Abstract
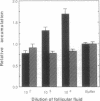
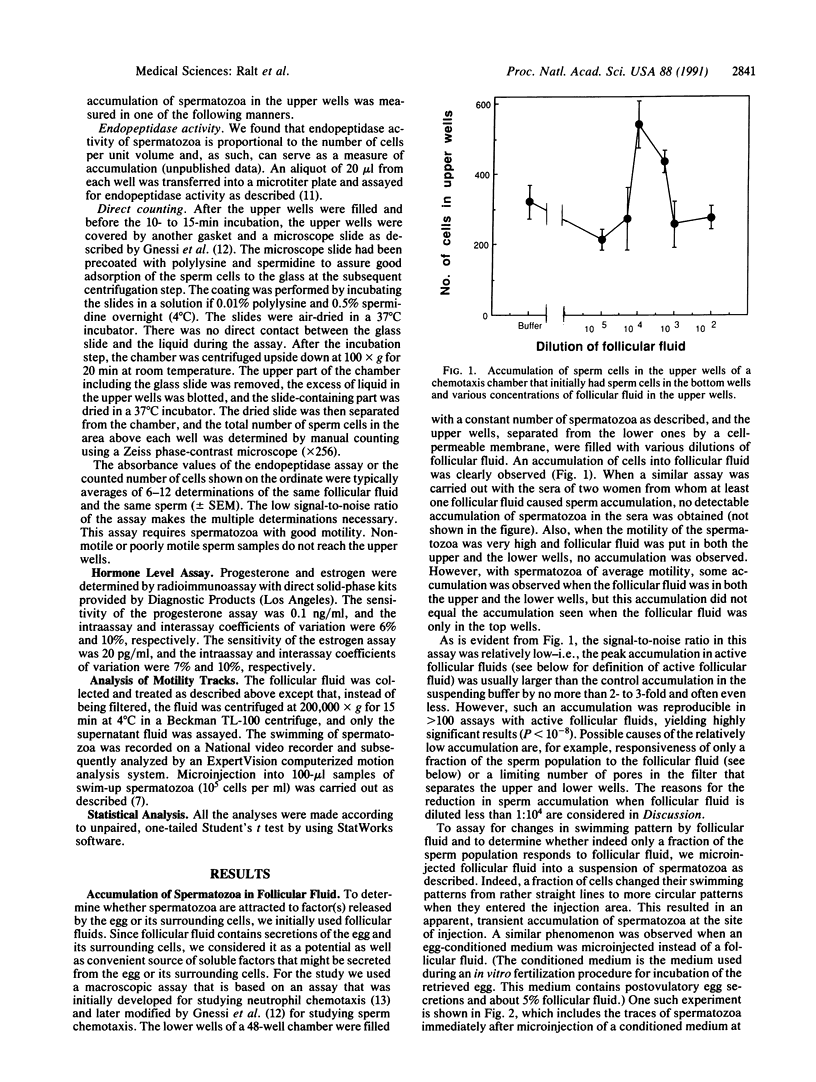
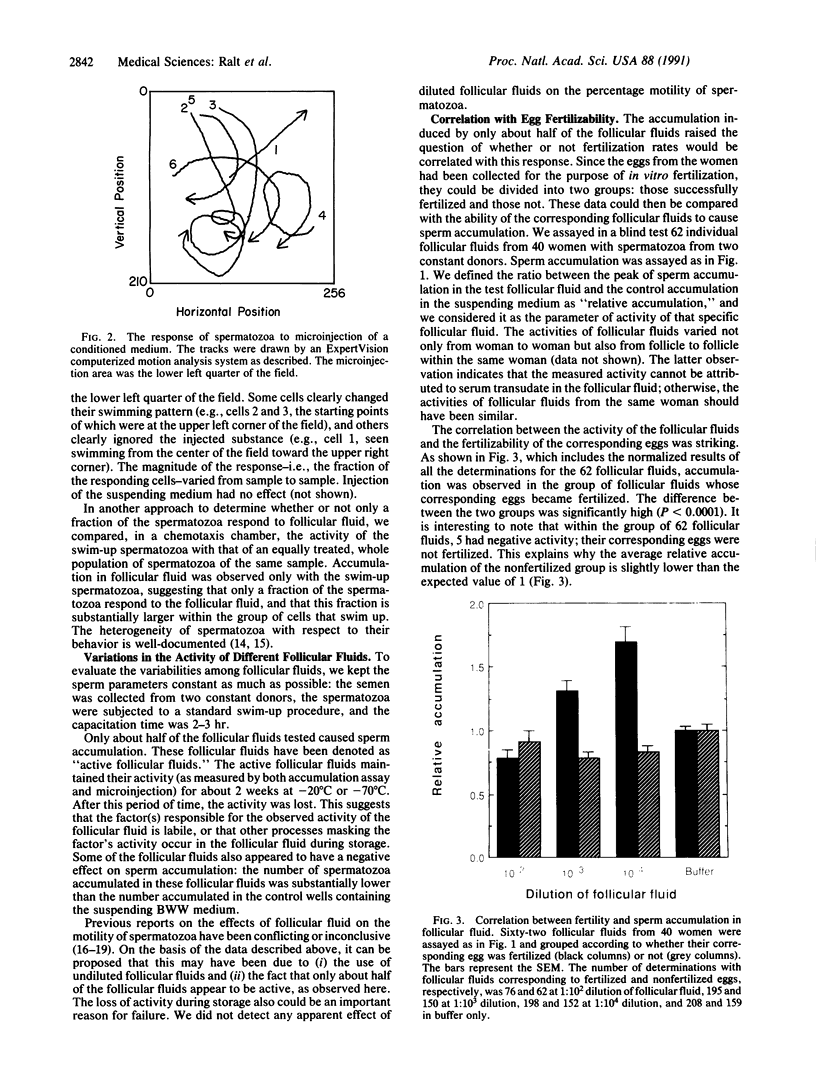
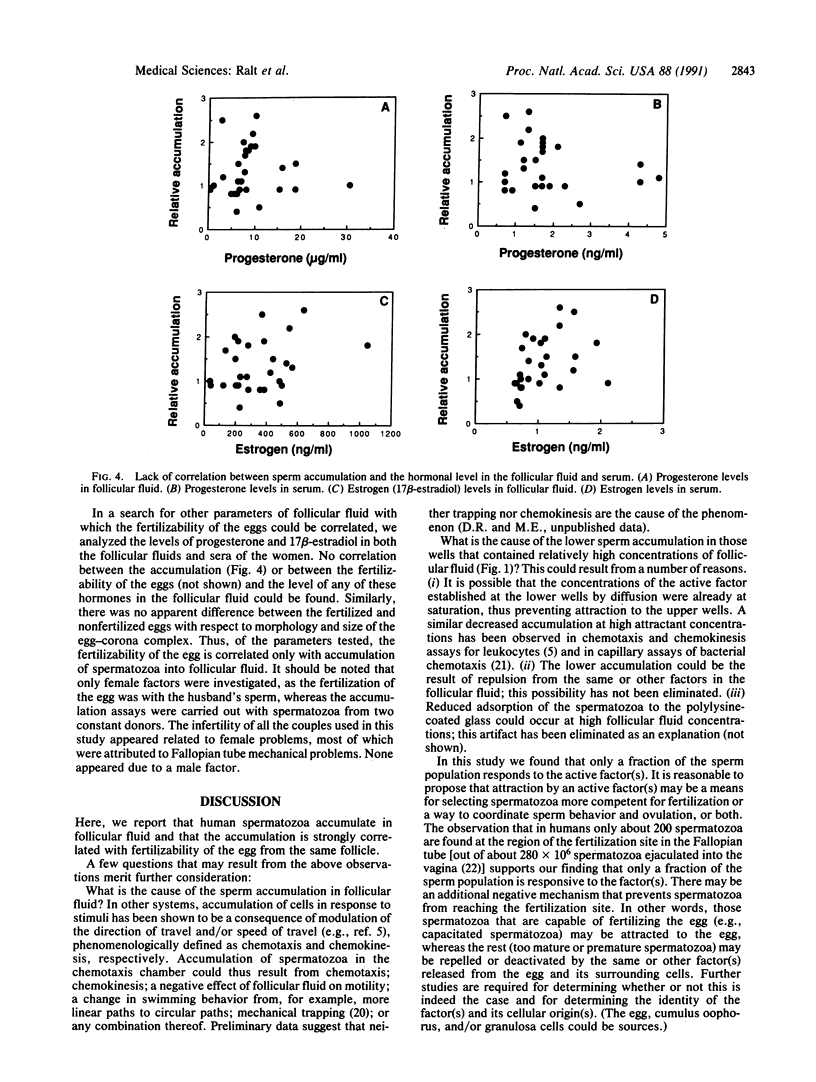
Spermatozoa normally encounter the egg at the fertilization site (in the Fallopian tube) within 24 hr after ovulation. A considerable fraction of the spermatozoa ejaculated into the female reproductive tract of mammals remains motionless in storage sites until ovulation, when the spermatozoa resume maximal motility and reach the fertilization site within minutes. The nature of the signal for sperm movement is not known, but one possible mechanism is attraction of spermatozoa to a factor(s) released from the egg. We have obtained evidence in favor of such a possibility by showing that human spermatozoa accumulate in follicular fluid in vitro. This accumulation into follicular fluid was higher by 30-260% than that observed with buffer alone and was highly significant (P less than 10(-8)). Not all of the follicular fluids caused sperm accumulation; however, there was a remarkably strong correlation (P less than 0.0001) between the ability of follicular fluid from a particular follicle to cause sperm accumulation and the ability of the egg, obtained from the same follicle, to be fertilized. These findings suggest that attraction may be a key event in the fertilization process and may give an insight into the mechanism underlying early egg-sperm communication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Arnal F., Humeau C., Lainé H., Hédon B., Flandre O., Catayée G. Effets in vitro du liquide folliculaire humain préovulatoire sur la migration des spermatozoïdes humains. C R Seances Acad Sci III. 1983;297(13):605–608. [PubMed] [Google Scholar]
