Abstract
Treating to target by monitoring disease activity and adjusting therapy to attain remission or low disease activity has been shown to lead to improved outcomes in chronic rheumatic diseases such as rheumatoid arthritis and spondyloarthritis. Patient-reported outcomes, used in conjunction with clinical measures, add an important perspective of disease activity as perceived by the patient. Several validated PROs are available for inflammatory arthritis, and advances in electronic patient monitoring tools are helping patients with chronic diseases to self-monitor and assess their symptoms and health. Frequent patient monitoring could potentially lead to the early identification of disease flares or adverse events, early intervention for patients who may require treatment adaptation, and possibly reduced appointment frequency for those with stable disease. A literature search was conducted to evaluate the potential role of patient self-monitoring and innovative monitoring of tools in optimising disease control in inflammatory arthritis. Experience from the treatment of congestive heart failure, diabetes and hypertension shows improved outcomes with remote electronic self-monitoring by patients. In inflammatory arthritis, electronic self-monitoring has been shown to be feasible in patients despite manual disability and to be acceptable to older patients. Patients' self-assessment of disease activity using such methods correlates well with disease activity assessed by rheumatologists. This review also describes several remote monitoring tools that are being developed and used in inflammatory arthritis, offering the potential to improve disease management and reduce pressure on specialists.
Keywords: Rheumatoid Arthritis, Patient perspective, Disease Activity
Key messages.
What is already known about this subject?
Treating to target in chronic rheumatic diseases such as rheumatoid arthritis and spondyloarthritis by monitoring disease activity and adjusting therapy to attain remission or low disease activity has been shown to lead to improved outcomes.
Patient-reported outcomes add the patient's perspective of disease activity to that of clinical measures.
What does this study add?
Diseases outside of inflammatory arthritis show improved outcomes with remote self-monitoring by patients.
Frequent remote patient monitoring could lead to early identification of disease flares, early intervention for patients requiring treatment adaptation or reduced appointment frequency for stable patients.
How might this impact on clinical practice?
There are various remote monitoring tools for inflammatory arthritis in use or being developed with the potential to help improve disease management.
Introduction
A tight control or treat-to-target management strategy has become the standard of care for rheumatic diseases such as rheumatoid arthritis (RA) and spondyloarthritis (SpA), including ankylosing spondylitis (AS) and psoriatic arthritis (PsA). Integral to the principle of treating to target is that disease activity is measured on a regular basis and therapy is adjusted accordingly to achieve a target agreed by the physician and the patient.1 2 Targeting low-disease activity or remission in the management of RA is part of the European League Against Rheumatism (EULAR) recommendations and, as has previously been widely reported (eg, the DREAM, TICORA and CAMERA studies), this has been shown to lead to improved outcomes.3–7 In a recent study, patients with RA who achieved guideline-recommended low disease activity (Disease Activity Score (DAS)28-CRP <2.6) used fewer healthcare resources, including a 36–45% lower hospital admission rate (p<0.05), compared with patients who did not achieve target disease activity levels.8 In outpatient clinics that monitor patients using outcome measures as standard practice, ∼75% of patients with RA have been reported to be in remission or in low disease activity.9 Furthermore, patients in remission who are used to modern technology have started to request the possibility of reporting their disease status by using their personal technology devices, for example, home PCs.
The treat-to-target strategy for RA was originally adopted from the treatment of hypertension and diabetes, where it resulted in considerable improvements in outcomes, however, with the difference that hypertension and diabetes are diseases not providing the patient with immediate alerts, namely with pain. Treat-to-target strategy in inflammatory arthritis usually uses clinical measurements of disease activity such as DAS28 or Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). While EULAR recommends the use of composite indices to assess disease activity, the American College of Rheumatology (ACR) recommends both composite indices or patient-driven composite tools (PAS, PAS-II or Routine Assessment of Patient Index Data (RAPID) 3).10 As such, in addition to clinical disease activity measures, the patient's perspective of disease state and burden is increasingly being recognised as an important consideration,11–13 and the most recent ACR guidelines refer to the use of patient-reported outcomes (PROs) as activity measures.14 The US Food and Drug Administration defines a PRO as: “any report of the status of a patient's health condition that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else. The outcome can be measured in absolute terms (eg, severity of a symptom, sign or state of a disease) or as a change from a previous measure”.15 PROs for inflammatory arthritis have been developed and validated to correlate closely with clinical measures of disease activity. PROs have been described as ‘critical, relevant and complementary’ in the context of the physician–patient interaction.16 It is of fundamental importance for the treating physician to have PRO information before making decisions on treatment and/or new interventions.16 Pincus et al17 have shown that the combination of the three PRO measures from the seven ACR core data set measures is as informative as the ACR20 responses and DAS scores in distinguishing between placebo and effective treatment. The Rheumatoid Arthritis Disease Activity Index (RADAI)-5 PRO showed a similar outcome concerning remission rates as the Simple Disease Activity Index (SDAI)-remission and even has a higher sensitivity to indicate the 2011 EULAR/ACR Boolean remission criteria than the SDAI-remission criterion.18 Thus, PROs could act as a surrogate for clinical measurement and enable remote monitoring of patients with RA.
