Summary
Objectives
To analyse whether the histological subtype of renal cell carcinoma (RCC) impacts survival post-surgical resection in contemporary patients, and if so, whether prognostic significance differs according to type of surgical resection or tumour stage.
Materials and methods
From 2006 to 2014, 2237 patients underwent surgical resection (25% radical nephrectomy [RN], 75% partial nephrectomy [PN]) for non-metastatic RCC at a tertiary referral centre. Estimated survival function curves and Cox regression models evaluated impact of histological subtype on recurrence-free survival (RFS) and overall survival (OS). Interaction analyses tested whether the impact of histological subtype depends on type of surgical resection or tumour stage.
Results
Patients with RCC stage T2 or lower, and those with low-grade conventional clear cell, papillary or chromophobe RCC of any stage had 5-yr RFS probabilities > 90%. Patients with clear cell papillary RCC stage T3 or greater had predicted 5-yr RFS of 81%. However, 5-yr OS probabilities were >94% for clear cell papillary RCC of any stage. High-grade conventional clear cell and papillary RCC stage T2 or lower, low-grade conventional clear cell and chromophobe RCC of any stage conferred 5-yr OS probabilities of > 93%. Unclassified RCC demonstrated the lowest OS probabilities at any stage.
In multivariable analyses, histological subtype impacted RFS (p<0.0001) and OS (p=0.026) following surgical resection, with no differences in this association for RN versus PN (RFS p=0.2, OS p=0.4), and across pathologic stages (RFS p=0.1, OS p=0.3). Compared to low-grade conventional clear cell RCC, chromophobe (hazard ratio [HR] 0.72, 95% confidence interval [CI] 0.30, 1.75) and papillary RCC (HR 0.30, 95% CI 0.09, 0.97) conferred lower risk of recurrence. Chromophobe (HR 0.67, 95% CI 0.30, 1.52) and clear cell papillary RCC (HR 0.91, 95% CI 0.12, 6.78) conferred the lowest risk of all-cause mortality.
Conclusions
In the era of PN for RCC, histological subtype remained a significant predictor of survival, regardless of type of surgical resection or tumour stage.
Keywords: renal cell carcinoma, histological subtype, recurrence, survival
1. Introduction
Pathologic stage, tumour grade, and performance status are the most established prognostic factors in renal cell carcinoma (RCC) (1). The question remains whether histological subtype impacts risk of recurrence or death in cases of RCC treated with surgical resection. Although early studies evaluating the impact of histological subtype yielded conflicting results (2–6), it is usually accepted that conventional clear cell histology portends a worse prognosis (7).
In 2012 the International Society of Urological Pathology (ISUP) consensus conference proposed five new epithelial neoplasias: tubulocystic RCC, acquired cystic disease-associated RCC, clear cell papillary RCC, microphthalmia family translocation RCC, and hereditary leiomyomatosis RCC syndrome-associated tumours (8). Furthermore, the majority of previous studies addressing the impact of the histological subtype of RCC on survival outcomes included mostly patients who underwent radical nephrectomy (RN). However, the role of kidney-sparing surgery has seen great expansion in recent years (9).
In light of these recent developments, it is not well known whether survival outcomes vary according to histological subtype in contemporary patients. A population-based study reported that histological subtype does not confer prognostic value in patients undergoing partial nephrectomy (PN) (10). Conflicting results have suggested that histological assignment allows better prognostic stratification for advanced and high-grade tumours only (3), or, conversely, for low-stage tumours only (5). Here, we evaluate the impact of histological subtype on survival following surgical resection of clinically localised RCC in the era of elective PN.
2. Materials and Methods
2.1 Patients
After obtaining institutional review board approval, we identified 2518 patients who underwent surgical resection of renal cortical tumours at Memorial Sloan Kettering Cancer Center (MSKCC) from 2006 to 2014. Exclusion criteria were age <18 yrs (n = 1), T0 or Tx tumours (n = 2), metastatic disease at diagnosis (n=182), and, to reduce heterogeneity, bilateral tumours or hereditary RCC (n=96), leaving 2237 patients for final analysis. Baseline patient characteristics were abstracted from a prospectively maintained database and included age, gender, body mass index, and American Society of Anesthesiologists (ASA) score.
