Abstract
Development of lung cancer is closely related to smoking in a majority of patients. Most smokers, however, do not develop lung cancer in spite of a high mutational load accumulating in the lung tissue. Here we investigate whether a cancer-specific footprint can be revealed by investigating circulating inflammatory markers in patients with non-small cell lung cancer (NSCLC) compared with patients with chronic obstructive pulmonary disease (COPD), both cohorts characterised by similar smoking history. Serum concentrations of 57 cytokines and matrix metalloproteinases (MMPs) from 43 patients with advanced NSCLC were evaluated by multiplex immunoassays and compared with serum samples from 35 patients with COPD. Unsupervised hierarchical clustering and non-parametric analyses were performed. False discovery rate was used to adjust for multiple testing. Clustering of cytokine and MMP concentrations in the serum revealed a distinct separation of the NSCLC patients from the COPD group. Individual concentrations of thymus and activation-regulated cytokine (C-C motif chemokine ligand 17), Gro-b (C-X-C motif chemokine ligand 2 (CXCL2)), CXCL13, interleukin (IL)-1ra, IL-6, IL-8 (CXCL8), IL-16, IL-17A, macrophage migration inhibitory factor (MIF), granulocyte colony-stimulating factor, platelet-derived growth factor subunit B, MMP-2, MMP-8 and MMP-12 were significantly different in serum from NSCLC and COPD patients. Moreover, the interferon-γ/IL-10 ratio was lower in cancer patients compared with COPD patients, consistent with a cytokine milieu favouring tumour tolerance. Our results suggest that NSCLC is characterised by a distinct inflammatory signature in serum. The different cytokine profiles in NSCLC and COPD patients may represent tumour-promoting and tumour-suppressing immune responses developing in response to mucosal inflammation and mutations induced by smoking.
The severity of lung cancer is well known and lung cancer remains the leading cause of cancer-related death worldwide.1 Treatment and prognosis rely heavily on disease stage at diagnosis. A majority of patients are diagnosed in advanced stages, not eligible for treatment with curative intent. Although scientific endeavours are comprehensive, there is a definite need for further insight into lung cancer biology aiming at improved diagnostics, more efficient cancer treatment and ultimately an increase in survival.
Inflammation is linked to multiple tumour-promoting effects and is in general recognised to have an important part in cancer evolution.2 The lungs are vulnerable for air-borne environmental factors, and tobacco smoke in particular is implicated in lung inflammation.3, 4 An association between the inflammatory disease chronic obstructive pulmonary disease (COPD) and lung cancer is evident from epidemiological and clinical studies.5, 6 There is, however, limited knowledge if or how the host immune response influence whether an individual with COPD develops lung cancer.
We know that heavy smokers accumulate a high mutational load in the lung tissue, but still only a minority develop cancer. Carcinogenesis is not merely a malignant transformation of cells; it is dependent on multiple interactions with different cell types making up the tumour microenvironment. Cells of the immune system, endothelial cells and fibroblasts are among others found adjacent to malignant cells in a tumour and are of importance in cancer development and progression.7 Genetic events in cells are followed by an interaction with the immune system.2 The immune response can either suppress the development of a tumour by eliminating cancer cells or promote tumour growth by selecting cancer cells that escape the immune control.8 It is evident that immune cells in the tumour microenvironment are relevant for tumour characteristics and patient outcome. Tumour-infiltrating lymphocytes are of clinical impact in several cancers, including lung cancer where high levels are correlated to better prognosis.9 To target the non-malignant cells in the tumour environment is an interesting approach to the treatment of cancer. Certainly, recent clinical trials with immune-associated mediators have proven efficient in non-small cell lung cancer (NSCLC) treatment.10, 11
Proteins and enzymes make up complex networks responsible for cell-to-cell communication in the tumour microenvironment. Cytokines are essential modulators. A shift from an immunological pattern with a T helper type 1 (TH1) orientation to a TH2 pattern mediated by cytokines is reported as a biological event in the carcinogenesis.12 Indeed, increased serum levels of specific cytokines are associated with a risk of developing lung cancer and is linked to survival both in early- and advanced-stage lung cancer.13, 14, 15 Matrix metalloproteinases (MMPs) are proteolytic enzymes originally known to degrade extracellular matrix. Moreover, they also regulate the activity of growth factors, cytokines, cell receptors and other proteases, thus influencing a variety of inflammatory processes and biological activities in cancer.16
Here we analyse a comprehensive panel of circulating cytokines and MMPs in patients with advanced-stage NSCLC using COPD patients as a control group. Both cohorts represent current or former smokers. Our aim is to investigate the cancer-specific footprint in the serum mirroring lung cancer carcinogenesis.
