Abstract
Prospective cohort studies of the relationship between coffee consumption and liver cancer risk have drawn different conclusions. Therefore, a dose-response meta-analysis of prospective cohort studies was performed to disentangle this causal relationship. Prospective cohort studies of the association between coffee consumption and liver cancer risk published prior to Jan 9, 2016 were identified by searching in the PubMed and EMBASE databases. Extracted data were analyzed using a random-effects model. Of the 2892 records identified using the search strategy, a total of twenty cohort studies from ten publications were included in the final meta-analysis. The pooled estimate of relative risk (RR) with 95% confidence interval (CI) for highest vs. non/occasional coffee drinkers was 0.55(0.44–0.67). No evidence of publication bias was observed (p for Egger’s test = 0.229). Sensitivity analysis indicated the results were robust. Dose-response analysis revealed a significant linear dose-response relationship between coffee consumption and liver cancer risk (p = 0.36). Subgroup analyses stratified by pre-specified variables (gender, geographic region, and adjusted factors) indicated similar results within individual subgroups. Our meta-analysis suggested that coffee consumption is inversely associated with liver cancer risk.
Primary liver cancer represents the second most common cancer-related cause of death and sixth most frequently diagnosed cancer worldwide1,2. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the two dominant histologic types of primary liver cancer3. The aetiology of liver cancer is poorly studied. At present, well-documented unfavorable risk factors for liver cancer included chronic infection with hepatitis B virus (HBV) or C virus (HCV), excessive alcohol intake, and dietary aflatoxin4. The association between liver cancer and other risk factors, including obesity, diabetes mellitus, and smoking, is less clear4. Evidence from a recent meta-analysis indicated that increased intake of vegetables, but not fruits, appears to have a protective effect on HCC incidence4.
The effect of coffee intake on cancer risk has been a topic of considerable interest, because coffee is the second most popular beverage worldwide, ranking behind tea only. Coffee contains large amounts of compounds with antioxidant, anti-inflammatory, and anticarcinogenic properties such as caffeine, chlorogenic acid, caffeic acid, and polyphenols, as well as diterpenes and other antioxidants were found in that beverage5,6. Over the last two decades, numerous case-control and cohort studies have assessed the relationship between coffee intake and liver cancer risk; however, these studies have yielded inconsistent results. To disentangle this potentially confusing association, four meta-analyses of case-control and cohort studies had been performed7,8,9,10. All meta-analyses have suggested an inverse association between coffee intake and liver cancer risk. However, the tendency of retrospective case-control studies to be subject to recall and selection biases must be considered, as these biases may limit to the generalizability of the findings of these studies. In addition, the pattern of the dose-response curve between coffee exposure and liver cancer risk has not yet been described. Therefore, an updated dose-response meta-analysis of prospective cohort studies was performed.
Methods
We report this meta-analysis according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) guidelines11. There was no registered protocol. Ethical approval and patient consent were not necessary, because we conducted a meta-analysis of published studies.
Inclusion Criteria
Studies were included if they fulfilled all the following criteria: (1) a prospective cohort study design was employed (prospective nested case-control studies were considered cohort studies); (2) the exposed population was patients with liver cancer; (3) the end point was incidence of or death from liver cancer; (4) the exposure of interest was coffee intake; and (5) adjusted RRs with corresponding 95 CIs were reported or other data provided were sufficient for their calculation. When more than one study studied the same population, the study reporting the most detailed information for both exposure and outcome was included in this meta-analysis.
Data sources
A systematic literature search of the PubMed and EMBASE databases was performed to identify studies published prior to Jan 9, 2016. No restrictions on language or publication year were imposed. References of included studies, reviews, and meta-analyses were also reviewed to identify other potential studies, not identified using the initial search terms. Furthermore, studies citing the included studies in Google Scholar were manually reviewed.
