Abstract
Ames dwarf mice (Prop1df/df) are long-lived due to a loss of function mutation, resulting in deficiency of GH, TSH, and prolactin. Along with a marked extension of longevity, Ames dwarf mice have improved energy metabolism as measured by an increase in their oxygen consumption and heat production, as well as a decrease in their respiratory quotient. Along with alterations in energy metabolism, Ames dwarf mice have a lower core body temperature. Moreover, Ames dwarf mice have functionally altered epididymal white adipose tissue (WAT) that improves, rather than impairs, their insulin sensitivity due to a shift from pro- to anti-inflammatory cytokine secretion. Given the unique phenotype of Ames dwarf epididymal WAT, their improved energy metabolism, and lower core body temperature, we hypothesized that Ames dwarf brown adipose tissue (BAT) may function differently from that of their normal littermates. Here we use histology and RT-PCR to demonstrate that Ames dwarf mice have enhanced BAT function. We also use interscapular BAT removal to demonstrate that BAT is necessary for Ames dwarf energy metabolism and thermogenesis, whereas it is less important for their normal littermates. Furthermore, we show that Ames dwarf mice are able to compensate for loss of interscapular BAT by using their WAT depots as an energy source. These findings demonstrate enhanced BAT function in animals with GH and thyroid hormone deficiencies, chronic reduction of body temperature, and remarkably extended longevity.
Obesity is a major risk factor for cardiovascular disease, cancer and type 2 diabetes mellitus, among other pathologies. Adding to the current burden on health care systems, it is projected that there will be 2 billion overweight and 1 billion obese individuals globally by 2030 (1). Because weight gain occurs when energy intake exceeds energy expenditure, many have focused on decreasing energy intake as a means to combat obesity. The beneficial impact of exercise on body composition is another key element of antiobesity interventions, whereas increasing energy expenditure by other means has received far less emphasis. This was largely due to the belief that human adults only possessed white adipose tissue (WAT) and that brown adipose tissue (BAT) was essentially nonexistent in humans after infancy (2). In 2009, paradigm shifting studies using 18F-fluorodeoxyglucose as a radiotracer during concurrent positron emission tomography and computed tomography scanning identified metabolically active BAT in adult humans located primarily in the cervical-supraclavicular region (3–6).
BAT is the major thermogenic tissue in mammals. It is mainly composed of brown adipocytes that are characterized by multilocular lipid droplets (7). BAT also possesses an abundance of uncoupling protein 1 (UCP-1) and mitochondria, and is richly innervated by sympathetic nerves (8). Innervation with sympathetic nerves is critical because thermogenesis is controlled by norepinephrine (NE) stimulation of β-adrenergic receptors (the β3 variant is most prevalent in rodents) (8). UCP-1, as well as other proteins involved in thermogenesis, use free fatty acids (FFAs) as an energy source. The use of FFAs has made BAT an attractive tissue to combat obesity (2).
Ames dwarf mice are characterized by a loss of function Prop1 mutation that results in the lack of differentiation of somatotrophs, thyrotrophs, and lactotrophs in the anterior pituitary (9). Without these endocrine cell lineages, Ames dwarf mice are deficient in GH, TSH, and prolactin, with secondary effects including greatly reduced circulating levels of IGF-1 and thyroid hormone (TH) (9, 10). These mutant mice have a marked extension of longevity: 40%–60% increase compared with their normal littermates (males and females, respectively) (11). Whereas Ames dwarf mice are deficient in several metabolic hormones, studies in the long-lived GH receptor knockout (12) and GHRH knockout (GHRH-KO) (13) mice demonstrate that GH may be the key to the extended longevity of Ames dwarf mice. Some of the mechanisms believed to be responsible for the remarkable extension of longevity in Ames dwarf mice are improved insulin sensitivity and glucose tolerance (10), lower body temperature (14), and improved energy metabolism as measured by decreased respiratory quotient (RQ) and increased oxygen consumption (VO2) and heat production (15).
Recently our laboratory demonstrated that Ames dwarf mice have functionally modified epididymal white adipose tissue (eWAT) that improves, rather than impairs, their insulin sensitivity (16). This improvement is due to a shift from pro- to anti-inflammatory cytokine secretion. Moreover, we reported that eWAT transplanted from an Ames dwarf mouse fed a high-fat diet (HFD) improves insulin sensitivity in a normal mouse fed a HFD (17). As expected, eWAT transplanted from a normal mouse fed a HFD impaired insulin sensitivity in a dwarf mouse fed a HFD. Together these studies demonstrated that adipose tissue in the absence of GH and TH signaling is beneficial.
Because Ames dwarf mice have altered WAT secretory function, energy metabolism, and body temperature, we hypothesized they may have functionally unique BAT. Here we show that Ames dwarf mice have phenotypically distinct BAT that has increased expression of genes involved in thermogenesis and lipid metabolism. We also show that removal of interscapular BAT (iBAT) elicits opposite physiological responses in dwarf and normal mice, in which the normal mice increase their adiposity and the dwarf mice decrease their adiposity. Together the findings in this study illustrate that BAT in the absence of GH and TH, along with lower body temperature, is functionally enhanced. This may provide useful insights toward the effort of activating BAT to combat the growing global obesity epidemic.