Results
Patient and disease characteristics
The median age of the NSCLC patients at inclusion was 70 years (range 47–88) and 72% of the patients were male. All NSCLC patients had a history of smoking either as current (26%) or former (74%) smokers. Twenty six of the patients (61%) had not received previous chemotherapy. Fourteen NSCLC patients (33%) were treated with systemic steroids at the time of inclusion. The median age of the COPD patients were 72 years (range 50–87) and 40% were male. All but three patients (91%) had a smoking history. Only 4 patients (11%) in the COPD group received systemic steroids at the time of serum sampling. A majority had grade III COPD disease (46%). The NSCLC and COPD cohorts were fairly well balanced at baseline with no significant differences in age and smoking history. A higher percentage of patients in the NSCLC cohort (33%) used systemic steroids compared with the COPD cohort (11%), but this difference was not significant. There was, however, a significant difference in sex (P=0.006) with a larger percentage of males relative to females in the NSCLC group compared with the COPD group. Baseline characteristics of the NSCLC and COPD patients are shown in Table 1.
Table 1. Characteristics of NSCLC and COPD patients included in the cytokine/MMP analysis.
| Variable |
NSCLC cohort |
COPD cohort |
P-value | ||
|---|---|---|---|---|---|
| N=43 | % | N=35 | % | ||
| Age at inclusion (years) | |||||
| Mean/median/range | 69/70/47–88 | 71/72/50–87 | 0.333a | ||
| Sex | |||||
| Male | 31 | 72.1 | 14 | 40.0 | 0.006b |
| Female | 12 | 27.9 | 21 | 60.0 | |
| Smoking history | |||||
| Current | 11 | 25.6 | 13 | 37.1 | 0.057c |
| Former | 32 | 74.4 | 19 | 54.3 | |
| Never | 0 | 0 | 3 | 8.6 | |
| Pack years | |||||
| Mean/median/range | 34/30/4–144 | 32/35/12–60 | 0.698a | ||
| Use of systemic steroids | |||||
| Yes | 14 | 32.6 | 4 | 11.4 | 0.153b |
| No | 29 | 67.4 | 31 | 88.6 | |
| COPD grade | |||||
| I | 1 | 2.9 | |||
| II | 10 | 28.6 | |||
| III | 16 | 45.7 | |||
| IV | 8 | 22.8 | |||
| ECOG performance status | |||||
| 0 | 8 | 18.6 | |||
| 1 | 22 | 51.2 | |||
| 2 | 13 | 30.2 | |||
| Stage | |||||
| III | 11 | 25.6 | |||
| IV | 32 | 74.4 | |||
| Histology | |||||
| Adenocarcinoma | 26 | 60.5 | |||
| Squamous cell carcinoma | 13 | 30.2 | |||
| NOS | 4 | 9.3 | |||
| Previous chemotherapy | |||||
| Yes | 17 | 39.5 | |||
| No | 26 | 60.5 | |||
Abbreviations: COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; MMP, matrix metalloproteinase; NOS, not otherwise specified; NSCLC, non-small cell lung cancer.
Two sided t-test for continuous variables.
Fisher's exact test for categorical variables.
Chi-square test for categorical variables.
Distinct signatures of serum proteins separating NSCLC from COPD patients
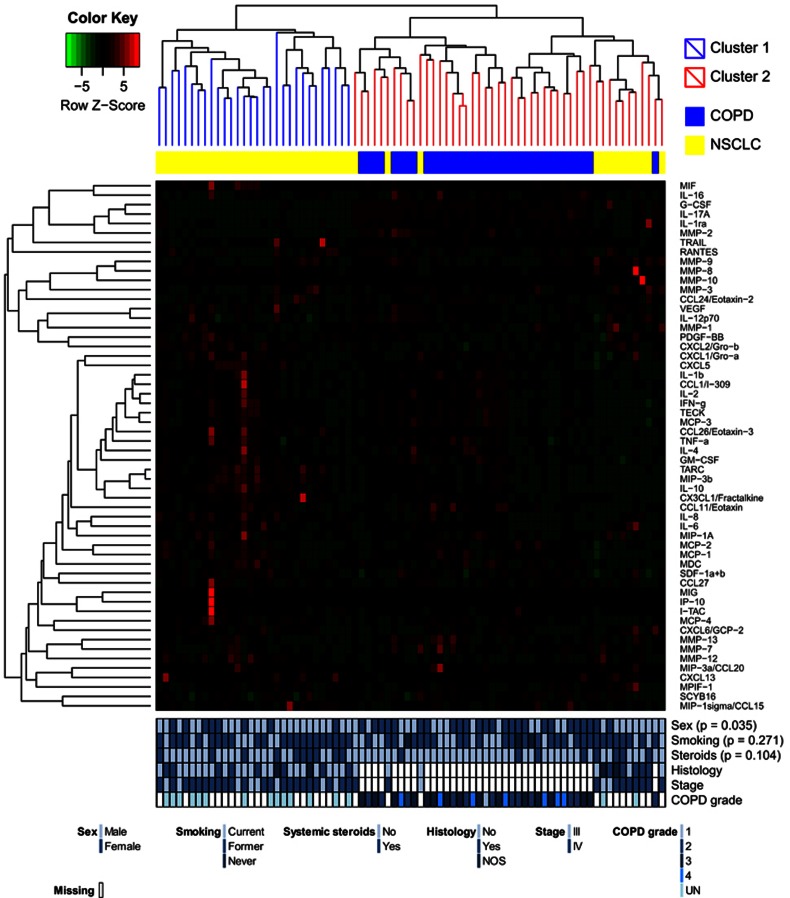
Hierarchical clustering of cytokines and MMPs separated the serum samples into two clusters (Figure 1) illustrating a clear separation of cytokine levels in NSCLC and COPD patients. There was a significant difference between the two clusters regarding sex (P=0.035), concurrent with the difference in baseline characteristics with fewer men among the COPD patients. No significant differences in smoking history or in the use of systemic steroids were seen between the clusters.