- Bignold L. P. Measurement of chemotaxis of polymorphonuclear leukocytes in vitro. The problems of the control of gradients of chemotactic factors, of the control of the cells and of the separation of chemotaxis from chemokinesis. J Immunol Methods. 1988 Apr 6;108(1-2):1–18. doi: 10.1016/0022-1759(88)90396-1. [DOI] [PubMed] [Google Scholar]
- Dandekar P. V., Quigley M. M. Laboratory setup for human in vitro fertilization. Fertil Steril. 1984 Jul;42(1):1–12. doi: 10.1016/s0015-0282(16)47949-5. [DOI] [PubMed] [Google Scholar]
- Dor J., Rudak E., Rotmench S., Levran D., Blankstein J., Lusky A., Nebel L., Serr D. M., Mashiach S. The role of early post-implantation beta-HCG levels in the outcome of pregnancies following in-vitro fertilization. Hum Reprod. 1988 Jul;3(5):663–667. doi: 10.1093/oxfordjournals.humrep.a136763. [DOI] [PubMed] [Google Scholar]
- Gnessi L., Ruff M. R., Fraioli F., Pert C. B. Demonstration of receptor-mediated chemotaxis by human spermatozoa. A novel quantitative bioassay. Exp Cell Res. 1985 Nov;161(1):219–230. doi: 10.1016/0014-4827(85)90506-3. [DOI] [PubMed] [Google Scholar]
- Hicks J. J., Martínez-Manautou J., Pedron N., Rosado A. Metabolic changes in human spermatozoa related to capacitation. Fertil Steril. 1972 Mar;23(3):172–179. [PubMed] [Google Scholar]
- Hoshi K., Yanagida K., Aita T., Yoshimatsu N., Sato A. Changes in the motility pattern of human spermatozoa during in vitro incubation. Tohoku J Exp Med. 1988 Jan;154(1):47–56. doi: 10.1620/tjem.154.47. [DOI] [PubMed] [Google Scholar]
- Hunter R. H. Human fertilization in vivo, with special reference to progression, storage and release of competent spermatozoa. Hum Reprod. 1987 May;2(4):329–332. doi: 10.1093/oxfordjournals.humrep.a136544. [DOI] [PubMed] [Google Scholar]