Materials and Methods
Animals
Male Ames dwarf (Prop1df/df) homozygous mice (df/df), and their normal littermates, were produced in our breeding colony at Southern Illinois University School of Medicine (SIUSOM) by mating heterozygous females and homozygous mutant males. The Prop1df/df mutation is maintained on a heterogeneous genetic background. GHRH-KO mice, and their normal littermates, were produced in our breeding colony at SIUSOM by mating heterozygous females and heterozygous males on a mixed C57/BL6 and 129SV background. All mice were housed under standard conditions (20°C-23°C and a 12 h light, 12 h dark cycle), and allowed ad libitum access to water and food (LabDiet 5001, with 29% calories from protein, 13% calories from fat, and 56% calories from carbohydrates; Nestle Purina). All animal protocols for this study were approved by the SIUSOM Laboratory Animal Care and Use Committee.
iBAT removal surgery
At three months of age, male Ames dwarf mice, and their normal littermates, entered the study and were randomly assigned to undergo a sham or iBAT removal surgery. After anesthesia using a ketamine and xylazine mixture (the ketamine and xylazine mixture was prepared by mixing 220 μL of xylazine and 650 μL of ketamine in 9.13 mL of 0.9% saline and was injected 180 μL per 10 g body weight), a 1-cm vertical incision was made between the scapulae exposing the iBAT depot. The total iBAT depot was removed by blunt dissection, without removing the surrounding sc white adipose tissue. Mice in the sham group had their iBAT mobilized but not removed (ie, iBAT was gently separated from the overlying connective tissue and neighboring WAT without disturbing its blood supply). All experiments began 4 weeks after surgery to ensure any residual inflammation has subsided as previously described (17).
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed as previously described (18). For the glucose tolerance test, mice were fasted overnight for 16 hours. Blood glucose was measured (time 0) by tail bleed using a glucometer (AgaMatrix). Glucose (Sigma) was injected ip at a dose of 2 g per kilogram of body weight. Glucose was prepared by mixing 10 g of glucose in 50 mL of 0.9% saline and was injected at 100 μL per 10 g body weight. Sequential glucose measurements were taken at 15, 30, 45, 60, and 120 minutes. For the insulin tolerance test, blood glucose was measured (time 0) by tail bleed in nonfasted mice using a glucometer. Porcine insulin (Sigma) was injected ip at a dose of 1 IU per kilogram of body weight. Insulin was prepared by mixing 40 μL of insulin (0.05 U/μL) in 20 mL of 0.9% saline and was injected at 100 μL per 10 g of body weight. Sequential glucose measurements were taken at 15, 30, 45, 60, and 120 minutes.
Indirect calorimetry and norepinephrine challenge
Indirect calorimetry was performed as previously described (15) using the PhysioScan metabolic system (AccuScan Instruments, Inc). This system uses zirconia and infrared sensors to monitor oxygen (O2) and carbon dioxide (CO2), respectively, inside respiratory chambers in which the mice are housed individually. All comparisons are based on animals studied simultaneously to minimize the effects of environmental and calibration variations; therefore, data from dwarf and normal mice are plotted separately to compare mice only tested simultaneously. After a 24-hour acclimation period, the mice were monitored in the metabolic chamber for 24 hours, with ad libitum access to food and water. Gas samples for each mouse were collected and analyzed every 10 minutes. Output parameters include spontaneous locomotor activity (centimeters), oxygen consumption (milliliters per kilogram per minute), respiratory quotient (CO2 production/VO2), and heat production (calories per hour per gram). For the NE challenge, mice were anesthetized using a ketamine and xylazine mixture as described above. Once anesthetized, the mice were injected ip with NE (Sigma) at a dose of 1 mg per kilogram of body weight (19). NE was prepared by mixing 5 mg of NE in 50 mL of distilled H2O, and was injected at 100 μL per 10 gram of body weight. VO2 and heat production were measured for 25 minutes after the NE treatment.
Body composition measurements
Mice were anesthetized using a ketamine and xylazine mixture, as described above, before body composition measurements were taken. Body composition was measured by dual-energy x-ray absorptiometry (DEXA) scanning using the PIXI-mus small animal densitometer (Lunar), as previously described (18). This system directs low-energy x-rays through a mouse to a radiation detector, which in turn digitally processes the radiation for analysis using the PIXI-mus software. Output parameters include percent lean mass.
Body temperature measurements
Body temperature measurements were performed using a thin, long-probe digital rectal thermometer (Nasco) at 9:00 am. Body temperature values measured in this study were similar to those previously reported at 9:00 am using ip transmitters (14) and sc transmitters (18), validating our method of body temperature measurement.
Histology
Fixation, sectioning, and hematoxylin and eosin (H&E) staining was performed as previously described (7). In short, sc, epididymal, perirenal, and iBAT were placed in paraformaldehyde, embedded in paraffin, and sectioned at 3 μm before being stained with H&E. The sections were examined using light microscopy at ×40 magnification. All the images were captured using an IX70 microscope (Olympus) and CellSens software (Olympus).
Reverse transcription-polymerase chain reaction
Quantitative RT-PCR was performed using the StepOne system (Applied Biosystems) and SYBR Green master mix (Applied Biosystems). Tissue was homogenized, and RNA was extracted using an RNeasy lipid tissue mini kit (QIAGEN) following the manufacturer's instructions. RNA was reverse transcribed to yield cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories). Amplification was performed over 40 cycles of denaturation (95°C for 15 sec), annealing (60°C for 30 sec), and elongation (72°C for 30 sec). The mRNA-specific primers (Integrated DNA Technologies) used in this study are listed in Supplemental Table 1. There was no change in Actin expression between mutants (dwarf or knockout) and their respective normal siblings. Therefore, Actin was used as a housekeeping gene. Calculations using B2M as a housekeeping gene showed no variance with actin in statistical calculations, verifying our results. Relative expression was calculated as previously described (20).
Statistical analysis
A Student's t test was used when comparing two groups. Analyses comparing more than two groups were performed by a two-way ANOVA test for significance of the effects of genotypes (df/df vs normal) and surgical treatment (iBAT removal vs sham). Values are reported throughout the figures as mean ± SEM. All statistical analyses and graphs were done using Prism 6 for Mac (GraphPad Inc).