2.2 Pathology
Pathologic data included tumour and nodal stage according to the 2009 AJCC TNM classification, tumour size, and histological subtype. Histological subtypes according to the Heidelberg classification included conventional clear cell carcinoma, chromophobe carcinoma, papillary carcinoma, and unclassified RCC (11). Conventional clear cell carcinoma was stratified into low-grade (grade 1-2) conventional clear cell carcinoma, and high-grade (grade 3-4) conventional clear cell carcinoma to take into account the prognostic value of tumour grade. Tumour grade was not incorporated into analyses of non-conventional clear cell RCC because it is not routinely assigned for these tumours at our institution (4). In addition, clear cell papillary carcinoma, the most common of the newly recognised histological subtype (8), was added as a prognostic category. The rarer RCC variants had too few cases to be analysed separately and were therefore reported together as “other histology”; these included collecting duct, medullary, mucinous tubular and spindle cell, tubulocystic, acquired cystic disease-associated and microphthalmia family translocation. We chose not to differentiate between papillary RCC type 1 and type 2 in the analyses because the distinction was not available for all patients. Furthermore, the clinical significance of sub typing papillary RCC has been questioned recently (12).
2.3 Outcome measures
Patients were typically followed every 6 months for 3 years, and annually thereafter. Surveillance included patient history, physical examination, comprehensive metabolic panel, abdominal computed tomography or ultrasound, and chest radiography. Recurrence information was based on clinical and radiologic findings, and categorised as the first evidence of local recurrence or distant relapse. New tumours on the contralateral side were not considered recurrences. Death was documented according to medical records or death certificates. For patients who were followed up outside our institution, there was regular correspondence with their physician to ensure that recurrences and deaths were recorded. In cases where 14 months passed without a report from the physician, we wrote to the patient to request information (reply rate approximately 40%).
2.4 Statistical analyses
The Chi-square test and Mann-Whitney U test were used to compare baseline variables between RN and PN patients. Estimated survival function curves were created, stratified by histological subtype and adjusted for age, gender, type of surgical resection, pathologic stage, nodal stage, tumour size, and, for the outcome of overall survival (OS), ASA score. We then aimed to determine whether histological subtype was predictive of recurrence-free survival (RFS) or OS after surgical resection, and whether its predictive value differed between patients who had undergone RN versus PN, or based on pathologic stage. To this end, we used Cox regression models adjusting for age, gender, type of surgical resection, pathologic stage (T1/T2 or T3/T4), nodal stage (N0/Nx or N+), tumour size (cm), and histological subtype (low-grade conventional clear cell, high-grade conventional clear cell, chromophobe, papillary, clear cell papillary, or unclassified). Of the 2237 patients, 39 (1.7%) with rare variants that were classified as “other histology” were excluded from these models. As mentioned above, it was unclear before beginning this analysis whether histological subtype impacted survival differently according to type of surgical resection (RN versus PN) (10) or tumour stage (3,5). Therefore, interaction terms between either type of surgical resection and histological subtype or between pathologic stage and histological subtype were included in the Cox regression models. For OS, models were generated with the addition of ASA score as a covariate. All analyses were performed using Stata 13 (StataCorp, College Station, TX). A two-sided p value < 0.05 was considered significant.
3. Results
Of 2237 patients, 551 (25%) underwent RN and 1686 (75%) underwent PN (Table 1). Clear cell papillary carcinoma comprised 1.8% of all tumours. As expected, patients undergoing RN had larger tumours and were more likely to have more advanced pathologic stage (all p<0.0001).
Table 1.