Figure 1.
Hierarchical clustering of 57 proteins (cytokines and MMPs) in serum samples from 43 patients with NSCLC and 35 patients with COPD. Clinical parameters with tests for significant differences between the clusters are visualised below the heat map. AD, adenocarcinoma; NOS, none otherwise specified; SCC, squamous cell carcinoma; UN, unknown COPD grade.
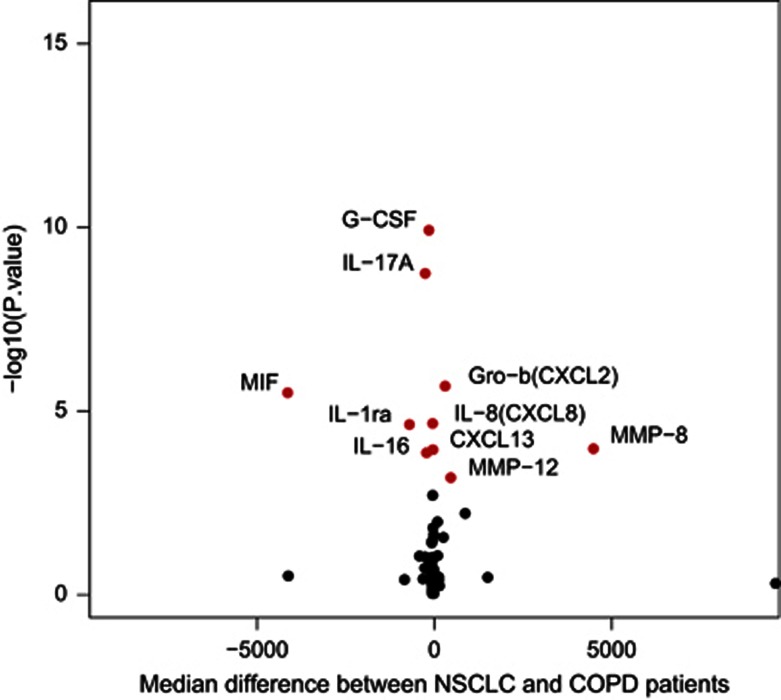
Several individual cytokines and MMPs had different concentrations in serum between the two cohorts (Table 2, Figure 2). After correction for multiple testing, NSCLC patients had a significant higher median level of thymus and activation regulated cytokine (TARC; C-C motif chemokine ligand 17 (CCL17)), Gro-b (C-X-C motif chemokine ligand 2 (CXCL2)), CXCL13, interleukin (IL)-6, IL-8 (CXCL8), platelet-derived growth factor subunit B (PDGF-BB), MMP-8 and MMP-12 in the serum compared with the COPD controls. For IL-1ra, IL-16, IL-17A, macrophage migration inhibitory factor (MIF), granulocyte colony-stimulating factor (G-CSF) and MMP-2, the median serum concentration value in NSCLC patients was significantly lower compared with COPD patients. Box plots of significantly different levels of cytokines/MMPs, and a complete table of all proteins investigated in the two cohorts are included in Supplementary Information (Supplementary Figure S1 and Supplementary Table S1).
Table 2. Levels of circulating cytokines and MMPs with a significant difference in NSCLC and COPD patients.
| Cytokine/MMP | NSCLC median | COPD median | P-value |
|---|---|---|---|
| CCL1/I-309 | 77 | 89 | 0.041 |
| MIP-3a (CCL20) | 24 | 17 | 0.036 |
| MIP-3b (CCL19) | 919 | 606 | 0.029 |
| TARC (CCL17) | 400 | 253 | 0.011a |
| Eotaxin (CCL11) | 63 | 72 | 0.037 |
| Gro-b (CXCL2) | 878 | 514 | <0.001a |
| CXCL13 | 54 | 36 | 0.000a |
| Fractalkine (CX3CL1) | 254 | 212 | 0.025 |
| IL-1ra | 434 | 1079 | <0.001a |
| IL-6 | 27 | 16 | 0.002a |
| IL-8 (CXCL8) | 31 | 21 | 0.000a |
| IL-10 | 76 | 56 | 0.016 |
| IL-16 | 370 | 532 | <0.001a |
| IL-17A | 73 | 273 | <0.001a |
| MIF | 808 | 4895 | <0.001a |
| G-CSF | 33 | 127 | <0.001a |
| PDGF-BB | 3288 | 2357 | 0.003a |
| MMP-2 | 37 242 | 89 243 | <0.001a |
| MMP-8 | 9436 | 5072 | <0.001a |
| MMP-12 | 871 | 347 | <0.001a |
Abbreviations: CCL, C-C motif chemokine ligand; COPD, chronic obstructive pulmonary disease; CXCL, C-X-C motif chemokine ligand; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; MIF, macrophage migration inhibitory factor; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; NSCLC, non small cell lung cancer; PDGF-BB, platelet-derived growth factor subunit B; TARC, thymus and activation regulated cytokine. Concentrations measured in pg ml−1.