- Indig F. E., Ben-Meir D., Spungin A., Blumberg S. Investigation of neutral endopeptidases (EC 3.4.24.11) and of neutral proteinases (EC 3.4.24.4) using a new sensitive two-stage enzymatic reaction. FEBS Lett. 1989 Sep 25;255(2):237–240. doi: 10.1016/0014-5793(89)81098-1. [DOI] [PubMed] [Google Scholar]
- Lambert H., Overstreet J. W., Morales P., Hanson F. W., Yanagimachi R. Sperm capacitation in the human female reproductive tract. Fertil Steril. 1985 Feb;43(2):325–327. [PubMed] [Google Scholar]
- Morales P., Overstreet J. W., Katz D. F. Changes in human sperm motion during capacitation in vitro. J Reprod Fertil. 1988 May;83(1):119–128. doi: 10.1530/jrf.0.0830119. [DOI] [PubMed] [Google Scholar]
- Mortimer D., Camenzind A. R. The role of follicular fluid in inducing the acrosome reaction of human spermatozoa incubated in vitro. Hum Reprod. 1989 Feb;4(2):169–174. doi: 10.1093/oxfordjournals.humrep.a136866. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Lippes J. Effect of human follicular and tubal fluids on human, mouse and rat spermatozoa in vitro. Can J Genet Cytol. 1972 Mar;14(1):167–174. doi: 10.1139/g72-019. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Brokaw C. J., Garbers D. L., Vacquier V. D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985 Dec;101(6):2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Zinaman M., Drobnis E. Z., Morales P., Brazil C., Kiel M., Cross N. L., Hanson F. W., Overstreet J. W. The physiology of sperm recovered from the human cervix: acrosomal status and response to inducers of the acrosome reaction. Biol Reprod. 1989 Nov;41(5):790–797. doi: 10.1095/biolreprod41.5.790. [DOI] [PubMed] [Google Scholar]