Clinicopathologic characteristics for patients undergoing radical nephrectomy (RN) or partial nephrectomy (PN) for renal cell carcinoma (RCC).
| All patients (N=2237) | RN (N=551; 25%) | PN (N=1686; 75%) | p value | |
|---|---|---|---|---|
| Age, yrs, median (IQR) | 60 (61, 68) | 62 (52, 69) | 60 (51, 68) | 0.004 |
| Female, n (%) | 737 (33) | 182 (33) | 555 (33) | >0.9 |
| BMI, median (IQR) (n=2193) | 29 (26, 33) | 29 (26, 33) | 29 (26, 33) | 0.8 |
| ASA score, n (%) (n=2236) | ||||
| I/II | 993 (44) | 221 (40) | 772 (46) | 0.0001 |
| III/IV | 1243 (56) | 330 (60) | 913 (54) | |
| Histological subtype, n (%) | ||||
| Low-grade conventional clear cell | 711 (32) | 96 (17) | 615 (36) | <0.0001 |
| High-grade conventional clear cell | 764 (34) | 316 (57) | 448 (27) | |
| Chromophobe | 243 (11) | 58 (11) | 185 (11) | |
| Papillary | 311 (14) | 36 (7) | 275 (16) | |
| Clear cell papillary | 41 (1.8) | 5 (0.9) | 36 (2.1) | |
| Unclassified | 128 (5.7) | 27 (4.9) | 101 (6.0) | |
| Other | 39 (1.7) | 13 (2.4) | 26 (1.5) | |
| Pathologic T stage, n (%) | ||||
| T1 | 1574 (70) | 136 (25) | 1438 (85) | <0.0001 |
| T2 | 101 (4.5) | 71 (13) | 30 (1.8) | |
| T3 | 554 (25) | 338 (61) | 216 (13) | |
| T4 | 8 (<1) | 6 (1.1) | 2 (0.1) | |
| Nodal stage, n (%) | ||||
| Nx | 1578 (71) | 124 (23) | 1454 (86) | <0.0001 |
| N0 | 625 (28) | 398 (72) | 227 (13) | |
| N1 | 11 (0.5) | 9 (1.6) | 2 (0.1) | |
| N2 | 23 (1.0) | 20 (3.6) | 3 (0.2) | |
| Tumour size, cm, median (IQR) (n=2943) | 3.5 (2.3, 5.4) | 7.2 (5.0, 9.8) | 3.0 (2.0, 4.0) | <0.0001 |
IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists. Percentages may not sum due to rounding.
In our cohort, 149 patients experienced recurrence during follow-up and 150 patients died from any cause. Median follow-up among survivors was 2.8 yrs (interquartile range 1.1 – 5.0). Overall, RFS and OS estimates at 5 years were 92% (95% confidence interval [CI] 90 – 93%) and 90% (95% CI 88 – 91%), respectively. RFS and OS curves stratified by histological subtype and adjusted for age, gender, type of surgical resection, pathologic stage, nodal stage, and tumour size, are depicted in Figure 1. Survival curves stratified by histological subtype and by stage (T1/T2 versus T3/T4) are depicted in Figure 2. Corresponding RFS and OS probabilities at 5 years after surgical resection are shown in Table 2. Patients with RCC stage T2 or lower, and those with low-grade conventional clear cell, papillary or chromophobe RCC of any stage had 5-yr RFS probabilities > 90%. Patients with clear cell papillary RCC stage T3 or greater had the highest probability of recurrence, with predicted 5-yr RFS of 81% (Table 2A). However, 5-yr OS probabilities were >94% for clear cell papillary RCC of any stage (Table 2B). Furthermore, high-grade conventional clear cell and papillary RCC stage T2 or lower, low-grade conventional clear cell and chromophobe RCC of any stage conferred 5-yr OS probabilities of > 93%. Unclassified RCC demonstrated the lowest OS probabilities at any stage.
Figure 1.