Statistically significant P-values retained after correction for multiple.
Figure 2.
Volcano plot illustrating the magnitude and significance of the differences in cytokine/MMP serum concentration levels in patients with NSCLC and COPD. Dots marked in red are cytokines/MMPs with a significantly different median serum concentration after correction for multiple testing.
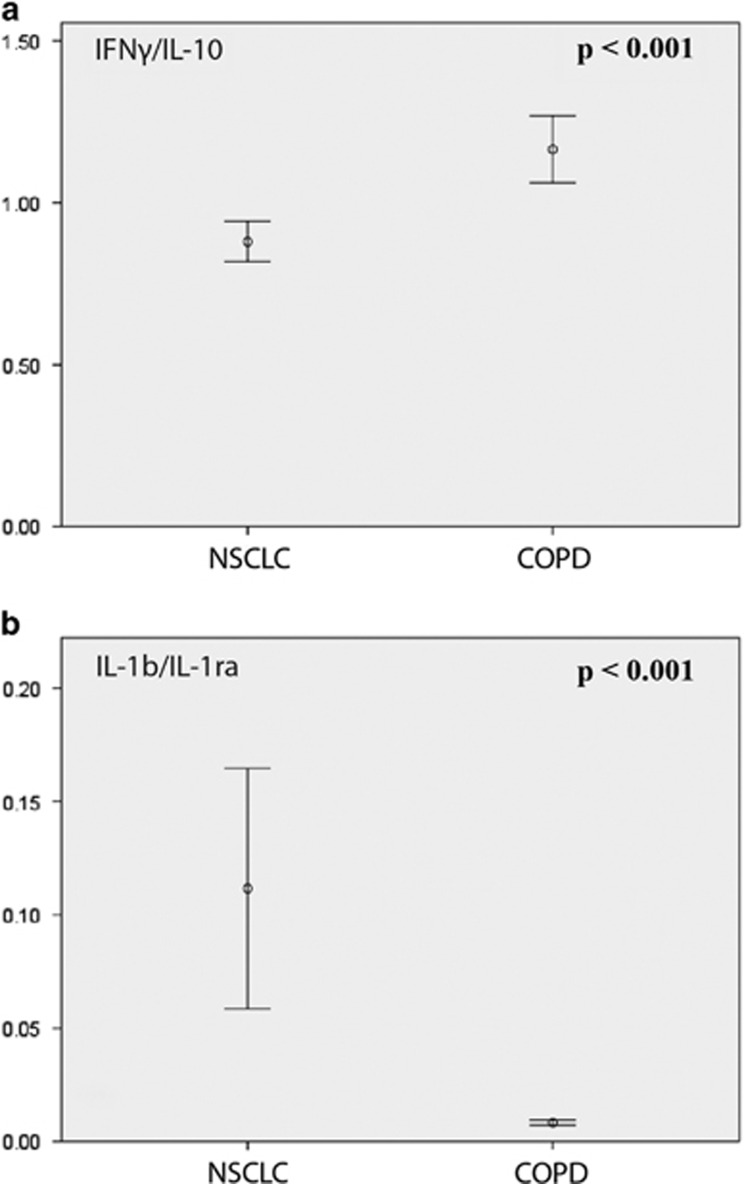
Interferon gamma (IFNγ) and IL-10 are considered hallmark cytokines for antitumour TH1 responses and immunological tumour tolerance, respectively. The serum concentration of IFNγ did not vary between NSCLC and COPD patients. The IL-10 levels were higher in NSCLC patients but did not retain significance after correction for multiple testing. IFNγ/IL-10 ratio, however, was significantly different between NSCLC and COPD patients (P<0.001), with a median ratio of 0.84 in the NSCLC group versus 1.11 in the COPD cohort (Figure 2).
Another interesting connection at the biological level in this panel of cytokines was the association between the pro-inflammatory protein IL-1b and its naturally occurring antagonist IL-1ra. No difference was seen in the level of IL-1b between the groups, but the IL-1ra concentration in the serum samples was significantly lower in the NSCLC group. Accordingly, the IL-1b/IL-1ra ratio in the NSCLC group was significantly higher compared with the COPD patients (P<0.001; Figure 3).
Figure 3.
Differences in IFNγ/IL-10 (a) and IL-1b/IL-1ra (b) ratios comparing patients with NSCLC and COPD.