In many other disease areas, such as diabetes, hypertension and congestive heart failure, patient self-monitoring is a well-accepted and common practice in supporting a tight control strategy. For example, among patients with hypertension at high risk of cardiovascular disease, self-monitoring with self-titration of antihypertensive medication resulted in a 9.2 mm Hg lower systolic blood pressure at 12 months compared with usual care.19 Advances in e-health tool technology are helping patients with chronic diseases to self-monitor and assess their symptoms and health, facilitating the incorporation of routine collection of PROs into clinical practice.20
Need for frequent patient monitoring in inflammatory arthritis
The consequences of poor control of inflammatory arthritis include swollen and painful joints, irreversible joint damage, functional disability, decreased work productivity, sleep disturbance and a reduced ability of patients to lead a normal active life. In addition to advocating treat-to-target strategy, the EULAR treatment recommendations suggest that clinic visits should be scheduled every 1–3 months when treating rheumatic disease with biologics.3 However, this frequency of visits may not always be possible due to specific barriers such as geographical and health-system-related constraints and, even in the case of high-quality care, patients' lives may not be predictable due to disease activity fluctuations.21
Frequent remote patient monitoring could potentially lead to the early identification of disease flares, prioritisation of patients who may require a treatment review, and possibly reduced appointment frequency for those with stable disease. Evidence has shown that fluctuations in disease activity do have a direct effect on the destruction of joints.22 Therefore, after the newly diagnosed patient has reached a state of remission or low disease activity, there might be a potential benefit of remote monitoring carried out in between regular scheduled clinic visits to ensure that disease activity remains tightly controlled. Remote patient monitoring may also reduce the number of visits to the physician's office and be more convenient for many patients, especially those who are functionally incapacitated or who live far away from the nearest rheumatology clinic.23
There is evidence to support a correlation between higher patient engagement in their treatment and improved adherence to therapy.24 Self-monitoring by patients is one method that can potentially increase engagement with their treatment. Self-monitoring may also lead to more consistent reporting in the long term, as outcomes are reported by the same person over time. While it is generally acknowledged that PROs are a subjective measurement, patients are best placed to provide evaluations of their pain and global estimates of well-being.
In summary, self-monitoring of PROs by patients may lead to improved disease control, potential early identification of disease flares and improved convenience for patients over clinic visits. Patients may also be more engaged in their treatment and improve their adherence to therapy.
Aims
This review considers the role of PROs and patient self-monitoring in inflammatory arthritis. A search of the literature was conducted to look at the potential of electronic patient monitoring tools to support this role. The authors' personal experiences of certain tools are included, as well as examples of electronic monitoring tools currently in use from disease areas other than inflammatory arthritis.
Literature search methodology
Patient self-monitoring and remote monitoring with innovative monitoring tools is a well-accepted and common practice in several chronic disease areas. A literature search was conducted to evaluate the potential role of patient self-monitoring and innovative monitoring tools in optimising disease control in inflammatory arthritis. Readers should note that this was a narrative review and the methods of the review are explained below.