Search strategy
Two authors independently conducted the literature search. In our meta-analysis, we used the following key words or MeSH terms: (“coffee” OR “caffeine” OR “diet” OR “beverages” OR “lifestyle” OR “drinking”) AND (“hepatocellular carcinoma” OR “hepatic carcinoma” OR “liver cancer” OR “liver tumors” OR “liver neoplasms”) AND (“risk assessment” OR “risk” OR “risk factors”).
Data extraction
The following data were independently extracted by two authors: first author last name, year of publication, cohort name, country, age of subjects at baseline, simple size, duration of follow-up (years), adjusted RRs with corresponding 95% CIs for each exposure level of coffee intake, and adjusted factors. Risk estimates that adjusted for the largest number of confounding factors were considered in the current meta-analysis. Any disagreements were resolved by discussion.
Assessment the risk of bias
For observational studies, no well-established standard appraisal tools are currently available12. Therefore, the 9-star Newcastle-Ottawa Scale (NOS), which is recommended by the Cochrane Collaboration13, was adopted to assess the risk of bias (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). Studies that scored seven stars or more were considered to be less prone to bias.
Statistical Analysis
We derived a pooled estimate of adjusted RRs and 95% CIs using a random-effects model14, as the results provided by this model were more conservative. Heterogeneity across studies was tested using Cochran’s Q statistic and the proportion of total variation was estimated using I2 test15. We applied the following interpretation: I2 < 25% = low heterogeneity; 25% < I2 < 75% = moderate heterogeneity; and I2 > 75% = high heterogeneity. Publication bias was evaluated using funnel plots and Egger’s test16, where a p-value of the Egger’s test less than 0.05 suggests the presence of publication bias. In the sensitivity analysis, exclusion of single study at one time was assessed to investigate its influence of individual study.
For the primary analysis, we computed a pooled RR with 95% CI for highest vs. lowest intake of coffee. The lowest exposure category was defined as no consumption or occasional drinkers (0–1cup/day) of coffee. In subgroup analyses, we investigated the variations (gender, study region, adjusted factors) between studies. In addition, we performed a dose-response meta-analysis using the method described by Greenland and Orsini et al.17,18. Briefly, this method requires the exposure distribution of cases and person-years at risk, and RRs with 95% CIs for at least three quantitative exposure categories were provided in the original studies. For each study, the median or mean level of exposure within each category was assigned a corresponding RR; however, when unavailable, the midpoint of the upper and lower boundaries of each category was assigned as the value of exposure. When the highest category was open-ended, we assumed the length of the open-ended interval to be 1.5 times that of the closest category. A potential nonlinear or linear dose-response relationship between coffee consumption (cups/day) and liver cancer risk was modelled by using restricted cubic splines with 3 knots at fixed percentiles (25%, 50%, and 75%). A p-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to 019. All statistical analyses were performed using STATA/SE 12.0 software (StataCorp, College Station, TX, USA).
Results
Search results
The PRISMA statement flowchart shows the process of identification and study selection. (PRISMA_Flowchart_SuppInfo.doc). In the initial search, 2892 records were identified. We first excluded duplicates. A second assessment based on the reading of titles and abstracts was performed, and 2184 records were considered to be potentially eligible for inclusion. During the process of full-text evaluation, 4 records were excluded20,21,22,23. Finally, ten records were included in this meta-analysis3,24,25,26,27,28,29,30,31,32.
Characteristics of the cohort studies
Table 1 shows the main characteristics of included studies. Ten records including twenty cohort studies, published from 2005 to 2015 were selected for inclusion. The studies were conducted in Japan, Singapore, the USA, Finland, Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom. Liver cancer cases in all included studies were identified by cancer or death registries. All included studies used cancer incidence as the end point with exception of the study conducted by Kurozawa et al.25, in which the endpoint was death from liver cancer. Of the studies, one reported data separately for HCC and ICC3; three assessed total primary liver cancer26,27,30, and the remaining included studies evaluated eHCC24,25,28,29,31,32.