Results
Gene expression and morphology of BAT are altered in Ames dwarf mice
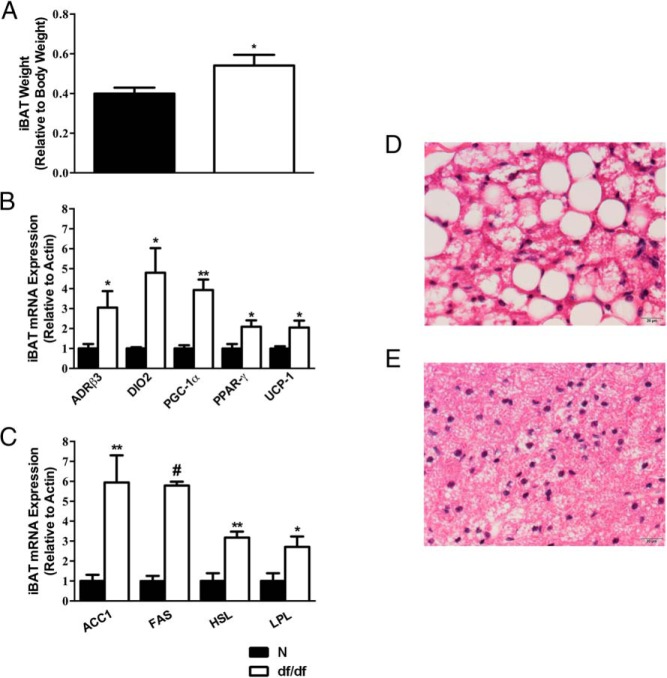
It has been previously reported that the relative iBAT weight, along with UCP-1 expression in iBAT, is inversely related to GH signaling (21). Therefore, we hypothesized that relative iBAT weight, and gene expression related to thermogenesis and lipid metabolism, may be altered in Ames dwarf mice. We found that dwarfs have an increase in their relative iBAT weight compared with their normal littermates (P = .03) (Figure 1A). We also observed an increase in the expression of several thermogenic genes in dwarf mice. ADRβ3 (P = .04), DIO2 (P = .02), peroxisomal proliferator-activated receptor-γ coactivator 1α (P = .006), peroxisomal proliferator-activated receptor-γ (P = .03), and UCP-1 (P = .02) (Figure 1B) were all increased in dwarf mice iBAT compared with their normal littermates. Moreover, we observed an increase in the expression of genes involved in lipid metabolism. ACC1 (P = .007), FAS (P < .0001), HSL (P = .003), and LPL (P = .03) (Figure 1C) were increased in dwarf mice iBAT compared with their normal littermates. Interestingly, the only change in gene expression observed in iBAT of GHRH-KO mice was an increase in UCP-1 expression (P = .02) (Supplemental Figure 1A). We also observed histological differences in the iBAT depot between the normal and Ames dwarf mice. The normal mice (Figure 1D) exhibited a typical histology of BAT with small, round lipid vacuoles that appear as unstained spaces in the H&E-stained preparations. By visual inspection, iBAT from dwarf mice (Figure 1E) have a reduction in the size of lipid droplets and increased nuclei per field, indicating a reduction in cell size.
Figure 1.
Gene expression and morphology of BAT are altered in Ames dwarf mice. A, iBAT weight relative to body weight (n = 10). B, iBAT thermogenic mRNA expression. C, iBAT lipid metabolism mRNA expression (n = 5 per group for panels B and C). D, Representative N iBAT H&E stain. E, Representative df/df iBAT H&E stain. *, P < .05; **, P < .01; #, P < .0001. df/df, homozygous; N, normal.
iBAT removal increases the size of WAT depots in normal mice, whereas it decreases the size of WAT depots in dwarf mice
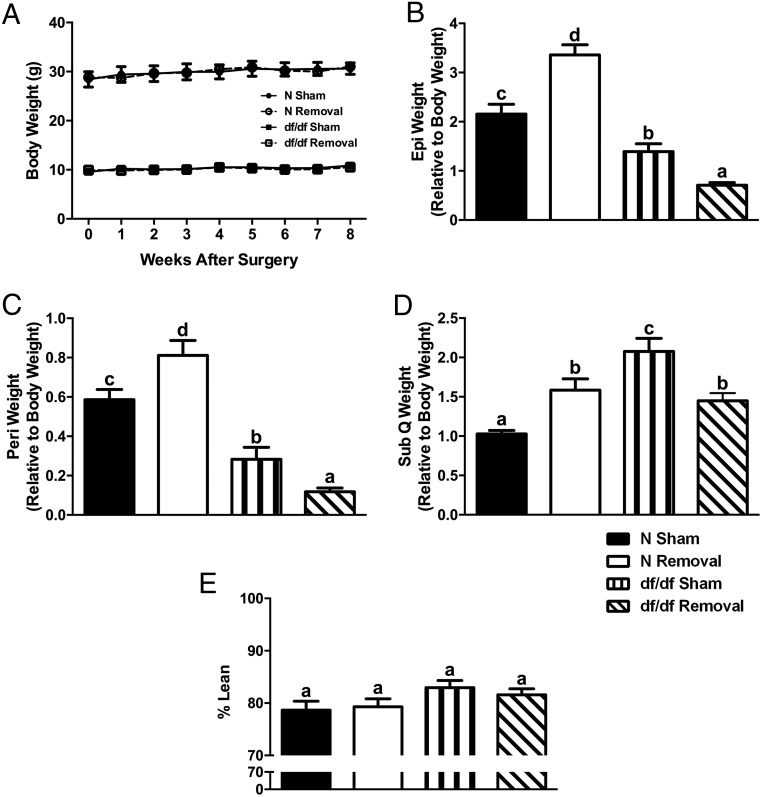
After observing genetic and morphological alterations in Ames dwarf iBAT, we used an iBAT removal surgery to identify metabolic characteristics of Ames dwarf mice that are impacted by BAT. We observed no change in body weight (Figure 2A) after iBAT removal. We did, however, observe significant differences between surgical treatments as seen by an increase in the weight of epididymal (P = .002) (Figure 2B), perirenal (P = .03) (Figure 2C), and sc (P = .0009) (Figure 2D) fat in normal mice and a decrease in epididymal (P = .0004), perirenal (P = .01), and sc (P = .006) fat in dwarf mice after iBAT removal. Consistent with the observed opposite changes in WAT weight, there appeared to be differences in adipocyte size and the number of nuclei per field upon visual analysis. We observed an increase in adipocyte size in epididymal (Figure 3, A and B), perirenal (Figure 3, E and 3F) and sc (Figures 3, I and J) fat in normal mice and a decrease in epididymal (Figure 3, C and D), perirenal (Figure 3, G and H) and sc (Figure 3, K and L) fat in dwarf mice after iBAT removal. Despite alterations in WAT depot weights and adipocyte sizes, we observed no significant differences between surgical treatments, or genotypes, in percentage of lean body mass as measured by whole-body DEXA scanning (Figure 2E).