Associations of cytokine and MMP levels in different clinical subsets of NSCLC patients
When omitting all NSCLC and COPD patients using corticosteroids, we observed a distinct serum signature separating NSCLC and COPD patients, similar to the one observed in the entire cohort (Supplementary Table S2). In addition, the levels of IL-10 and MMP-3 were observed to be significantly different, with a higher and lower median level in NSCLC patients compared with COPD patients, respectively. The significant association of TARC between the patient groups was lost.
Among NSCLC patients, two MMPs had a significant different concentration after correction for multiple testing, when comparing patients using systemic steroids or not (Table 3). MMP-3 (P<0.001) and MMP-9 (P=0.001) had a higher median concentration in the systemic steroid-user group.
Table 3. Cytokine and MMP serum concentration values in the NSCLC patients (N=43) with a significant different distribution in non-steroid users compared with patients using systemic steroids.
| Cytokine/MMP |
NSCLC cohort |
P-value | |
|---|---|---|---|
| No systemic steroids, N=29, median | Systemic steroids, N=14, median | ||
| MDC (CCL22) | 977 | 453 | 0.013 |
| IL-17a | 88 | 56 | 0.011 |
| MMP-3 | 5681 | 15 443 | <0.001a |
| MMP-7 | 3759 | 2676 | 0.036 |
| MMP-9 | 42 768 | 77 164 | 0.001a |
| MMP-12 | 1050 | 389 | 0.003 |
| MMP-13 | 148 | 75 | 0.011 |
Abbreviations: CCL, C-C motif chemokine ligand; IL, interleukin; MDC, macrophage-derived chemokine; MMP, matrix metalloproteinase; NSCLC, non-small cell lung cancer.
Concentrations measured in pg ml−1.
Statistically significant P-values adjusted for multiple testing.
Cytokine/MMP levels in other clinical subsets of NSCLC are presented in Supplementary Table S3. Of note, current smokers had a significantly higher level of cytokines CCL11 (P=0.001), Gro-a (CXCL1) (P=0.001), stromal-derived factor 1 alpha+beta (SDF-1a+b; CXCL12) (P=0.003) and IL-4 (P<0.001) compared with former smokers. Increased C-reactive protein (CRP) levels correlated with higher levels of multiple pro-inflammatory cytokines. The association between CRP, Gro-b (CXCL2) and IL-6 remained statistically significant after correction for multiple testing (P=0.001 and P<0.001, respectively). No significant differences were seen after correction for false discovery rate in NSCLC patients with a previous history of chemotherapy use compared with patients with no prior chemotherapy treatment.
Discussion
In the present study, we have investigated a panel of 57 circulating inflammatory markers consisting of cytokines and MMPs in the serum from NSCLC patients with advanced disease. The cytokines and MMPs were chosen based on previously published reports on potential biomarkers in lung cancer, and they represent a broad spectrum of inflammatory mediators of special interest in the tumour microenvironment. Obtaining peripheral blood samples is easy compared with samples of lung tumours. The concept of revealing essential markers of disease in blood tests have a particular allure in this cohort of advanced-stage NSCLC patients, where life expectancy is short and good quality of life is important.
To the best of our knowledge, this is the first study where COPD patients are chosen as controls in a serum cytokine/MMP analysis in NSCLC patients. Several cytokine/MMP studies have previously been conducted with healthy subjects as controls.15, 17 All of the NSCLC patients included in our study were either former or current smokers, and there were no significant differences in pack years between the NSCLC and COPD cohorts. COPD is an inflammatory disease strongly associated with smoking and is therefore likely to have an effect on the cytokine profile in patients. Higher levels of inflammatory markers have been reported among former and current smokers as well as among those with a history of chronic bronchitis or emphysema, compared with healthy subjects, supporting that notion.13 Respiratory function tests were not a requirement at inclusion in the Thoracal Radiotherapy and Tarceva (ThoRaT) study. Sixteen of the NSCLC patients reported to have COPD (degree unknown) in their medical history at baseline. Owing to the similar smoking histories in both cohorts, it is likely that even more of the NSCLC patients would be suffering from COPD. COPD patients, on the other hand, were followed for a minimum of 2 years after serum sampling, and none of the patients included in theseanalyses developed cancer later. In our study, we aimed at discovering a cytokine and MMP profile in the serum reflecting the malignant disease and not a confounding inflammatory pattern owing to smoking. The different cohorts have a similar background, the use of a highly carcinogenic substance, but only one group of patients developed cancer.
The study investigates serum cytokine profiles in a relatively small cohort of patients with advanced NSCLC. Previous chemotherapy could be a possible confounder in the evaluation of the differences in cytokine distribution between NSCLC and certainly untreated COPD patients. Sixty percent of the NSCLC patients included in the study, however, had not received treatment with chemotherapy prior to inclusion. Moreover, when comparing the cytokine concentration level in NSCLC with and without previous chemotherapy, no significant differences were found.