Table 1. Characteristics of included prospective studies of coffee consumption and risk of liver cancer.
| Study | Cohort Name | Country | Age | No. of Cases | No. of Cohort size | Duration of follow-up (years) | Coffee consumption | Relative risk (95% CI) | Adjustment |
|---|---|---|---|---|---|---|---|---|---|
| Inoue et al.24 | JPHC Study | Japan | 40–69 | 334 | 90,452 | 10 | ≥5 cups/day vs. Almost never | 0.24 (0.08–0.77) | Age, sex, study center, smoking, alcohol intake, vegetable consumption, and tea intake. |
| Kurozawa et al.25 | JACC Study | Japan | 40–79 | 258 | 83,966 | 11 | ≥1 cup/day vs. Non-drinkers | 0.50 (0.31–0.79) | Age, sex, education, history of diabetes and liver diseases, smoking, and alcohol intake. |
| Shimazu et al.26 | Cohort 1 | Japan | >40 | 70 | 22,404 | 9 | ≥1 cup/day vs. Non-drinkers | 0.53 (0.28–1.00) | Age, sex, history of liver disease, smoking, and alcohol intake |
| Cohort 2 | Japan | 40–64 | 47 | 38,703 | 6 | ≥1 cup/day vs. Non-drinkers | 0.68 (0.31–1.51) | Age, sex, history of liver disease, smoking, and alcohol intake | |
| Hu et al.27 | — | Finland | 25–74 | 128 | 60,323 | 19.3 | ≥8 cups/day vs. 0–1 cup/day | 0.32 (0.16–0.62) | Age, sex, study year, alcohol intake, smoking, education, diabetes, history of liver disease, and BMI |
| Ohishi et al.28 | AHSI | Japan | NA | 224 | 644 | 44 | Daily vs. Non-drinkers | 0.40 (0.16–1.02) | Age, sex, history of liver disease, alcohol intake, smoking, BMI, diabetes mellitus, and radiation dose to the liver. |
| Johnson et al.29 | SCH study | Singapore | 45–74 | 362 | 63,257 | 13 | ≥3 cups/day vs. Non-drinkers | 0.56 (0.31–1.00) | Age, sex, dialect group, study year, BMI, education, alcohol intake, smoking, tea intake, and history of diabetes |
| Lai et al.30 | ATBCP Study | Finland | 50–69 | 194 | 27,037 | 18.2 | ≥4 cups/day vs. 0–1 cup/day | 0.53 (0.30–0.95) | Age, BMI, education, marital status, history of diabetes, smoking, alcohol intake, tea intake, ATBC intervention arm, and serum cholesterol. |
| Bamia et al.31 | EPIC | European | 25–70 | 201 | 486,799 | 11 | Q5 vs. Q1 | 0.28 (0.16–0.50) | Age, sex, diabetes, education, BMI, smoking, physical activity, alcohol intake, energy intake, and tea intake. |
| Petrick et al.3 | LCPP | USA | 25–70 | 1,120 | 1,212,893 | 10–22 | >3 cups/day vs. Non-drinkers | MHCC:0.73 (0.53–0.99); MICC: 1.11 (0.52–2.35),WICC:0.89 (0.46–1.72) | Age, sex, race, cohort, BMI, smoking, and alcohol intake. |
| Setiawan et al.32 | MEC | USA | 45–75 | 451 | 162,022 | 18 | ≥4 cups/day vs. Non-drinkers | 0.59 (0.35–0.99) | Age, sex, race/ethnicity, education, BMI, alcohol intake, smoking, and diabetes. |
No., number; JACC Study: Japan Collaborative Cohort Study for Evaluation of Cancer Risk; JPHC Study: The Japan Public Health Center-based Prospective Study; AHSI, the Adult Health Study longitudinal cohort; SCH study, the Singapore Chinese Health Study; ATBCP Study:, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; EPIC, the European Prospective Investigation into Cancer and nutrition; LCPP, the Liver Cancer Pooling Project; MEC, the US Multiethnic Cohort; MHCC, hepatocellular carcinoma in men; WICC, intrahepatic cholangiocarcinoma in women; MICC, hepatocellular carcinoma in men.