Figure 2.
iBAT removal increases the size of WAT depots in normal mice, whereas it decreases the size of WAT depots in dwarf mice. A, Body weight curve after iBAT removal (n = 9–10 per group). B, Relative Epi weight after iBAT removal. C, Relative Peri weight after iBAT removal. D, Relative SubQ weight after iBAT removal (n = 5–8 per group for panels B–D). Epi, epididymal; Peri, perirenal; SubQ, subcutaneous. E, Percentage lean body mass (n = 7). Values not marked with the same superscript letters (a–d) are significantly different (P < .05). df/df, homozygous; N, normal.
Figure 3.
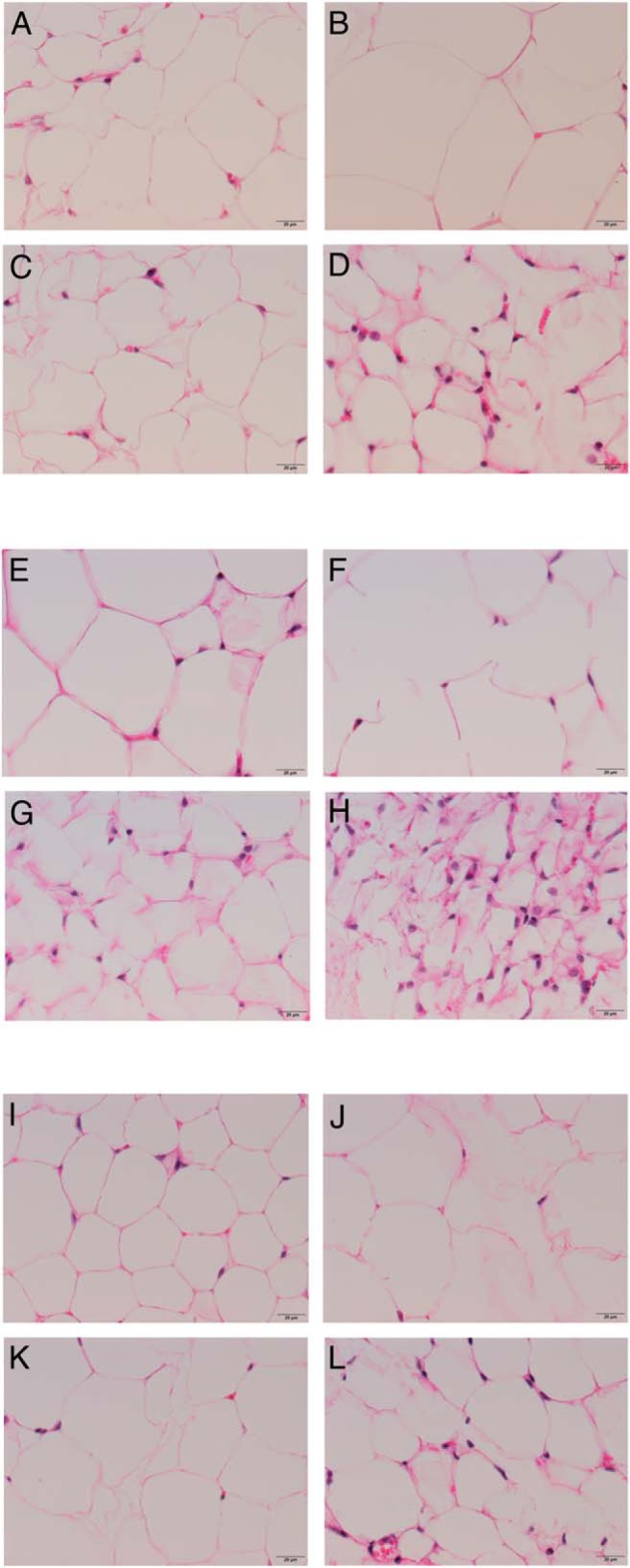
iBAT removal increases the size of white adipocytes in normal mice, whereas it reduces the size of white adipocytes in dwarf mice. A–D, Representative Epi H&E stains. E–H, Representative Peri H&E stains. I–L, Representative SubQ H&E stains. Epi, epididymal; Peri, perirenal; SubQ, subcutaneous.
BAT is not a key regulator of Ames dwarf glucose metabolism or insulin sensitivity
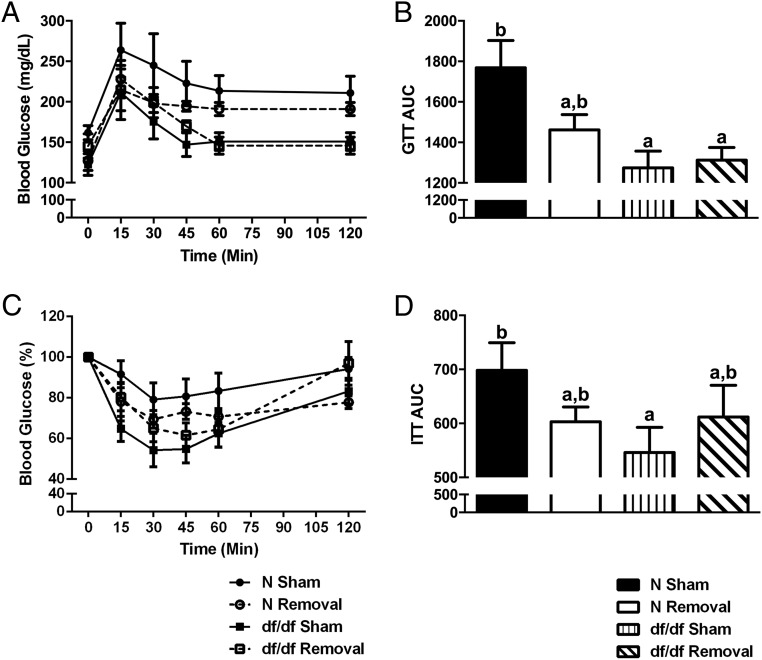
Because glucose homeostasis and insulin sensitivity are believed to be integral to the extended longevity of Ames dwarf mice (10) and BAT has been shown to play a role in glucose metabolism (22), we aimed to elucidate whether BAT has a role in the improved glucose tolerance and insulin sensitivity of Ames dwarf mice. As previously reported (10), there are significant differences between genotypes in glucose tolerance (P = .01) (Figure 4, A and B) and insulin sensitivity (P = .04) (Figure 4, C and 4D). However, we observed no significant differences between surgical treatments in glucose tolerance or insulin sensitivity.