Many former studies have been conducted with only a few cytokines in parallel in the same individuals, but some have reported on larger panels. Kaminska et al.17 found elevated serum levels of tumour necrosis factor alpha (TNFα), IL-6, IL-8, IL-10, IL-1ra, vascular endothelial growth factor (VEGF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in a study including NSCLC patients of all stages, compared with healthy controls. Elevated levels of IL-6, IL-8 and IL-10 were consistent with our findings, but contrary to their study, we found lower levels of G-CSF and IL-1ra, and no differences in TNFα, VEGF and GM-CSF in comparison with COPD patients. In another study, Barrera et al.15 found higher levels of IL-6, IL-8, IL-12p70, IL-17A and IFNγ comparing an advanced NSCLC cohort with 50% non-smokers, with healthy controls. Again, our results confirm the high levels of IL-6 and IL-8 in NSCLC patients; however, no differences were seen between our cohorts in IL-12p70 or IFNγ concentrations, and IL-17A was found to be lower in our NSCLC group. COPD-associated inflammation can lead to elevated serum levels of multiple cytokines and the different observations with regard to some cytokines between our study and those of Karminska et al.17 and Barrera et al.15 may be explained by our use of COPD controls, rather than healthy individuals.
The concentration of IL-17A in serum was found approximately fourfold lower in NSCLC versus COPD patients in our study. We cannot, from our data set, decode if this finding reflects elevated levels of IL-17A in the COPD cohort or low levels among the NSCLC patients. Based on current knowledge, the first explanation appears more likely, as IL-17 has been found elevated in patients with a range of chronic inflammatory disorders.18 One former study revealed elevated levels of IL-17A in serum collected from COPD patients compared with control groups of both healthy smokers and non-smokers; IL-17A was also seen increased in accordance with advancing COPD stages.19 Studies in NSCLC patients have, contradictory to our results, found elevated IL-17A levels in circulation but then again compared with healthy controls.15, 20 It is known that naive CD4+ TH precursor cells can differentiate into a variety of different TH subsets, including TH1, TH2, TH17 and regulatory T cells.21 TH17 cells are considered the predominant producer of IL-17. Both tumour-suppressing and tumour-promoting functions have been attributed to IL-17A and TH17 cells, and the role of TH17 cells in tumour immunity remains ambiguous.22
The immune system has a pivotal role against cancer. The development of a successful immune response depends on the balance between the TH1 (antitumour) and TH2 responses and on the activity of immune cells that inhibit antitumour responses. T cells producing IL-10, in particular type 1 regulatory (Tr1) T cells and TH2 cells, are considered to be key suppressors of antitumour response and it is previously proposed that TH2-type inflammation facilitates tumour growth.23 It is further known that TH1 cells produce one particular set of cytokines such as IL-2, IFNγ and TNFα while TH2 cells produce others, for example, IL-4, IL-5, IL-6, IL-10 and IL-13.24 In our study, IL-6 and IL-10 concentration in the serum was found to be higher in NSCLC patients, although the differences in IL-10 did not retain significance after correction for multiple testing. IFNγ/IL-10 ratio was nonetheless found reduced in NSCLC patients compared with the COPD group, supporting the notion that the TH1 response in NSCLC patients was turned down, whereas the TH2 response was more prominent. These contrasting immune responses developing in different individuals may carry particular importance for the development of lung cancer. Intriguingly, most heavy smokers do not develop lung cancer in spite of a high mutation load accumulating in the lung tissue. There is increasing evidence suggesting that this resilience is related to the host immune response, known to be particularly potent for controlling tumours with a high mutation load and thus a high neoantigen frequency.25, 26, 27 We hypothesise that the different cytokine profiles identified among NSCLC patients and COPD subjects in our study represent a footprint of tumour-promoting versus tumour-suppressing immune responses developing in the host, in response to mucosal inflammation and mutations induced by smoking. It would be of interest to assess how this cytokine profile is influenced by immunological checkpoint inhibitors or other forms of therapy. Further, it is possible that a pro-cancer cytokine profile precedes the clinical manifestation of NSCLC and can be used for early detection of lung cancer.