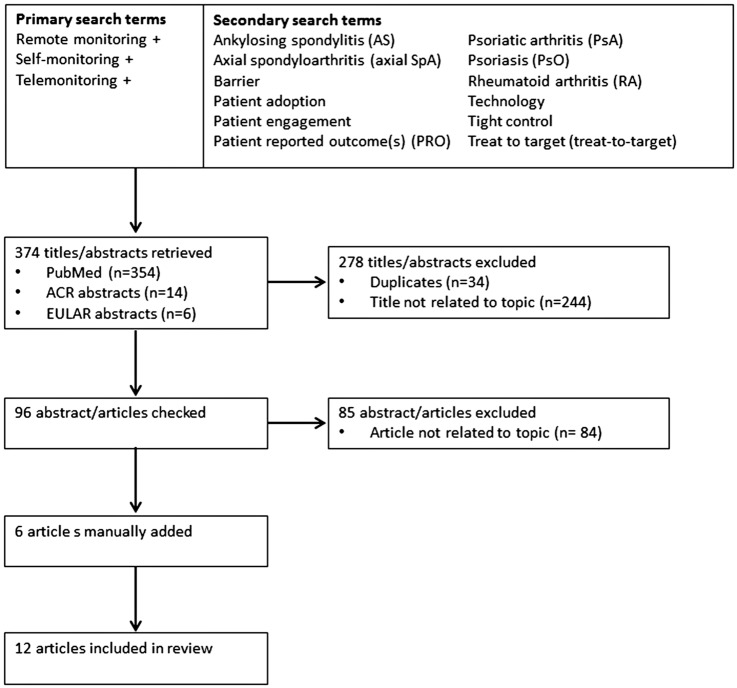
The following databases were searched: PubMed (January 2000 to June 2015), accepted abstracts from ACR and EULAR annual congresses (ACR 2012–2014 and EULAR 2011–2015). Figure 1 shows the search terms used. Searches were performed using a combination of a single primary search term in conjunction with each secondary term (primary term AND secondary term). Relevance to the topic was determined by scanning the title and, where available, the abstract of the retrieved articles. Hits were collated and manually de-duplicated.
Figure 1.
Literature search methodology and results flow diagram. ACR, American College of Rheumatology; EULAR, European League Against Rheumatism.
Examples that have been drawn from other disease areas were gathered from the authors' experience in order to add a wider healthcare context outside of rheumatology.
Literature search results
The literature search retrieved 374 titles/abstracts (354 titles from PubMed, 14 ACR abstracts, 6 EULAR abstracts), of which 278 were excluded by the initial scan and collation (34 duplicates, 244 titles not related to topic). The remaining 96 abstracts/articles were checked for relevance; 85 were excluded as not related to the topic, leaving 11 articles.20 23 25–33 The findings of the initial literature search were reviewed and the authors suggested additional articles for inclusion that were not found as a result of search terms or human error of the manual searching of databases. After manual addition of these 6 articles,8 9 34–37 17 articles were selected for inclusion in the review (see online supplementary appendix A and figure 1). Key evidence from the literature search is summarised below.
rmdopen-2016-000302supp_appendix.pdf (275.1KB, pdf)
Discussion—literature search findings
Innovative electronic remote monitoring and PRO reporting solutions could enable better data capture, easier incorporation of data into electronic medical records, and more frequent monitoring of disease activity in patients with RA between clinic visits.23 Remote data collection offers the additional advantage of convenience to patients, especially those who are functionally incapacitated or who live far away from the nearest rheumatology clinic, as the data can be collected at home. Remote monitoring and reporting of PROs may facilitate a treat-to-target approach and help to achieve a low disease activity state or remission among patients with RA.23 Results from a 2004 survey of 135 US physicians indicated that a large majority (83%) thought that remote patient monitoring would prove beneficial for the healthcare industry.33 Their main concerns related to the privacy of medical information on the internet and the security of online transactions.
Specific data on the use of patient-led remote PRO monitoring tools in inflammatory arthritis appear to be relatively sparse based on the search criteria employed in the current review. This apparent knowledge gap suggests that research into the utility of electronic PRO reporting tools in inflammatory arthritis is warranted. Areas to be investigated include: any differences in long-term outcomes in patients remotely monitoring PROs; patient satisfaction after long-term use of such a remote monitoring tool; any difference in number of clinic visits or healthcare resource usage among patients using remote monitoring tools versus those not; any difference in cost of treatment; potential barriers to implementation of such tools.
Potential for remote monitoring of inflammatory diseases
Electronic remote monitoring tools for inflammatory rheumatic diseases offer additional data to support clinical decision-making, may improve the quality of care by effective patient communication and contribute to empowerment of patients.32 The use of electronic remote monitoring tools to support tight control in such diseases is of great interest to rheumatologists, given the need for tight disease control to prevent symptoms, avoid joint damage and recognise complications early.
Technology for remote monitoring should be simple and practical to use. In addition, monitoring systems should be automated where possible in order to spare staff resources. There is a concern that use of IT applications by patients with RA may be limited by their age and manual disability. However, a recent study found that manual disability in patients with RA is not an obstacle for using mobile applications.26 The mobile application for smartphones that was tested comprised a simple questionnaire over four screens. Fifteen patients with RA with an average age of 63±10 years completed the questionnaire twice, taking 91±23 s the first time and 49±20 s the second time. All patients agreed that the application was generally easy to use and intuitive, and that the mobile visual analogue scale was at least as easy to complete as in paper form.