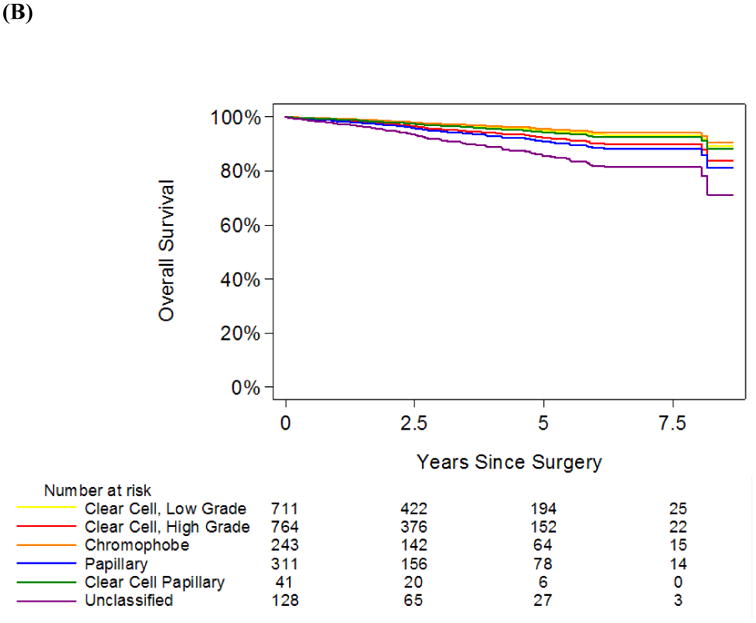
A, Recurrence-free survival and B, overall survival estimates, stratified by histological subtype and adjusted for age, gender, ASA score (for overall survival), type of surgical resection, pathologic stage, nodal stage, and tumour size. The yellow line is low-grade conventional clear cell carcinoma, the red line is high-grade conventional clear cell carcinoma, the orange line is chromophobe carcinoma, the blue line is papillary carcinoma, the green line is clear cell papillary carcinoma, and the purple line is unclassified carcinoma.
Figure 2.
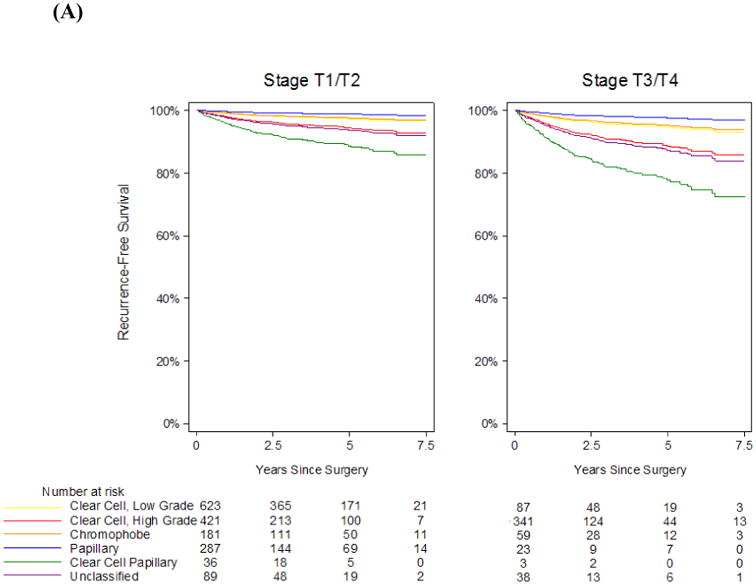

A, Recurrence-free survival and B, overall survival estimates stratified by histological subtype and pathologic stage, and adjusted for age, gender, ASA score (for overall survival), type of surgical resection, and tumour size. The yellow line is low-grade conventional clear cell carcinoma, the red line is high-grade conventional clear cell carcinoma, the orange line is chromophobe carcinoma, the blue line is papillary carcinoma, the green line is clear cell papillary carcinoma, and the purple line is unclassified carcinoma.
Table 2.