The pro-inflammatory cytokine IL-1 has been shown elevated in several cancer types such as breast, colon, melanoma, head and neck as well as lung cancer and is associated with an increase in metastases and poor prognosis.28 We did not observe any difference between serum levels of IL-1b between the NSCLC and COPD cohorts investigated. The NSCLC patients in our study did, however, have a median concentration level of IL-1ra approximately half the value seen in the COPD serum samples. Moreover, the median IL-1ra concentration values were lower in stage IV NSCLC patients compared with patients in stage III, although this did not retain significance after correction for multiple testing. IL-1ra is a naturally occurring IL1 antagonist. In IL-1ra-deficient mice, skin tumours developed more rapidly compared with wild-type mice in a model of IL-1-induced carcinogenesis.29 In lung cancer, elevated levels of IL-1ra in serum have previously been found associated with decreased risk of lung cancer.13 Here we discovered that the IL-1b/IL-1ra ratio was elevated in NSCLC patients compared with COPD patients, indicating that the pro-malignant IL-1b expression level was higher in NSCLC patients relative to the antagonist; the ‘cancer-protective' IL-1ra. Interestingly, therapeutic agents reducing IL-1 activity, such as recombinant IL-1ra, are commercially available for treatment of inflammatory diseases and could be an approach in cancer treatment.30
Several meta-analyses suggest MMPs as prognostic markers in lung cancer, although the results are conflicting.31, 32, 33 Many previous studies of MMPs comparing lung cancer patients with healthy individuals have also shown contradictory results.34 In our study, a higher level of circulating MMP-8 and MMP-12 was revealed, whereas MMP-2 was reduced in NSCLC compared with COPD patients. When excluding patients using steroids from both cohorts, MMP-3 was also found lower in NSCLC patients. In the NSCLC cohort, MMP-3 and MMP-9 levels were elevated in patients using systemic steroids. It is previously shown that MMP-3 can be induced by steroids.35 MMP-9 may also be released from an increasing amount of neutrophils accompanying treatment with steroids. In COPD, tissue destruction and remodeling are important processes where MMPs are likely to have a central part.36 The most likely explanation for MMPs in the pathogenesis of cancer is also through degradation of extracellular matrix enabling tumour cell invasion and metastasis. However, MMPs are also recognised as important participants in the communication between cancer cells and the non-malignant stroma and can modulate most stages of tumour progression.16
Our study suggests that NSCLC is mirrored by a distinct inflammatory signature in the serum. The different cytokine profiles in NSCLC and COPD patients may represent tumour-promoting and tumour-suppressing immune responses. Lack of independent validation remains a limitation in the interpretation of the data as well as the relatively small number of patients. However, the results are encouraging for a follow-up in a larger cohort owing to its possible implications for disease etiology, diagnostics and treatment. There is an increasing interest in the use of radiological screening for early detection of lung cancer. In this setting, serum biomarkers may serve as a cost-effective, high-throughput tool for identifying subjects at risk who may be referred to computed tomographic scans.
Methods
Study population and data collection
The cytokine analyses were performed using serum samples obtained from 43 patients with advanced-stage NSCLC included in an ongoing clinical study (ThoRaT) from December 2011 until June 2015. The patients were referred for palliative radiotherapy. Clinical characteristics of the NSCLC patients were collected from the hospital medical records. Tumours were staged according to the Union for International Cancer Control, Tumour, Node, Metastasis 7. Histopathological evaluations were retrieved from pathology reports.
Blood sample processing
The blood samples from the NSCLC patients were collected at inclusion in the ThoRaT study, prior to radiotherapy. Blood was collected in serum tubes, kept in room temperature appending coagulation and then processed at 2450 g for 15 min within 1 h after sampling. Finally, the samples were transferred in 250 μl aliquots into cryovials and stored at −80 °C until usage.
COPD cohort
Serum samples from 35 patients with COPD were obtained at the Department of Medicine, Vestfold Hospital Trust, Tønsberg, Norway. Clinical information was acquired from the hospital records (Table 1). All COPD patients included were in a regular follow-up and had no sign of lung cancer prior to blood sampling. The patients were also followed for a minimum of 2 years after blood sampling with no sign of cancer. The serum samples in the COPD cohort were preprocessed under strictly defined and equal conditions as the samples obtained from the NSCLC patients, stored at the same site as the NSCLC samples and processed further at the same centre by the same personnel.
Cytokine analyses
Serum concentration levels of the cytokines CCL1/I-309, monocyte chemotactic chemokine-1 (MCP-1) (CCL2), MCP-2 (CCL8), MCP-3 (CCL7), MCP-4 (CCL13), RANTES (CCL5), macrophage inflammatory protein (MIP) 1alpha (CCL3), MIP-1sigma (CCL15), MIP-3alpha (CCL20), MIP-3beta (CCL19), TARC (CCL17), eotaxin (CCL11), eotaxin-2 (CCL24), eotaxin-3 (CCL26), CCL-21, macrophage-derived chemokine (CCL22), myeloid progenitor inhibitory factor 1 (CCL23), thymus expressed cytokine (CCL25), CCL27, growth-regulated protein alpha (Gro-a) (CXCL1), growth-regulated protein beta (Gro-a) (CXCL2), CXCL5, granulocyte chemotactic protein-2 (CXCL6), monokine induced by gamma interferon (CXCL9), interferon gamma induced protein-10 (CXCL10), interferon inducible T-cell alpha chemoattractant (CXCL11), SDF-1a+b (CXCL12), CXCL13, SCYB16 (CXCL16), fractalkine (CX3CL1), IL-1b, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL12p70, IL-16, IL17a, IFNγ, TNFα, TNF-related apoptosis-induced ligand, MIF, G-CSF, GM-CSF, PDGF-BB, VEGF and MMP-1, -2, -3, -7, -8, -9, -12 and -13 were quantified using a multiplex bioassay (BioRad, Hercules, CA, USA) according to the manufacturer's instructions. Concentrations were calculated using an eight-parameter standard curve. Each serum sample was run in duplicates and averaged to calculate the concentrations.