A study in 153 patients with RA, systemic lupus erythematosus or SpA compared completion of standardised questionnaires using paper and pencil or electronically on a tablet PC.32 The scores obtained by the two methods did not differ, and patients reported no major difficulties using the tablet PC. Most patients (62%) expressed a preference for using remote data entry in the future, while 7 (5%) patients felt uncomfortable with the tablet PC due to their rheumatic disease.
Disease activity measured by patients and reported with an electronic tool has been shown to correlate well with DAS28 results from a clinical examination.28 A study of 51 patients with rheumatic disease reported a high correlation of 0.88 for DAS, with moderate correlation (0.63) for number of tender joints and a lower correlation (0.41) for number of swollen joints. In 37 (73%) patients, self-monitoring and the clinical examination by the physician resulted in an identical classification for low, moderate or high disease activity, with self-monitoring resulting in a higher classification in 12 (24%) cases and a lower classification in 2 (4%) cases.28
In another study, patients' self-assessment of disease activity (RAPID3 and 4) correlated strongly with that of rheumatologists (DAS44, Clinical Disease Activity Index (CDAI), SDAI).25 Ninety patients with RA with a mean age of 55±14 years were educated to use a smartphone application for self-assessment, with weekly questionnaires to complete. Strong correlations were seen between patient and rheumatologist assessment of disease activity when comparing RAPID3 and DAS44 (R=0.60), CDAI (R=0.53) and SDAI (R=0.49), with similar correlations seen with RAPID4.25
Use of electronic patient monitoring tools in other disease areas
A variety of electronic patient monitoring tools are already well accepted in other chronic disease areas (table 1). In cardiology and congestive heart failure, for example, patients undergoing cardiac resynchronisation therapy who were followed with quarterly in-office visits without a daily remote monitoring system had an 86% higher risk of delayed detection of adverse events, during a median follow-up of 7 months, than those who used remote monitoring.29
Table 1.
Summary of evidence of impact of remote patient monitoring tools on patient outcomes across various disease areas
| Disease area | Participants | Intervention | Follow-up | Outcome |
|---|---|---|---|---|
| Cardiology/congestive heart failure29 | 99 patients receiving cardiac resynchronisation therapy | Daily remote monitoring (RM) vs standard programme of in-office visits | 7 months | Rate of detection of clinical adverse events was 23.8% in the RM group vs 48.7%; HR 0.14 (95% CI 0.06 to 0.37) |
| Diabetes30 | 301 patients with type 2 diabetes | Automated telemonitoring, clinician notification and informal caregiver involvement | 3–6 months | Significant improvements over time in long-term medication non-adherence, physical functioning, depressive symptoms and diabetes-related distress (all p<0.001). Significant improvements in patient-reported frequency of weekly medication adherence, self-monitored blood glucose (SMBG) performance, checking feet and abnormal SMBG readings |
| Hypertension31 | 778 patients taking antihypertensive drugs | Usual care vs usual care with home blood pressure monitor (BPM) vs web-based pharmacist care with home BPM | 12 months | 55% of patients in the pharmacist-care group vs 37% in the usual care with home BPM group had BP <140/90 mm Hg. Home BPM accounted for 30.3% of the intervention effect, secure electronic messaging for 96%, and medication intensification for 29.3% |
In diabetes management, a combined programme of automated telemonitoring, clinician notification and informal caregiver involvement was associated with consistent improvements in adherence to treatment, diabetes self-management behaviours, physical functioning and psychological distress.30 A study on a remote monitoring tool in diabetes found that the ability to raise an automatic alert in case of measurements below or above certain limits offered a sense of security, and treating physicians were able to follow the therapeutic course in an easy and timely manner. Furthermore, the remote nature of the monitoring may be especially favourable for elderly, sometimes immobile patients.27
Another example comes from the treatment of hypertension. A study evaluated the role of home monitoring, communication with pharmacists, medication intensification, medication adherence and lifestyle factors in contributing to the effectiveness of an intervention to improve blood pressure control in patients with uncontrolled essential hypertension.31 Study arms analysed were usual care with a home blood pressure monitor and pharmacist-assisted care with a home blood pressure monitor delivered via a patient website. At 12 months follow-up, patients in the web-based pharmacist care group were more likely to have a blood pressure below 140/90 mm Hg compared with patients in the group with home blood pressure monitors only (55% vs 37%; p=0.001). The effect of web-based pharmacist care on improved blood pressure control was explained in part through a combination of home blood pressure monitoring, secure messaging and antihypertensive medication intensification.31
Discussion—remote monitoring tools for inflammatory arthritis
Further to the results of the literature search discussed above, several of the current authors have personal experience with remote monitoring tools being developed for use in inflammatory arthritis. These tools are described below and summarised in table 2. They represent only a sample of the existing tools; many rheumatology registries also make use of web-based tools.