Figure 4.
BAT is not a key regulator of Ames dwarf glucose metabolism or insulin sensitivity. A and B, Glucose tolerance test (GTT) after iBAT removal. C and D, Insulin tolerance test (ITT) after iBAT removal (n = 7 per group throughout this figure). Values not marked with the same superscript letters (a–d) are significantly different (P < .05). df/df, homozygous; N, normal.
BAT is critical to Ames dwarf energy metabolism and thermogenesis, whereas it has a minimal role in their normal littermates
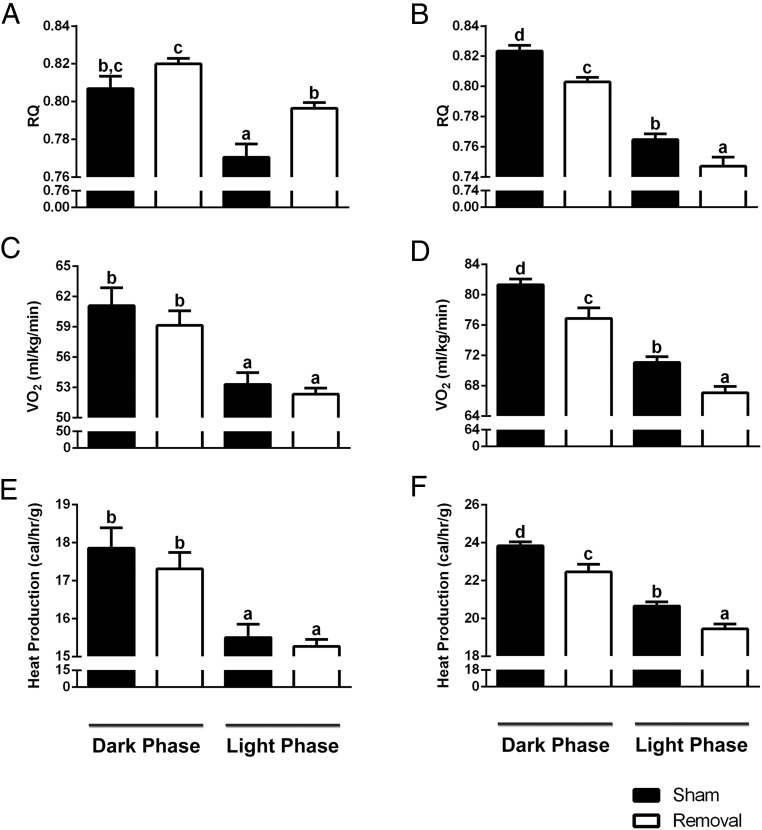
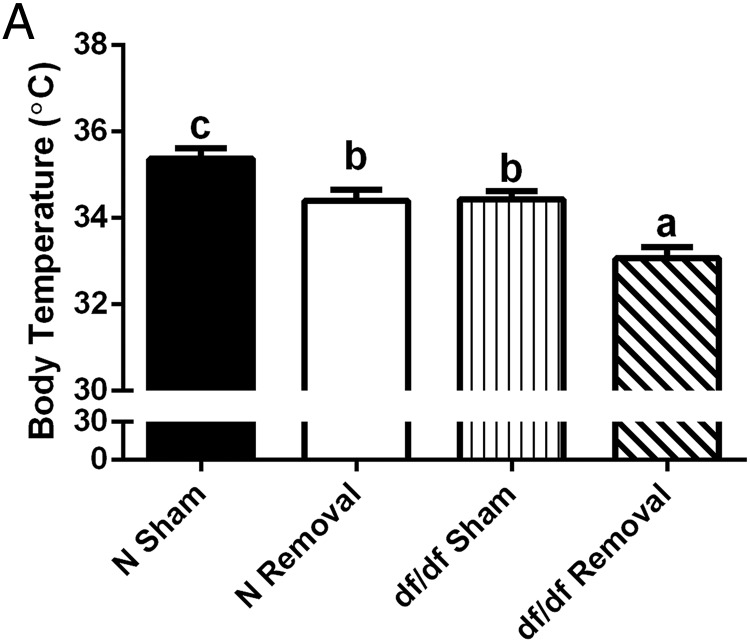
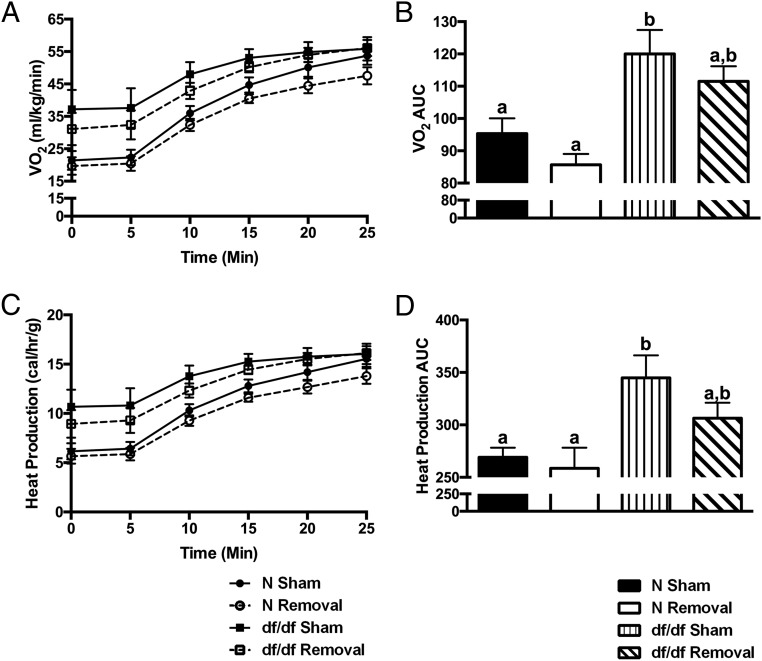
BAT is a major tissue involved in energy metabolism and regulation of body temperature (8). Because Ames dwarf mice have improved energy metabolism (15) and decreased body temperature (14), we looked to elucidate the influence of BAT on these altered phenotypes. After iBAT removal, we saw no significant differences between surgical treatments in locomotor activity in either normal or dwarf mice (Supplemental Figure 2). Both normal and dwarf mice demonstrated a normal diurnal rhythm of energy metabolism, consistent with previously published data (15). We did observe a significant treatment effects on RQ, as seen by an increase in normal mice during the light phase (P = .002) (Figure 5A) and a decrease in dwarf mice during the light (P = .02) and dark phases (P = .0004) (Figure 5B). Normal mice showed no significant difference between surgical treatments in VO2 (Figure 5C), whereas dwarf mice showed a significant decrease during the light (P = .002) and dark phases (P = .009) (Figure 5D). Moreover, normal mice demonstrated no significant difference between surgical treatments in heat production (Figure 5E), whereas dwarf mice demonstrated a significant decrease during the light (P = .001) and dark phases (P = .006) (Figure 5F). As previously described (14), there was a significant effect of genotype on body temperature; dwarf mice had a lower body temperature than their normal littermates (P = .01) (Figure 6A). Moreover, we observed a slight but statistically significant differences between surgical treatments in normal mice as seen by a slight decrease in body temperature (P = .01) and a more significant reduction in body temperature for dwarf mice (P = .0006) after iBAT removal.
Figure 5.
BAT is critical to Ames dwarf energy metabolism, whereas it has a minimal role in their normal littermates. A, Normal RQ after iBAT removal. B, Dwarf RQ after iBAT removal. C, Normal VO2 after iBAT removal. D, Dwarf VO2 after iBAT removal. E, Normal heat production after iBAT removal. F, Dwarf heat production after iBAT removal (n = 7 throughout this figure). Values not marked with the same superscript letters (a–d) are significantly different (P < .05).
Figure 6.
BAT is critical to Ames dwarf thermogenesis, whereas it has a lesser role in their normal littermates. A, Body temperature after iBAT removal (n = 7). Values not marked with the same superscript letters (a–d) are significantly different (P < .05). df/df, homozygous; N, normal.
iBAT removal does not impair sympathetic outflow in Ames dwarf mice
