fold change in firefly luciferase activity (normalized to Renilla luciferase activity) relative to vehicle-treated controls (n = 4/treatment). Asterisks indicate statistically significant differences between vehicle and T3-treated cells (P < .001; Student's independent sample t test).
To test for functionality of these putative TREs, we conducted transient transfection-reporter assays using luciferase reporter constructs with DNA fragments that correspond to regions A (281 bp) and B (322 bp) of the X. tropicalis genome. Treatment with T3 increased luciferase activity in cells transfected with these constructs (Figure 5C), which was abolished by single base pair substitutions introduced into each of the two half-sites of TRE-A1 and TRE-B2 (Figure 5C; see Table 3).
The dnmt3a TREs are highly conserved between X. tropicalis and X. laevis species, but not other vertebrates
The two TREs that we discovered are highly conserved between X. laevis and X. tropicalis (see Figure 4A). The divergence of these two lineages is estimated to have occurred 50 million years ago (29). The 5′ half-site of TRE-A1 has one nucleotide difference between the two species, but the remainder of the TRE, including the spacer, is identical between species. The TRE-B2 differs between the two species only in position 3 of the 4-base spacer.
We investigated DNA sequence conservation of the two frog genomic regions containing the identified TREs among different vertebrate taxa but found no clear orthology. The mouse dnmt3a gene has at least two TREs located at +30.3 and +49.3 kb from the TSS (30). The frog TRE-B2 and the mouse +30.3 kb TRE are found within the intron between the coding exons 1 and 2 of the respective dnmt3a genes. However, comparative genomic analysis does not support orthology between these two TREs. We also did not find orthology between the frog TRE-A1 and either of the mouse dnmt3a TREs, or sequence similarity of the two frog dnmt3a TREs with similar genomic regions of any other vertebrate taxon analyzed.
Discussion
Here we show that a critical gene for epigenetic modulation, dnmt3a, is under the control of T3 in developing tadpole tissues. The expression of dnmt3a is developmentally regulated, increasing as endogenous plasma [T3] rises, and can be induced in early prometamorphic tadpole brain, tail, and hind limb by exogenous T3. Our histochemical analysis shows that dnmt3a is expressed outside of neurogenic zones where newly born cells migrate and differentiate, suggesting that it may play a role in cell cycle exit and cell differentiation. Furthermore, we provide strong evidence that the gene is directly regulated by liganded TRs via at least two TREs. Our findings in the frog parallel similar results in mouse brain (30), although the evolutionary origin of T3 control of dnmt3a requires further investigation. We hypothesize that T3 regulation of dnmt3a plays a central role in T3 action via the regulation of DNA methylation patterns in tadpole tissues during metamorphosis.
Both biochemical (RTqPCR) and histochemical (ISHH) analyses showed that dnmt3a mRNA increased in tadpole brain during spontaneous metamorphosis, with peak levels at metamorphic climax when plasma [T3] reaches a maximum (17). Also, dnmt3a mRNA could be induced by T3 in early prometamorphic tadpole brain, tail, and hind limb with relatively early kinetics (by 8 hours). Furthermore, dnmt3a hnRNA, which can be used as a proxy for the transcription rate of a gene owing to the short half-life of precursor mRNA (hnRNA) (20–22), was elevated after 2 hours of T3 treatment in tadpole brain, continued to increase, and remained high at 8 and 16 hours in all three tissues, supporting the hypothesis that T3 increased the rate of dnmt3a gene transcription.
This T3 regulation of Xenopus dnmt3a may be explained, at least in part, by two functional DR+4 TREs located within the frog dnmt3a locus: one at −7.1 kb (TRE-A1), and another at +5.1 kb (TRE-B2) from the TSS. The TR ChIA-PET analysis on tadpole tail fin identified a third putative TR binding site at +9.6 kb from the predicted translation start site (region C), but we did not find TR association in tadpole brain by targeted ChIP assay, which could reflect tissue-specific TR binding among different tissues. Therefore, whereas we identified two functional TREs in tadpole dnmt3a, it is possible that there are other TREs associated with this locus. Future work using genome-wide analysis of TR binding sites in tadpole brain will be required to answer this question.