Table 2.
Some examples of remote monitoring tools available for inflammatory arthritis, based on authors' experience
| Tool | Disease | PROs/disease activity measures available | Platform | Automatic alerts for healthcare professionals | Patient's ability to view results | Data security |
|---|---|---|---|---|---|---|
| iMonitor(http://www.pfizer.co.uk/content/medical-and-educational-goods-and-services-megs-imonitor) | RA PsA AS |
BASFI BASDAI HAQ Pt-DAS28 RADAI5 RAID RAPID3 |
PC Tablet Smartphone |
✓ | ✓ | ✓ |
| GoTreatIT (http://www.diagraphit.com) | RA PsA Axial SpA |
DAS DAS28 BASDAI/ASDAS Patient reported joint pain HAQ MHAQ MDHAQ VAS pain fatigue QUEST RA questions PROMIS20 RAID BASFI/BASG |
PC Tablet (mobile phones soon to be supported) |
✓ (Alerts for patients when a report is due is under development) |
✓ | ✓ |
| Sanoïa (http://www.sanoia.com) | RA PsA AS |
HAQ RAID RAPID3 ASAS NSAID ASAS QoL ASAS HI BASDAI BASFI |
PC Tablet Smartphone |
✓ | ✓ | ✓ |
| Andar (http://www.sanoia.com) | RA | RAPID3 DAS28 SDAI CDAI |
PC | ✓ | ✓ | ✓ |
AS, ankylosing spondylitis; ASAS HI, Assessment of SpondyloArthritis international Society Health Index; ASAS NSAID, Assessment of SpondyloArthritis international Society Nonsteroidal Anti-inflammatory Drug; ASAS QoL, Assessment of SpondyloArthritis international Society Quality of Life; ASDAS, Ankylosing Spondylitis Disease Activity Score; Axial SpA, spondyloarthritis; BASFI, Bath Ankylosing Spondylitis Functional Index; BASG, Bath Ankylosing Spondylitis Global assessment; HAQ, Health Assessment Questionnaire; MDHAQ, Multidimensional Health Assessment Questionnaire; MHAQ, Modified Health Assessment Questionnaire; PROMIS20, Patient-Reported Outcomes Measurement Information System20; Pt-DAS28, Patient Derived Disease Activity Score28; QUEST RA, Quantitative Patient Questionnaires in Standard Monitoring of Patients with Rheumatoid Arthritis; RA, rheumatoid arthritis; RAID, Rheumatoid Arthritis Impact of Disease; PROs, patient-reported outcomes; PsA, psoriatic arthritis; VAS, Visual Analog Scale.
GoTreatIT Rheuma (Norway)
The GoTreatIT tool (http://www.diagraphit.com) was developed as a hospital computer system for patient monitoring in clinical practice. The tool incorporates disease measures (all in Norwegian and English) and PRO tools (most of them available in more than 20 languages). It is currently used in 13 hospital centres and by 3 private practising rheumatologists in Norway, and other centres have plans to use it. The tool is used for data collection to the national arthritis registry (NorArtritt). Furthermore, GoTreatIT is also used by more than 10 rheumatology centres in Finland and used for data collection to the Finnish arthritis registry (ROB-FIN). It has been used in a cross-sectional study reporting similar disease burdens in RA, PsA and axial SpA, to compare disease status and treatment in RA between Norway and Finland, and to explore the change in disease status and treatment in patients with RA in a 10-year period at an outpatient clinic.
A technical solution called GoTreatIT Web has recently been developed which allows the patient to report their disease status via the internet directly into the hospital system using secure transfer of information to the hospital server. The self-reported data become immediately visible for healthcare personal at the outpatient clinic. A 2012 presentation at the EULAR congress reported clinical workflow efficiencies with use of the tool, by combining patient monitoring and registry data collection in a single workflow.
Sanoïa (France)
Sanoïa (http://www.sanoia.com), launched in 2010, provides online secure health records that allow patients to track and store their own health data. It is available in 14 languages on PCs, tablet computers and smartphones. Forms such as BASDAI, Health Assessment Questionnaire, ASAS-QoL, RAPID3 and treatment trackers are available, and the patient can generate and print reports and graphical summaries. The patient decides whether to allow the physician to see their data. In September 2015, 4695 patients with RA were registered, and patients with AS and PsA started using the tool. A randomised controlled trial is underway to evaluate the effect of the tool on the quality of patient–doctor interactions (http://www.clinicaltrials.gov/ct2/show/NCT02200068).