A, Recurrence-free survival and B, overall survival estimates at 5 years after surgical resection for renal cell carcinoma (RCC), adjusted for age, gender, type of surgical resection, pathologic stage, tumour size, and histological subtype.
| (A) | ||
|---|---|---|
| T1 and T2, % (95% CI) | T3 and T4, % (95% CI) | |
| All | 97.0 (95.7-97.9) | 93.8 (90.5-95.9) |
| Low-grade conventional clear cell | 97.5 (95.7-98.5) | 94.8 (90.7-97.1) |
| High-grade conventional clear cell | 93.8 (91.0-95.8) | 87.5 (82.0-91.4) |
| Chromophobe | 98.2 (96.1-99.1) | 96.2 (91.7-98.3) |
| Papillary | 99.2 (97.8-99.7) | 98.4 (94.9-99.5) |
| Clear cell papillary | 90.5 (67.0-97.6) | 81.2 (42.1-95.1) |
| Unclassified | 94.7 (89.8-97.2) | 89.1 (79.1-94.5) |
| (B) | ||
| T1 and T2, % (95% CI) | T3 and T4, % (95% CI) | |
| All | 94.7 (93.0-96.0) | 91.3 (87.6-93.9) |
| Low-grade conventional clear cell | 96.2 (94.0-97.6) | 93.8 (89.6-96.3) |
| High-grade conventional clear cell | 94.7 (91.8-96.5) | 91.3 (86.6-94.4) |
| Chromophobe | 97.4 (94.6-98.8) | 95.8 (90.8-98.1) |
| Papillary | 94.0 (90.3-96.3) | 90.2 (82.7-94.6) |
| Clear cell papillary | 96.5 (77.3-99.5) | 94.3 (64.8-99.2) |
| Unclassified | 90.5 (83.5-94.7) | 84.7 (73.2-91.6) |
CI, confidence interval.
Using a Cox regression model adjusted for age, gender, type of surgical resection, pathologic stage, nodal stage, and tumour size, histological subtype was found to be a significant predictor of RFS (p<0.0001 for all histological subtypes) and OS (p=0.026 for all histological subtypes) (Table 3). Overall, chromophobe and papillary RCC conferred the best prognosis regarding risk of recurrence. Chromophobe and clear cell papillary RCC conferred the best prognosis regarding risk of all-cause mortality.
Table 3.
Multivariable Cox model for the association between histological subtype and A) recurrence, B) all-cause mortality.
| (A) | ||||
|---|---|---|---|---|
| Variable | HR | 95% CI | p value | |
| Age (per 10 years) | 1.19 | 1.03, 1.39 | 0.02 | |
| Female gender | 0.60 | 0.40, 0.91 | 0.02 | |
| Partial nephrectomy | 0.93 | 0.56, 1.53 | 0.8 | |
| Pathologic T3/T4 | 2.09 | 1.32, 3.33 | 0.002 | |
| Nodal stage N+ | 6.70 | 3.87, 11.60 | <0.0001 | |
| Tumour size (cm) | 1.24 | 1.17, 1.31 | <0.0001 | |
| Histological subtype | Low-grade conventional clear cell | Ref. | - | <0.0001 |
| High-grade conventional clear cell | 2.50 | 1.41, 4.43 | ||
| Chromophobe | 0.72 | 0.30, 1.75 | ||
| Papillary | 0.30 | 0.09, 0.97 | ||
| Clear cell papillary | 3.89 | 0.88, 17.1 | ||
| Unclassified | 2.15 | 0.96, 4.81 | ||
| (B) | ||||
| Variable | HR | 95% CI | p value | |
| Age (per 10 years) | 1.66 | 1.40, 1.96 | <0.0001 | |
| Female gender | 0.49 | 0.32, 0.74 | 0.001 | |
| Partial nephrectomy | 0.43 | 0.28, 0.68 | <0.0001 | |
| ASA score III/IV | 1.34 | 0.93, 1.92 | 0.1 | |
| Pathologic T3/T4 | 1.67 | 1.11, 2.50 | 0.01 | |
| Nodal stage N+ | 5.08 | 2.84, 9.10 | <0.0001 | |
| Tumour size (cm) | 1.04 | 0.98, 1.10 | 0.2 | |
| Histological subtype | Low-grade conventional clear cell | Ref. | - | 0.026 |
| High-grade conventional clear cell | 1.42 | 0.87, 2.30 | ||
| Chromophobe | 0.67 | 0.30, 1.52 | ||
| Papillary | 1.60 | 0.89, 2808 | ||
| Clear cell papillary | 0.91 | 0.12, 6.78 | ||
| Unclassified | 2.58 | 1.34, 4.95 | ||
HR, hazard ratio; CI, confidence interval.