Statistical analyses
Data are reported using descriptive statistics with percentages, means, medians and ranges. Unsupervised hierarchical clustering was performed using Spearman correlation and average linkage with scaled cytokine/MMP values owing to differences in interprotein concentration values. Differences in the clinical groups and between clusters were calculated with two-sided t-tests (assuming equal variance) and chi-squared/Fisher's exact tests for continuous and categorical data, respectively. Non-parametric tests, Mann–Whitney U-test and Kruskal–Wallis test, were used to explore the differences in individual cytokine levels between NSCLC and COPD patients as well as in different clinical subsets. The median difference between serum levels in the two cohorts was utilised to make the volcano plot, also owing to lack of normally distributed data. The Benjamini–Hochberg false discovery rate was used in order to reduce the possibility for significant independent test results by chance alone owing to multiple testing.
Data were analysed using the SPSS software package version 21 (SPSS, Chicago, IL, USA). Hierarchical clustering performed in R version 3.2.2 (R Project for Statistical Computing, Vienna, Austria). Two sided P-values<0.05 were considered statistical significant. Stricter levels of significance due to correction for multiple testing are noted in the text and tables where applicable.
Ethics approval
This study was approved by the regional ethics committee in Norway (reference number 2012/320) and the institutional review board. A written consent was obtained from all the participants in this study.
Acknowledgments
We thank the Department of Cell Therapy, Oslo University Hospital — The Norwegian Radium Hospital, Oslo, Norway for allowing us to use their facility in order to perform the multiplex bioassays.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Clinical and Translational Immunology website (http://www.nature.com/cti)
Supplementary Material
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2014; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc 2008; 5: 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 2010; 17: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration 2011; 81: 265–284. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Hata A, Koh E, Hiroshima K. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg 2014; 62: 415–421. [DOI] [PubMed] [Google Scholar]
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012; 125: 5591–5596. [DOI] [PubMed] [Google Scholar]
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 2014; 27: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes RM, Busund L-T, Kilvær TL, Andersen S, Richardsen E, Paulsen EE et al. The role of tumor infiltrating lymphocytes in development, progression and prognosis of non-small cell lung cancer. J Thorac Oncol 2016; 11: 789–800. [DOI] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev 2010; 21: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Hildesheim A, Engels Ea, Kemp TJ, Park JH et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013; 105: 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodelon C, Polley MY, Kemp TJ, Pesatori aC, McShane LM, Caporaso NE et al. Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann Oncol 2013; 24: 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera L, Montes-Servin E, Barrera A, Ramirez-Tirado La, Salinas-Parra F, Banales-Mendez JL et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann Oncol 2014; 26: 428–435. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141: 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska J, Kowalska M, Kotowicz B, Fuksiewicz M, Glogowski M, Wojcik E et al. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis: M-CSF - an independent prognostic factor. Oncology 2006; 70: 115–125. [DOI] [PubMed] [Google Scholar]
- Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 2012; 181: 8–18. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cheng Z, Liu W, Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD 2013; 10: 459–465. [DOI] [PubMed] [Google Scholar]
- Duan M-C, Han W, Jin P-W, Wei Y-P, Wei Q, Zhang L-M et al. Disturbed Th17/Treg balance in patients with non-small cell lung cancer. Inflammation 2015; 38: 2156–2165. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol 2014; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454: 436–444. [DOI] [PubMed] [Google Scholar]
- Abbas AK, Murphy KM SA. Functional diversity of helper T lymphocytes. Nature 1996; 383: 787–793. [DOI] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med 2006; 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M et al. Interleukin-1β-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res 2007; 67: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev 2010; 29: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q, Wang Q, Zhan P, Peng L, Wei S-Z, Shi Y et al. The role of matrix metalloproteinase 2 on the survival of patients with non-small cell lung cancer: a systematic review with meta-analysis. Cancer Invest 2010; 28: 661–669. [DOI] [PubMed] [Google Scholar]
- Peng W-J, Zhang J-Q, Wang B-X, Pan H-F, Lu M-M, Wang J. Prognostic value of matrix metalloproteinase 9 expression in patients with non-small cell lung cancer. Clin Chim Acta 2012; 413: 1121–1126. [DOI] [PubMed] [Google Scholar]
- Liang Y, Guo S, Zhou Q. Prognostic value of matrix metalloproteinase-7 expression in patients with non-small cell lung cancer. Tumor Biol 2014; 35: 3717–3724. [DOI] [PubMed] [Google Scholar]
- Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol 2013; 34: 2041–2051. [DOI] [PubMed] [Google Scholar]
- Ribbens C, Martin y Porras M, Franchimont N, Kaiser M-J, Jaspar J-M, Damas P et al. Increased matrix metalloproteinase-3 serum levels in rheumatic diseases: relationship with synovitis and steroid treatment. Ann Rheum Dis 2002; 61: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington PTG, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006; 61: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.