Andar (Spain)
Andar (http://www.proyectoandar.com) is a standardised monitoring tool in which the patient completes the RAPID3 questionnaire and clinical and laboratory measurements can be added by the healthcare professional, allowing calculation of composite indices (DAS28, SDAI, CDAI). Initially, this was developed as a paper-based questionnaire that patients completed before each clinic visit. It has now been developed as a web-based tool. Patients determine their own treatment targets and can view the evaluations. Physicians add blood test results, and nurses decide whether patients need urgent visits on the basis of monthly alerts.
iMonitor
iMonitor is a web-based software application that allows patients to report information about their disease state for RA, PsA and AS. It can be accessed by PC, tablet or smartphone. Data are protected during storage and transmission and are encrypted using a PIN code entered by the user. Physicians can choose from a selection of PROs and set individual treatment targets and thresholds for each patient. The physician can then review PRO results entered by patients before an appointment, and real-time monitoring keeps them up to date with their patient's condition. Physicians receive alerts when established thresholds are not met or if PROs are not completed on time. Those patients with poor disease control can be prioritised, contacted and reviewed, as needed.37
Patient groups most likely to benefit
Certain patients may particularly benefit from the use of remote monitoring tools. For example, patients with early RA who are most likely to benefit from a treat-to-target strategy may be the first candidates to adopt such tools. Others who may be suitable include patients with a high technological understanding, those with high engagement with their own disease management, those with barriers to frequent clinic visits (eg, poor mobility or great distance from the clinic), and those at high risk of flare or with a high need for monitoring (eg, patients whose disease activity fluctuates greatly between clinic visits). In addition, patients with stable disease may also be a target group for use of remote monitoring tools which allow them to report a stable condition without needing to attend a clinic for assessment.
Discussion—future perspective
As the cultural trend of moving towards digital monitoring and record keeping in healthcare develops, we anticipate that further work to develop the current and future range of remote PRO monitoring tools will continue. Our current review and search criteria highlighted a low number of published articles specifically relating to remote PRO reporting tools. While we recognise the limits of our search, there is a need for greater interest and research in the potential benefits of these tools.
Conclusions
A treat-to-target strategy targeting low disease activity or remission in the management of RA is the standard of care and has been shown to lead to better outcomes. Remote monitoring and reporting of PROs may facilitate a treat-to-target approach and help to achieve a low disease activity state or remission among patients with RA.23 PROs used in conjunction with rheumatologist-led disease activity monitoring may add an important perspective on disease activity, as it is perceived by the patient. Several validated PROs exist for inflammatory arthritis.
There is an unmet need for more frequent patient monitoring in chronic inflammatory arthritis to improve disease management and potentially to reduce pressure on specialists, as well as to achieve a better understanding of the disease course, which should be considered as more than just the linear path between two consecutive observation points. Evidence from several disease areas suggests that electronic tools that allow patients to give feed back on their disease may be beneficial. Innovative electronic tools that allow more frequent monitoring have the potential to improve disease management and may be more widely adopted in the future. Multiplatform availability of electronic monitoring devices is an important consideration in encouraging the widest usage possible. Innovative electronic tools, such as iMonitor, GoTreatIT, Sanoïa and Andar, may help to support physician time management, to reduce the burden on clinic time, and to prioritise patients who may need further attention.
Footnotes
Contributors: PvR, RA and BC are joint lead authors and contributed equally. DA, PB, MC, CC, AG-C, GH, BL, KP, AR, BR and PS-P contributed equally.
Funding: Initial drafting and subsequent medical writing support was provided by Clare Griffith, Synergy, London, UK and funded by Pfizer.