NE is a catecholamine that initiates thermogenesis (8). Responsiveness to a NE challenge has been used to measure sympathetic outflow (19, 23). Using a NE challenge, we observed a significant difference between genotypes in sympathetic outflow as seen by the increased VO2 (P = .01) (Figure 7, A and B) and heat production (P = .02) (Figure 7, C and D) in Ames dwarf mice. We did not, however, observe any significant differences between surgical treatments in sympathetic outflow after the iBAT removal.
Figure 7.
iBAT removal does not impair sympathetic outflow in Ames dwarf mice. A and B, VO2 after a NE challenge. C and D, Heat production after a NE challenge (n = 7 throughout this figure). Values not marked with the same superscript letters (a–d) are significantly different (P < .05). df/df, homozygous; N, normal.
Discussion
Alternative therapeutics, such as BAT activation, are currently under investigation to combat the alarming increase in global obesity (2). Ames dwarf mice are long lived (11) with improved energy metabolism (15), lower body temperature (14), and functionally unique eWAT (16, 17). Because of this, we hypothesized that BAT in Ames dwarf mice may be functionally enhanced. As previously reported in mutant mice deficient in GH signaling (21), we observed that Ames dwarf mice have an increase in their relative iBAT weight. Along with an increase in relative weight, we observed differences in morphology of the iBAT depot between dwarf and normal mice. Normal mice have brown adipocytes that are multilocular and occupied by numerous circular lipid droplets. Cold exposure alters the morphology of BAT by decreasing the size and number of lipid droplets (7). We observed a histological decrease in lipid content in iBAT in dwarf mice that is consistent with cold exposure. We believe this is to be expected, given their dramatically decreased body temperature, which is partially due to their lack of TH and a consequent increased demand for thermogenesis.
Beyond alterations in the relative amount and morphology of BAT in Ames dwarf mice, we observed alterations in both thermogenic and lipid metabolism gene expression. It is well established that UCP-1 is the main protein involved in BAT thermogenesis (8). We observed a significant increase in UCP-1 gene expression along with its two transcriptional coactivators peroxisomal proliferator-activated receptor-γ and peroxisomal proliferator-activated receptor-γ coactivator 1α in dwarf mice, compared with their normal littermates (8). Interestingly in GHRH-KO mice, we observed an increase in UCP-1 expression in BAT, whereas no other thermogenic genes were effected. This suggests that alterations in BAT in Ames dwarf mice may in large part be due to TH deficiency because GHRH-KO have normal thyroid function, and Ames dwarf mice are severely hypothyroid. Moreover, because both Ames and GHRH-KO are both dwarf, the differences in gene expression observed cannot be solely explained by the increased surface to body ratio that promotes increased thermogenesis to support body temperature. Furthermore, we observed an increase in both ADRβ3 and DIO2 gene expression in Ames dwarf mice compared with their normal littermates, which are both involved in the thermogenic processes. As mentioned above, ADRβ3 is the main adrenergic receptor in rodent BAT (8). DIO2 is responsible for converting T4 to its active form of T3 at target tissues such as BAT (24). The conversion of T4 to T3 is important because T3 potentiates the effects of catecholamines such as NE (8).