Our recently published findings show that dnmt3a is regulated by T3 in neonatal mouse brain, suggesting that this hormone regulation could represent an ancient signaling pathway in vertebrates. The TREs that we identified in the mouse dnmt3a gene are highly conserved among mammals, but we did not find similar sequences in reptiles, including birds (30). Similarly, the genomic regions containing the two TREs that we found within Xenopus dnmt3a genes are not conserved in fish, reptiles, or mammals. It is possible that our failure to find orthologous sequences could be due to divergent, missing, or incomplete sequences of genomic regions that may potentially contain TREs in these lineages. Alternatively, T3 regulation of dnmt3a in Xenopus and mammals may have arisen through convergent evolution. We do not know whether the orthologous dnmt3a genes are similarly regulated by T3 in other amphibians or reptiles. Further study is needed to determine when during the evolution of tetrapods the gene came under thyroid control, whether this evolved once or multiple times using the same regulatory regions, and whether hormone regulation has been lost in some lineages.
Our histochemical analysis showed widespread distribution of dnmt3a mRNA in the tadpole brain, which increased at metamorphic climax. The mRNA was notably absent from cells located in neurogenic regions (VZ/SVZ) where neural stem cells proliferate. The rate of cell proliferation in the tadpole VZ/SVZ peaks at midprometamorphosis (NF stage 56), then declines through late prometamorphosis and metamorphic climax (23). As cell proliferation declines, newborn cells migrate out of the VZ/SVZ (23) and differentiate, which correlates with an increase in dnmt3a mRNA. The rise in dnmt3a mRNA parallels a decline in many genes involved in cell cycle control in developing tadpole brain (Y. Kyono and R. J. Denver, unpublished RNA sequencing data). Recent findings in different cell types supports that DNMT3a is important for establishing and/or maintaining cell differentiation and cell cycle exit (31–34). We hypothesize that increased expression of dnmt3a modulated by T3 promotes DNA methylation at regulatory regions of cell cycle control genes. This may lead to long-term, stable transcriptional repression to maintain cell cycle exit and differentiation.
Modulation of DNA methylation is well known to play a central role in development and disease in humans (15, 35–39). Recent findings have shown dynamic changes in DNA methylation during mammalian brain development (14, 15), but little is known about how DNA methylation is regulated in neural cells. There are dynamic changes in DNA methylation in the tadpole brain throughout metamorphosis, which we discovered by analysis of global 5-methylcytosine and 5-hydroxymethylcytosine quantities and by genome-wide analysis using Methyl Capture sequencing (Y. Kyono, S. Raj, L. M. Sachs, N. Buisine, and R. J. Denver, unpublished findings). We do not yet know the role that DNMT3a plays in these epigenetic changes, but we are investigating this question as well as the consequences of changes in DNA methylation for gene transcription. Our findings reported here and our previous work in mouse brain support direct transcriptional regulation of the gene coding for the de novo DNA methyltransferase dnmt3a. We hypothesize that such hormone regulation of a key epigenetic modifier has the potential to widely influence DNA methylation during animal development.
Acknowledgments
We thank Yun-Bo Shi (National Institute of Child Health and Development, National Institutes of Health, Bethesda, Maryland) for the PB antiserum and the pSp64a-TRβ and pSp64a-RXRα plasmids, and Richard Harland (University of California, Berkley) for sharing draft X. laevis genome data (Build 5.0). We are grateful to Dr. Ronald Bonett for assistance with the bioinformatics analyses and César Rodriguez for technical support.
This work was supported by grants from the National Science Foundation (IOS 0922583 and IOS 1456115) to R.J.D. Y.K. was supported by the National Institutes of Health (NIH) Early Stage Training in the Neurosciences Training Grant T32-NS076401 and by a Ruth Kirschstein National Research Service Award from the National Institute of Neurological Disorders and Stroke (1F31NS073294-01A1). L.M.S. was supported by “CRESCENDO,” an Integrated Project from FP6 (LSHM-CT-2005-018652); by “IDEAL,” a large Integrated Project from FP7 (259679); and “MethylDev,” a CNRS PICS Program. This research used the Cell and Molecular Biology Core of the Michigan Diabetes Research and Training Center funded by NIH P60 DK20572, National Institute of Diabetes and Digestive Kidney Diseases.