Competing interests: RA has received research grants and honoraria from the speaker's bureau from Pfizer. BC has received honorarium from Pfizer. DA has received honoraria from Pfizer, MSD and UCB. PB is an employee of Pfizer. CC is an employee of Pfizer. GH is a founder and shareholder of the company DiaGraphIT, manufacturing GoTreatIT Rheuma. BL has received research grants as well as honoraria from Centocor, Abbott, Amgen, Aesca, UCB, Roche, MSD, Celltrion, Schering-Plough, Wyeth, Pfizer, BMS, Jannssen-Cilag, Eli-Lilly, Novartis, Sandoz and Celgene. KP has received honoraria from Abbvie, BMS, MSD, Pfizer, Roche and UCB. AR has received honoraria or research grants from Abbvie, BMS, Centocor, Eli-Lilly, Novartis, Pfizer, Roche and Wyeth. BR has received research grants as well as honoraria from Centocor, Abbott, Amgen, Aesca, UCB, Roche, MSD, Celltrion, Schering-Plough, Wyeth, Pfizer, BMS, Jannssen-Cilag, Eli-Lilly and Novartis. PS-P has received research grant honoraria from Abbvie, UCB, Roche, MSD, Pfizer, BMS, and Eli-Lilly.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Smolen JS, Breedveld FC, Burmester GR et al. . Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. 10.1136/annrheumdis-2015-207524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Braun J, Dougados M et al. . Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6–16. 10.1136/annrheumdis-2013-203419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeer M, Kuper HH, Hoekstra M et al. . Implementation of a treat-to-target strategy in very early rheumatoid arthritis: results of the Dutch Rheumatoid Arthritis Monitoring Remission Induction Cohort Study. Arth Rheum 2011;63:2865–72. 10.1002/art.30494 [DOI] [PubMed] [Google Scholar]
- 5.Grigor C, Capell H, Stirling A et al. . Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. 10.1016/S0140-6736(04)16676-2 [DOI] [PubMed] [Google Scholar]
- 6.Verstappen SM, Jacobs JW, van der Veen MJ et al. . Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007;66:1443–9. 10.1136/ard.2007.071092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates LC, Moverley AR, McParland L et al. . Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. 10.1016/S0140-6736(15)00347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemao E, Joo S, Kawabata H et al. . Effects of achieving target measures in RA on functional status, quality of life and resource utilization: analysis of clinical practice data. Arthritis Care Res (Hoboken) 2016;68:308–17. 10.1002/acr.22678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugeberg G, Hansen IJ, Soldal DM et al. . Ten years of change in clinical disease status and treatment in rheumatoid arthritis: results based on standardized monitoring of patients in an ordinary outpatient clinic in southern Norway. Arthritis Res Ther 2015;17:219 10.1186/s13075-015-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson J, Caplan L, Yazdany J et al. . Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64:640–7. 10.1002/acr.21649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leeb BF, Andel I, Leder S et al. . The patient's perspective and disease activity indexes. Rheumatology (Oxford) 2005;44:360–5. 10.1093/rheumatology/keh484 [DOI] [PubMed] [Google Scholar]
- 12.Tillett W, Eder L, Goel N et al. . Enhanced Patient Involvement and the Need to Revise the Core Set—report from the Psoriatic Arthritis Working Group at OMERACT 2014. J Rheumatol 2015;42:2198–203. 10.3899/jrheum.141156 [DOI] [PubMed] [Google Scholar]
- 13.Bykerk VP, Lie E, Bartlett SJ et al. . Establishing a core domain set to measure rheumatoid arthritis flares: report of the OMERACT 11 RA flare Workshop. J Rheumatol 2014;41:799–809. 10.3899/jrheum.131252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arth Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Guidance for Industry. Patient-reported outcome measures: use in medical product development to support labeling claims. US FDA, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peláez-Ballestas I. Patient reported outcome measures: what is their importance? Reumatol Clin 2012;8:105–6. 10.1016/j.reuma.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 17.Pincus T, Strand V, Koch G et al. . An index of the three core data set patient questionnaire measures distinguishes efficacy of active treatment from that of placebo as effectively as the American College of Rheumatology 20% response criteria (ACR20) or the Disease Activity Score (DAS) in a rheumatoid arthritis clinical trial. Arthritis Rheum 2003;48:625–30. 10.1002/art.10824 [DOI] [PubMed] [Google Scholar]
- 18.Rintelen B, Sautner J, Haindl P et al. . Remission in rheumatoid arthritis: a comparison of the 2 newly proposed ACR/EULAR remission criteria with the rheumatoid arthritis disease activity index-5, a patient self-report disease activity index. J Rheumatol 2013;40:394–400. 10.3899/jrheum.120952 [DOI] [PubMed] [Google Scholar]