The major metabolites used in BAT thermogenesis are FFAs (8). FFAs used in BAT thermogenesis are derived from hormone-sensitive lipase (HSL)-mediated lipolysis of locally stored triglycerides (25) or by lipoprotein lipase (LPL)-mediated uptake of circulating triglycerides (26). Here we observed an increase in the gene expression of both HSL and LPL in dwarf mice compared with their normal littermates. We also observed an increase in ACC1 and FAS gene expression. ACC1 converts acetyl CoA to malonyl CoA, which acts as a substrate for fatty acid elongation by fatty acid synthase (FAS) (19). This de novo lipogenesis appears to be necessary for maximal thermogenesis in BAT (7, 8). The alterations in relative BAT weight, morphology, and gene expression related to thermogenesis demonstrate a clear distinction between normal and Ames dwarf mice BAT. Further studies would need to be conducted to elucidate whether these changes are primarily a result of alterations in major hormonal axes (GH and TH) or reflect alterations in body temperature.
To further understand how BAT influences the metabolic traits of Ames dwarf mice, we examined the effects of iBAT removal. After surgical iBAT removal, we monitored the body weights of normal and dwarf mice and observed no differences between removal and sham mice. We did, however, see an inverse response in normal and dwarf mice in terms of relative WAT depot weights. Normal mice gained weight in their major WAT depots after iBAT removal, whereas dwarf mice lost weight. Moreover, we observed changes in white adipocyte size that would indicate an increased accumulation of lipids in the WAT of normal mice and lipolysis in the WAT of dwarf mice. Furthermore, we observed an increase in RQ in normal mice and a decrease in RQ in dwarf mice, which would indicate that normal mice are burning fewer lipids after iBAT removal, whereas dwarf mice are burning more lipids. We believe the accumulation of lipids in normal mice is to be expected because BAT is a major lipolytic tissue, and lower BAT activity is associated with obesity (8, 27–31). The increased use of lipids from WAT in dwarf mice after iBAT removal was unexpected; however, we believe it is related to their increased energy demand for thermogenesis, which may be further accentuated by their lower body temperature. Presumably, after iBAT removal, their already reduced body temperature dropped below a critical point, resulting in lipolysis of the WAT depots in an effort to maintain their body temperature at a viable level. This hypothesis is supported by previous work in our laboratory that showed the increased VO2 and decreased RQ in long-lived mice lacking GH signaling is reverted to the values measured in their normal littermates in thermoneutral (30°C) conditions (32). The effects of thermoneutral housing conditions on other metabolic parameters in these mice has yet to be examined.
In the present study, we did observe alterations in WAT depot weights, whereas whole-body DEXA scanning revealed no alterations in percent lean body mass. We believe that the alterations in WAT are beyond the detection of DEXA scanning and that an analysis of specific adipose depots is more representative of the changes observed after iBAT removal.
It is believed that improved insulin sensitivity and energy metabolism are key mechanisms of the extended longevity of Ames dwarf mice (10). Because BAT plays a major role in energy metabolism (8) and has been shown to play a role in glucose homeostasis (22), we hypothesized that BAT may be important in the improved energy metabolism and insulin sensitivity in Ames dwarf mice. However, we found that iBAT removal had no significant effect on glucose homeostasis or insulin sensitivity in normal or dwarf mice. This is interesting because iBAT removal altered the amount of eWAT in both dwarf and normal mice, which has been shown to play a role in their glucose homeostasis (16). Stanford et al (22) used C57/BL6 mice to show that BAT transplanted into the visceral cavity can influence glucose homeostasis 8 weeks after transplantation. It is possible that the removal of iBAT for longer periods than that of our study may lead to statistically significant alterations in glucose homeostasis. It is also important to note that the study by Stanford et al does not mention the effects of BAT transplantation on the resident visceral adipose tissue, which may also be a mechanism of improved glucose homeostasis that would be absent in our study. Our study showed a decrease in Ames dwarf eWAT and a numerical trend toward insulin resistance. This may vary from the study by Menon et al (16), which showed complete removal of eWAT in Ames dwarf mice causes insulin resistance because our mice still had intact (albeit decreased) eWAT. After the iBAT removal, we did observe a significant decrease in both VO2 and heat production in dwarf mice, whereas we did not observe any alterations in these parameters in normal mice. This was similar to our findings regarding body temperature in which the dwarf mice suffered a dramatic reduction in body temperature, whereas the normal mice showed only a slight, although significant decrease in body temperature. This illustrates that BAT is more critical for energy metabolism and thermoregulation in dwarf mice than in their normal littermates.
In rodents, BAT thermogenesis begins with NE binding to ADRβ3 (8). Because of this, a NE challenge can be used to measure sympathetic outflow (19, 23). As measured by both VO2 and heat production after a NE challenge, Ames dwarf mice have a more robust sympathetic outflow compared with their normal littermates. Interestingly, iBAT removal did not significantly alter sympathetic outflow, although a numerical decrease was observed in the dwarf mice.
New therapies are needed to fight the unprecedented increase in obesity. BAT is currently being examined as a means to reverse or prevent obesity; however, ways of enhancing the function of BAT are not well understood. Our current study resulted in three novel findings. First, we show that Ames dwarf mice have BAT that is functionally enhanced from that of their normal littermates. By increasing the amount of BAT and expression of genes related to thermogenesis, Ames dwarf mice are able to improve their energy metabolism, which we believe to be a key regulator of their extended longevity because improved energy metabolism in Ames dwarf mice is associated with lower reactive oxygen species production and maintenance of mitochondrial DNA (33–36) and favorable alterations in the activity of electron transport chain complexes (37, 38), which are all associated with increased longevity (39). Moreover, phosphatase and tensin homolog transgenic mice have hyperactive BAT and are long lived (40), and mice with increased uncoupling in skeletal muscle due to increased UCP-1 have a delay in aging and age-related diseases (41). Furthermore, increased uncoupling appears to affect longevity in humans as well (42, 43). Second, removal of Ames dwarf iBAT results in their use of WAT as a source of metabolic fuel. We believe this unexpected finding to be the result of a critical drop in body temperature, which supports various recent studies, suggesting that living in cooler conditions may decrease body fat. Lastly, we report for the first time that Ames dwarf mice have an increased sympathetic outflow compared with their normal littermates. We believe these findings are central to understanding the improved energy metabolism of Ames dwarf mice. Our study also illustrates that chronic, mild cold stress and lower body temperature increase sympathetic outflow and BAT thermogenesis, which may provide unique means to activate BAT and subsequently reducing adiposity.