Current address for Y.K.: Department of Human Genetics, The University of Michigan, Ann Arbor, Michigan 48109.
Disclosure Summary: The authors have nothing to disclose.
Appendix
See Table 4.
Table 4.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Xenopus laevis TH receptor β | Full length protein | PB | Yun-Bo Shi | Rabbit, polyclonal | 1:100 ChIP |
| Anti-histone 3 (H3) | Anti-H3 | Millipore, 07-670 | Rabbit, polyclonal | 1:100 ChIP | |
| Anti-acetylated H3 (AcH3) | Anti-acH3 | Millipore, 06-599 | Rabbit, polyclonal | 1:100 ChIP | |
| Anti-digoxigenin conjugated to alkaline phosphatase | Anti-Dig | Roche, 11333089001 | Sheep, polyclonal | 1:500 ISHH |
Footnotes
- acH3
- acetylated H3
- ChIA
- chromatin interaction analysis
- ChIP
- chromatin immunoprecipitation
- CpG
- cytosine-guanine
- DMSO
- dimethylsulfoxide
- DNMT
- DNA methyltransferase
- DR+4
- direct repeat plus four-base spacer
- EMSA
- electrophoretic mobility shift assay
- H3
- histone 3
- hnRNA
- heteronuclear RNA
- ISHH
- in situ hybridization histochemistry
- NF
- Nieuwkoop and Faber
- NRS
- normal rabbit serum
- PET
- paired-end tag
- RTqPCR
- real-time quantitative PCR
- RXR
- retinoid X receptor
- T3
- thyroid hormone
- TR
- T3 receptor
- TRE
- T3 response element
- TSS
- transcription start site
- VZ/SVZ
- ventricular/subventricular zones.
References
- 1. Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002;25(3):268–288. [DOI] [PubMed] [Google Scholar]
- 2. Anderson GW, Schoonover CM, Jones SA. Control of thyroid hormone action in the developing rat brain. Thyroid. 2003;13(11):1039–1056. [DOI] [PubMed] [Google Scholar]
- 3. Gudernatsch JF. Feeding experiments on tadpoles. I. The influence of specific organs given as food on growth and differentiation. A contribution to the knowledge of organs with internal secretion. Wilhelm Roux Arch Entwickl Mech Org. 1912;35(3):457–483. [Google Scholar]
- 4. Denver RJ. Neuroendocrinology of amphibian metamorphosis. In: Shi YB, ed. Current Topics in Developmental Biology: Animal Metamorphosis. Vol 103 Chap 7 San Diego, CA: Elsevier; 2013:195–227. [DOI] [PubMed] [Google Scholar]
- 5. Brown DD, Cai L. Amphibian metamorphosis. Dev Biol. 2007;306(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchholz DR. More similar than you think: frog metamorphosis as a model of human perinatal endocrinology. Dev Biol. 2015;408(2):188–195. [DOI] [PubMed] [Google Scholar]
- 7. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9(6):465–476. [DOI] [PubMed] [Google Scholar]
- 9. Feng S, Cokus SJ, Zhang X, et al. Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci USA. 2010;107(19):8689–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328(5980):916–919. [DOI] [PubMed] [Google Scholar]
- 11. Rottach A, Leonhardt H, Spada F. DNA methylation-mediated epigenetic control. J Cell Biochem. 2009;108(1):43–51. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe D, Suetake I, Tada T, Tajima S. Stage- and cell-specific expression of Dnmt3a and Dnmt3b during embryogenesis. Mech Dev. 2002;118(1–2):187–190. [DOI] [PubMed] [Google Scholar]
- 13. MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88(3):170–183. [DOI] [PubMed] [Google Scholar]
- 14. Shin J, Ming G, Song H. DNA modifications in the mammalian brain. Philos Trans R Soc B Biol Sci. 2014;369(1652). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isles AR. Neural and behavioral epigenetics; what it is, and what is hype. Genes Brain Behav. 2015;14(1):64–72. [DOI] [PubMed] [Google Scholar]
- 16. Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leloup J, Buscaglia M. Triiodothyronine, hormone of amphibian metamorphosis. C R Acad Sci. 1977;284(22):2261–2263. [Google Scholar]
- 18. Krain LP, Denver RJ. Developmental expression and hormonal regulation of glucocorticoid and thyroid hormone receptors during metamorphosis in Xenopus laevis. J Endocrinol. 2004;181(1):91–104. [DOI] [PubMed] [Google Scholar]