- 19.McManus RJ, Mant J, Haque MS et al. . Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014;312:799–808. 10.1001/jama.2014.10057 [DOI] [PubMed] [Google Scholar]
- 20.Wicks P, Stamford J, Grootenhuis MA et al. . Innovations in e-health. Qual Life Res 2014;23:195–203. 10.1007/s11136-013-0458-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puchner R, Brezinschek HP, Fritz J et al. . Is the state of health of rheumatoid arthritis patients receiving adequate treatment, predictable?—results of a survey. BMC Musculoskelet Disord 2015;16:109 10.1186/s12891-015-0567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsing PMJ, Landewe RBM, van Riel PLCM et al. . The Relationship between disease activity and radiologic progression in patients with rheumatoid arthritis. Arth Rheum 2004;50;2082–93. 10.1002/art.20350 [DOI] [PubMed] [Google Scholar]
- 23.Sargious A, Lee SJ. Remote collection of questionnaires. Clin Exp Rheumatol 2014;32(Suppl 85):S168–72. [PubMed] [Google Scholar]
- 24.Stevenson FA, Cox K, Britten N et al. . A systematic review of the research on communication between patients and healthcare professionals about medicines: the consequences for concordance. Health Expect 2004;7:235–45. 10.1111/j.1369-7625.2004.00281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller R, Walker U, Kyburz D et al. . Patient's self-monitoring via smartphone: the Compass study correlation between patient self-assessment of rheumatoid arthritis disease activity via smartphone technology and physicians’ validated scores. Arthritis Rheum 2014;66(Suppl 10):S1298 [abstract 2971]. [Google Scholar]
- 26.Sikorska-Siudek K, Przygodzka M, Bojanowski S et al. . Mobile application for patients with rheumatoid arthritis (RA) as a supporting tool for disease activity monitoring: its usability and interoperability. Ann Rheum Dis 2015;74(Suppl 2):986 [abstract AB0280] 10.1136/annrheumdis-2015-eular.1158 [DOI] [Google Scholar]
- 27.Mlekusch W. [Perspectives of mobile communication in the management of diabetics]. Wien Med Wochenschr 2011;161:359–60. 10.1007/s10354-011-0012-9 [DOI] [PubMed] [Google Scholar]
- 28.Langer A, Langer H. Computer-aided self-monitoring of disease activity in chronic arthritis: a new tool for disease management? Presented at EULAR 2004 [abstract HP0016]. http://www.abstracts2view.com/eular/view.php?nu=EULAR04L1_2004HP0016.
- 29.De Ruvo E, Gargaro A, Sciarra L et al. . Early detection of adverse events with daily remote monitoring versus quarterly standard follow-up program in patients with CRT-D. Pacing Clin Electrophysiol 2011;34:208–16. 10.1111/j.1540-8159.2010.02932.x [DOI] [PubMed] [Google Scholar]
- 30.Aikens JE, Rosland AM, Piette JD. Improvements in illness self-management and psychological distress associated with telemonitoring support for adults with diabetes. Prim Care Diabetes 2015;9:127–34. 10.1016/j.pcd.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ralston JD, Cook AJ, Anderson ML et al. . Home blood pressure monitoring, secure electronic messaging and medication intensification for improving hypertension control. Appl Clin Inform 2014;5:232–48. 10.4338/ACI-2013-10-RA-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter JG, Becker A, Koch T et al. . Self-assessments of patients via Tablet PC in routine patient care: comparison with standardised paper questionnaires. Ann Rheum Dis 2008;67:1739–41. 10.1136/ard.2008.090209 [DOI] [PubMed] [Google Scholar]
- 33.Parekh SG, Nazarian DG, Lim CK. Adoption of information technology by resident physicians. Clin Orthop Relat Res 2004;421:107–11. 10.1097/01.blo.0000126865.22310.59 [DOI] [PubMed] [Google Scholar]
- 34.Michelsen B, Fiane R, Diamantopoulos AP et al. . A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS ONE 2015;10:e0123582 10.1371/journal.pone.0123582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokka T, Haugeberg G, Asikainen J et al. . Similar clinical outcomes in rheumatoid arthritis with more versus less expensive treatment strategies. Observational data from two rheumatology clinics. Clin Exp Rheumatol 2013;31:409–14. [PubMed] [Google Scholar]
- 36.Rødevand E, Haavardsholm E, Bader L et al. . The Norwegian BIORHEUMA project—achieving patient benchmarking and patient register in one work flow using the GoTreatIt computer software system. Ann Rheum Dis 2012;71(Suppl 3):455 [abstract FRI0419] 10.1136/annrheumdis-2011-200372 [DOI] [PubMed] [Google Scholar]
- 37.Hendrikx J, Fransen J, Toniolo A, et al. Moving towards personalized healthcare: a patient reported outcome based algorithm can aid rheumatologists and patients in monitoring rheumatoid arthritis in daily clinical practice. Presented at ACR 2013 [abstract 2663]. http://acrabstracts.org/abstract/moving-towards-personalized-healthcare-a-patient-reported-outcome-based-algorithm-can-aid-rheumatologists-and-patients-in-monitoring-rheumatoid-arthritis-in-daily-clinical-practice/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2016-000302supp_appendix.pdf (275.1KB, pdf)