We then assessed whether the predictive value of histological subtype for RFS was different for patients who underwent RN versus PN by adding an interaction term between type of surgical resection and histological subtype. There was no evidence of an interaction between type of surgical resection and histological subtype (p=0.2). Similarly, we found no evidence of an interaction between type of surgical resection and histological subtype for OS (p=0.4).
Next, we tested whether the predictive value of histological subtype for RFS or OS was different based on pathologic stage, using an interaction term between pathologic stage and histological subtype in the Cox regression model. There was no significant interaction between histological subtype and pathologic stage for RFS (p = 0.1) or for OS (p=0.3).
4. Discussion
Due to widespread use of modern imaging techniques and possibly the continuing epidemic of obesity (13), the incidence of RCC is increasing with the majority of newly diagnosed patients presenting with low-stage disease (14). The downward stage migration of RCC, together with a better understanding of the functional advantages of kidney-sparing surgery, have bolstered the role of PN in recent years (9). Furthermore, additional histological subtypes have been recognised in recent years as distinct epithelial tumours within the ISUP Vancouver classification of renal neoplasia (8). In this evolving context of RCC, it remains essential to determine whether histological subtype retains prognostic value in a cohort of contemporary patients. From our study, histological subtype remains an important predictor of RFS and OS in a large cohort of patients undergoing primarily PN for clinically localised RCC.
To date, reports evaluating the impact of histology on RCC outcomes utilising multivariable analyses have documented conflicting results (2–6). Patard et al studied 4063 patients with RCC treated surgically at eight academic centres (six of them European) and found that histological subtype predicts cancer-specific survival in univariate analysis, but not in multivariable analysis (2). Similarly, two studies including a total of 1120 European patients documented that histological subtype was not an independent predictor of survival (3,6). These results were contradicted by later studies from American centres (4,5) including the Mayo Clinic and MSKCC where histological subtype was shown to be an independent predictor of outcome. Specifically, conventional clear cell RCC was associated with a significantly higher risk of metastasis and cancer-specific death compared to chromophobe and papillary RCC. The above-mentioned studies, however, included predominantly patients who underwent RN and may not reflect the current paradigm shift towards PN. It is also noteworthy that conventional clear cell carcinoma represented 66% of all cases in our study, while the proportion ranged from 79% to 89% in other series (2,3,5,6). This difference could represent increased appreciation of the renal histological subtypes on the part of our pathologists. Finally, the majority of these studies included patients with metastatic disease at presentation (2,3,5,6), which limits definitive conclusions for patients who undergo surgical resection with curative intent.
In the current study, histological subtype did not predict either RFS or OS differently for patients who underwent RN versus PN. In a Surveillance, Epidemiology, and End Results-based study, Crépel et al evaluated patients who underwent PN from 1988 and 2004 and found no difference in cancer-specific survival based upon histological subtype (10). However, this population-based study most likely included patients for whom PN had imperative indications, precluding comparison with our cohort. Importantly, subtypes other than conventional clear cell carcinoma were underrepresented in the Crépel study, comprising only 15% of all cases, in contrast to 34% in the current study. Furthermore, we documented that histological subtype did not predict either RFS or OS differently based on pathologic stage. It has been suggested that smaller tumours exhibit slow growth rates and reduced propensity to metastasise (15), thus raising the question whether biologic behaviour differs between low-stage and high-stage tumours, even within a specific histological subtype. This is exemplified by the study by Ficarra et al, in which histological subtype allowed better prognostic stratification only for locally advanced and high grade tumours (3). In contrast, Leibovich et al found that the impact of histological subtype was stronger in low-stage tumours when comparing conventional clear cell, papillary and chromophobe RCC (5). However, despite the inclusion of patients treated from 1970 to 2003, their series included a higher proportion of low-stage patients than ours, and, as admitted by the authors, was likely underpowered to detect survival differences for high-stage, high-grade patients.