- 19. Crespi EJ, Denver RJ. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc Natl Acad Sci USA. 2006;103(26):10092–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao M, Stenzel-Poore M, Denver RJ. Structural and functional conservation of vertebrate corticotropin-releasing factor genes: evidence for a critical role for a conserved cyclic AMP response element. Endocrinology. 2007;148(5):2518–2531. [DOI] [PubMed] [Google Scholar]
- 21. Bagamasbad P, Ziera T, Borden SA, et al. Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology. 2012;153(11):5334–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagamasbad PD, Bonett RM, Sachs L, et al. Deciphering the regulatory logic of an ancient, ultraconserved nuclear receptor enhancer module. Mol Endocrinol. 2015;29(6):856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denver RJ, Hu F, Scanlan TS, Furlow JD. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol. 2009;326(1):155–168. [DOI] [PubMed] [Google Scholar]
- 24. Denver RJ, Williamson KE. Identification of a thyroid hormone response element in the mouse Kruppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology. 2009;150(8):3935–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoopfer ED, Huang L, Denver RJ. Basic transcription element binding protein is a thyroid hormone-regulated transcription factor expressed during metamorphosis in Xenopus laevis. Dev Growth Diff. 2002;44(5):365–381. [DOI] [PubMed] [Google Scholar]
- 26. Lebel JM, Dussault JH, Puymirat J. Overexpression of the β 1 thyroid receptor induces differentiation in neuro-2a cells. Proc Natl Acad Sci USA. 1994;91(7):2644–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samuels HH, Stanley F, Casanova J. Depletion of L-3,5,3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979;105(1):80–85. [DOI] [PubMed] [Google Scholar]
- 28. Buisine N, Ruan X, Bilesimo P, et al. Xenopus tropicalis genome re-scaffolding and re-annotation reach the resolution required for in vivo ChIA-PET analysis. PLoS One. 2015;10(9):e0137526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hellsten U, Khokha MK, Grammer TC, Harland RM, Richardson P, Rokhsar DS. Accelerated gene evolution and subfunctionalization in the pseudotetraploid frog Xenopus laevis. BMC Biol. 2007;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kyono Y, Subramani A, Ramadoss P, Hollenberg AN, Bonett RM, Denver RJ. Liganded thyroid hormone receptors transactivate the DNA methyltransferase 3a gene in mouse neuronal cells. Endocrinology. 2016;157(9):3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Challen GA, Sun D, Jeong M, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2011;44(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng J, Zhou Y, Campbell SL, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13(4):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen S, Meletis K, Fu D, Jhaveri S, Jaenisch R. Ablation of de novo DNA methyltransferase dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Dev Dyn. 2007;236(6):1663–1676. [DOI] [PubMed] [Google Scholar]
- 34. Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. [DOI] [PubMed] [Google Scholar]
- 35. Aus J, Martez de Paz A, Esteller M. MeCP2: the long trip from a chromatin protein to neurological disorders. Trends Mol Med. 2014;20(9):487–498. [DOI] [PubMed] [Google Scholar]
- 36. Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochem Biophys Res Commun. 2014;455(1–2):70–83. [DOI] [PubMed] [Google Scholar]
- 37. Hall L, Kelley E. The contribution of epigenetics to understanding genetic factors in autism. Autism. 2014;18(8):872–881. [DOI] [PubMed] [Google Scholar]
- 38. Falahi F, Sgro A, Blancafort P. Epigenome engineering in cancer: fairytale or a realistic path to the clinic? Front Oncol. 2015;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15(3):152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]