Acknowledgments
We thank Dr Cristal Hill for her invaluable insight into our work and Dr Wei Du for her assistance with microscopy. We also thank the Histology Department of the Memorial Medical Center for assisting with histological staining.
This work was supported by National Institute on Aging Grant AG0119899.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- DEXA
- dual-energy x-ray absorptiometry
- eWAT
- epididymal WAT
- FAS
- fatty acid synthase
- FFA
- free fatty acid
- GHRH-KO
- GHRH knockout
- H&E
- hematoxylin and eosin
- HFD
- high-fat diet
- HSL
- hormone-sensitive lipase
- iBAT
- interscapular BAT
- LPL
- lipoprotein lipase
- NE
- norepinephrine
- RQ
- respiratory quotient
- SIUSOM
- Southern Illinois University School of Medicine
- TH
- thyroid hormone
- UCP-1
- uncoupling protein 1
- VO2
- oxygen consumption
- WAT
- white adipose tissue.
References
- 1. Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation. 2012;125:2782–2791. [DOI] [PubMed] [Google Scholar]
- 2. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. [DOI] [PubMed] [Google Scholar]
- 5. Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. [DOI] [PubMed] [Google Scholar]
- 6. Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. [DOI] [PubMed] [Google Scholar]
- 7. Xiao XQ, Williams SM, Grayson BE, et al. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology. 2007;148:4150–4159. [DOI] [PubMed] [Google Scholar]
- 8. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 9. Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. [DOI] [PubMed] [Google Scholar]
- 10. Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93:571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. [DOI] [PubMed] [Google Scholar]
- 12. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. [DOI] [PubMed] [Google Scholar]
- 13. Sun LY, Spong A, Swindell WR, et al. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife. 2013;2:e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav. 1999;67:433–437. [DOI] [PubMed] [Google Scholar]
- 15. Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menon V, Zhi X, Hossain T, et al. The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell. 2014;13:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill CM, Fang Y, Miquet JG, Sun LY, Masternak MM, Bartke A. Long-lived hypopituitary Ames dwarf mice are resistant to the detrimental effects of high-fat diet on metabolic function and energy expenditure. Aging Cell. 2016;15(3):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darcy J, Fang Y, Hill CM, McFadden S, Sun LY, Bartke A. Metabolic alterations from early life thyroxine replacement therapy in male Ames dwarf mice are transient. Exp Biol Med (Maywood). 2016;241(16):1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sjogren M, Alkemade A, Mittag J, et al. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J. 2007;26:4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood). 2003;228:207–215. [DOI] [PubMed] [Google Scholar]
- 22. Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, Shi H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol Behav. 2014;125:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476. [DOI] [PubMed] [Google Scholar]
- 25. Holm C, Fredrikson G, Cannon B, Belfrage P. Hormone-sensitive lipase in brown adipose tissue: identification and effect of cold exposure. Biosci Rep. 1987;7:897–904. [DOI] [PubMed] [Google Scholar]
- 26. Klingenspor M, Ebbinghaus C, Hulshorst G, et al. Multiple regulatory steps are involved in the control of lipoprotein lipase activity in brown adipose tissue. J Lipid Res. 1996;37:1685–1695. [PubMed] [Google Scholar]
- 27. Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96:192–199. [DOI] [PubMed] [Google Scholar]
- 28. Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010;299:E601–E606. [DOI] [PubMed] [Google Scholar]
- 29. Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6:e17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chondronikola M, Volpi E, Borsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hibi M, Oishi S, Matsushita M, et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes (Lond). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Westbrook R. The Effects of Altered Growth Hormone Signaling on Murine Metabolism [dissertation] Springfield, Illinois: Southern Illinois University, Department of Molecular Biology, Microbiology and Biochemistry; 2012. [Google Scholar]
- 33. Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood). 2002;227:94–104. [DOI] [PubMed] [Google Scholar]
- 34. Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35:199–212. [DOI] [PubMed] [Google Scholar]
- 35. Hauck SJ, Bartke A. Effects of growth hormone on hypothalamic catalase and Cu/Zn superoxide dismutase. Free Radic Biol Med. 2000;28:970–978. [DOI] [PubMed] [Google Scholar]
- 36. Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. [DOI] [PubMed] [Google Scholar]
- 37. Brown-Borg HM, Johnson WT, Rakoczy SG. Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. Age (Dordr). 2012;34:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choksi KB, Nuss JE, DeFord JH, Papaconstantinou J. Mitochondrial electron transport chain functions in long-lived Ames dwarf mice. Aging (Albany NY). 2011;3:754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown-Borg HM. The somatotropic axis and longevity in mice. Am J Physiol Endocrinol Metab. 2015;309:E503–E510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. [DOI] [PubMed] [Google Scholar]
- 41. Gates AC, Bernal-Mizrachi C, Chinault SL, et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. [DOI] [PubMed] [Google Scholar]
- 42. Rose G, Crocco P, D'Aquila P, Montesanto A, Bellizzi D, Passarino G. Two variants located in the upstream enhancer region of human UCP1 gene affect gene expression and are correlated with human longevity. Exp Gerontol. 2011;46:897–904. [DOI] [PubMed] [Google Scholar]
- 43. Rose G, Crocco P, De Rango F, Montesanto A, Passarino G. Further support to the uncoupling-to-survive theory: the genetic variation of human UCP genes is associated with longevity. PLoS One. 2011;6:e29650. [DOI] [PMC free article] [PubMed] [Google Scholar]