The studies mentioned above did not elaborate on clear cell papillary RCC, one of the new entities incorporated into the ISUP Vancouver classification of renal neoplasia (8). As this study confirms, this tumour is the most common of the newly recognised histological subtypes, comprising 1.8% of all cases in our cohort. Clear cell papillary RCC was first reported in 2000 as renal angiomyoadenomatous tumour (16) and later at our institution in patients with end-stage kidney disease (17), but most cases actually occur in kidneys without any underlying intrinsic renal disease. It consists of variable papillary structures lined by cells with clear cytoplasm and low grade nuclei with a characteristic linear arrangement away from the basal aspect of cells (17). Despite its denomination, clear cell papillary RCC is genetically distinct from either conventional clear cell or papillary RCC (18,19). There is limited information regarding its clinical behaviour, especially for higher stage tumours, which have not been reported previously (20–22). In this study we noted that patients with clear cell papillary RCC stage T1 and T2 had a relatively indolent clinical course. Patients with T3 or T4 clear cell papillary RCC had the lowest RFS at 5 yrs (81%), yet 5-yr OS probability was excellent (94%). The discrepancy may be related to the relatively low number of patients in our cohort with clear cell papillary RCC, as evidenced by the wide CIs for RFS and OS probabilities, which may not allow for robust conclusions.
The present results also address the role of PN in case of papillary RCC. Papillary RCC is characterised by a higher risk of multifocality (up to 41%) (23), a point that has been raised to argue against kidney-sparing surgery in the presence of biopsy-proven papillary RCC. Our data, however, do not suggest that PN jeopardises survival in patients with papillary RCC, which is in agreement with findings from the study by Crépel et al (10).
Limitations of our analysis include a lack of consistency in diagnosis, sub classification, and staging inherent to a prospective study. However, pathologic specimens were assessed by urological pathologists accustomed to evaluating malignancy and experienced in the ISUP Vancouver classification of renal neoplasia (8). Furthermore, although regular correspondence takes place with all our patients, it is possible that we did not capture all events that occurred after hospital discharge. Follow-up was relatively short. These limitations notwithstanding, our study provides helpful insights into the impact of histological subtype in a large contemporary cohort of patients, using the most recent classification system of RCC. Modern genomic techniques have confirmed the genetic distinction between histological subtypes (24). A deeper understanding of genetic events and molecular pathways underlying the differences in RCC phenotypes would be highly relevant to the effective development of future targeted and individualised therapies. Currently, evidence is lacking to support the present targeted treatments in non-conventional clear cell RCC (25). Our findings also provide an evidence-based justification for the stratification of patients according to histological subtype in current adjuvant therapy trials (26).
5. Conclusion
In contemporary patients treated by surgical resection for RCC, histological subtype remained a significant predictor of RFS and OS, regardless of type of surgical resection or pathologic stage. Further research exploring the mechanisms underlying these differences may lead to the development of novel, individualised therapies.
Highlights.
The histologic subtype of renal cell carcinoma remains a predictor of survival
The predictive value of histologic subtype is independent of the surgical technique
The predictive value of histologic subtype is independent of the pathologic stage
Acknowledgments
Daniel P. Nguyen is a research fellow and is supported by research grants from these organisations: Nuovo-Soldati Foundation, Arnold U. und Susanne Huggenberger-Bischoff Foundation, Gottfried und Julia Bangerter-Rhyner Foundation, and the Swiss Urological Association. Funding for statistical support on this paper was provided by U.S. National Institutes of Health Cancer Center Support Grant P30 CA008748 to PI: Craig B. Thompson, MD
The authors thank MSKCC editor Amy Plofker for her review of the manuscript.
Footnotes
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–61. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 2.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra V, Martignoni G, Galfano A, et al. Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol. 2006;50:786–94. doi: 10.1016/j.eururo.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Teloken PE, Thompson RH, Tickoo SK, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol. 2009;182:2132–6. doi: 10.1016/j.juro.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309–16. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Gudbjartsson T, Hardarson S, Petursdottir V, et al. Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nationwide study of 629 patients. Eur Urol. 2005;48:593–600. doi: 10.1016/j.eururo.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490–504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 8.Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013;37:1469–89. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 9.Touijer K, Jacqmin D, Kavoussi LR, et al. The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur Urol. 2010;57:214–22. doi: 10.1016/j.eururo.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Crépel M, Isbarn H, Capitanio U, et al. Does histologic subtype affect oncologic outcomes after nephron-sparing surgery? Urology. 2009;74:842–5. doi: 10.1016/j.urology.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–3. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Sukov WR, Lohse CM, Leibovich BC, Thompson RH, Cheville JC. Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J Urol. 2012;187:54–9. doi: 10.1016/j.juro.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 13.Navai N, Wood CG. Environmental and modifiable risk factors in renal cell carcinoma. Urol Oncol. 2012;30:220–4. doi: 10.1016/j.urolonc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 15.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michal M, Hes O, Havlicek F. Benign renal angiomyoadenomatous tumor: A previously unreported renal tumor. Ann Diagn Pathol. 2000;4:311–5. doi: 10.1053/adpa.2000.17890. [DOI] [PubMed] [Google Scholar]
- 17.Tickoo SK, dePeralta-Venturina MN, Harik LR, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. 2006;30:141–53. doi: 10.1097/01.pas.0000185382.80844.b1. [DOI] [PubMed] [Google Scholar]
- 18.Rohan SM, Xiao Y, Liang Y, et al. Clear-cell papillary renal cell carcinoma: molecular and immunohistochemical analysis with emphasis on the von Hippel-Lindau gene and hypoxia-inducible factor pathway-related proteins. Mod Pathol. 2011;24:1207–20. doi: 10.1038/modpathol.2011.80. [DOI] [PubMed] [Google Scholar]
- 19.Deml KF, Schildhaus HU, Compérat E, et al. Clear cell papillary renal cell carcinoma and renal angiomyoadenomatous tumor. Two variants of a morphologic, immunohistochemical, and genetic distinct entity of renal cell carcinoma. Am J Surg Pathol. 2015;39:889–901. doi: 10.1097/PAS.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexiev BA, Drachenberg CB. Clear cell papillary renal cell carcinoma: Incidence, morphological features, immunohistochemical profile, and biologic behavior: A single institution study. Pathol Res Pract. 2014;210:234–41. doi: 10.1016/j.prp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Williamson SR, Eble JN, Cheng L, Grignon DJ. Clear cell papillary renal cell carcinoma: differential diagnosis and extended immunohistochemical profile. Mod Pathol. 2013;26:697–708. doi: 10.1038/modpathol.2012.204. [DOI] [PubMed] [Google Scholar]
- 22.Aydin H, Chen L, Cheng L, et al. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 2010;34:1608–21. doi: 10.1097/PAS.0b013e3181f2ee0b. [DOI] [PubMed] [Google Scholar]
- 23.Méjean A, Hopirtean V, Bazin JP, et al. Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol. 2003;170:764–7. doi: 10.1097/01.ju.0000081122.57148.ec. [DOI] [PubMed] [Google Scholar]
- 24.Bielecka ZF, Czarnecka AM, Szczylik C. Genomic analysis as the first step toward personalized treatment in renal cell carcinoma. Front Oncol. 2014;4:194. doi: 10.3389/fonc.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol. 2014;67:740–9. doi: 10.1016/j.eururo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Pal SK, Haas NB. Adjuvant therapy for renal cell carcinom a: past, present, and future. Oncologist. 2014;19:851–9. doi: 10.1634/theoncologist.2014-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]