Risk of bias assessment
Table S1_SuppInfo.doc shows that all included studies received a score of seven stars or more; thus, included studies were judged to be at low risk of bias.
Overall analysis
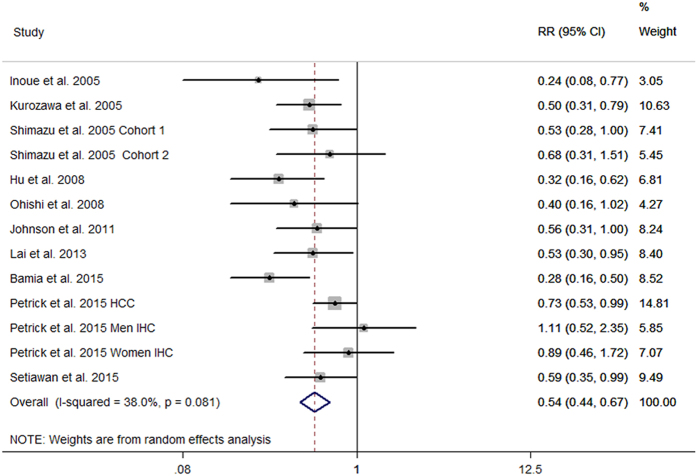
Study-specific multivariable-adjusted RRs with 95% CIs for the highest versus lowest categories of coffee intake are shown Fig. 1. The pooled results showed an inverse relationship between coffee consumption and liver cancer risk. The summary RR and 95% CI were 0.55 (0.44–0.67), with moderate heterogeneity observed (I2 = 38.0%, P = 0.081).
Figure 1. Forest plot for study-specific and pooled RRs and 95% CIs of liver cancer for highest versus lowest categories of coffee consumption.
MHCC, hepatocellular carcinoma in men; WICC, intrahepatic cholangiocarcinoma in women; MICC, hepatocellular carcinoma in men.
Sensitivity analysis
Sensitivity analysis indicated that the overall result was not substantially influenced by any single study, with RRs and 95 CIs ranging from 0.52 (0.41–0.65) to 0.59 (0.49–0.71), as shown in Table 2.
Table 2. Results of sensitivity analysis.
| Study excluded | Pooled RR with 95% CI | Heterogeneity | |
|---|---|---|---|
| I2 (%) | P | ||
| Inoue et al.24 | 0.56(0.45–0.69) | 35.7 | 0.105 |
| Kurozawa et al.25 | 0.55(0.43–0.69) | 42.3 | 0.060 |
| Shimazu et al.26 Cohort 1 | 0.54(0.43–0.68) | 43.0 | 0.056 |
| Shimazu et al.26 Cohort 2 | 0.53(0.43–0.67) | 42.5 | 0.059 |
| Hu et al.27 | 0.57(0.46–0.70) | 33.3 | 0.123 |
| Ohishi et al.28 | 0.55(0.44–0.69) | 41.5 | 0.065 |
| Johnson et al.29 | 0.54(0.43–0.68) | 43.1 | 0.055 |
| Lai et al.30 | 0.54(0.43–0.69) | 43.0 | 0.056 |
| Bamia et al.31 | 0.59(0.49–0.71) | 15.8 | 0.289 |
| Petrick et al.3 HCC | 0.52(0.41–0.65) | 30.4 | 0.149 |
| Petrick et al.3 Men IHC | 0.52(0.43–0.64) | 31.7 | 0.138 |
| Petrick et al.3 Women IHC | 0.52(0.42–0.65) | 36.7 | 0.097 |
| Setiawan et al.32 | 0.54(0.42–0.68) | 43.0 | 0.056 |
Publication bias
As shown in Fig. 2, the funnel plot did not show any asymmetry. Additionally, the result of Egger’s linear regression test suggested no evidence of publication bias (p = 0.229).
Figure 2. Funnel plot of liver cancer for highest versus lowest categories of coffee consumption.
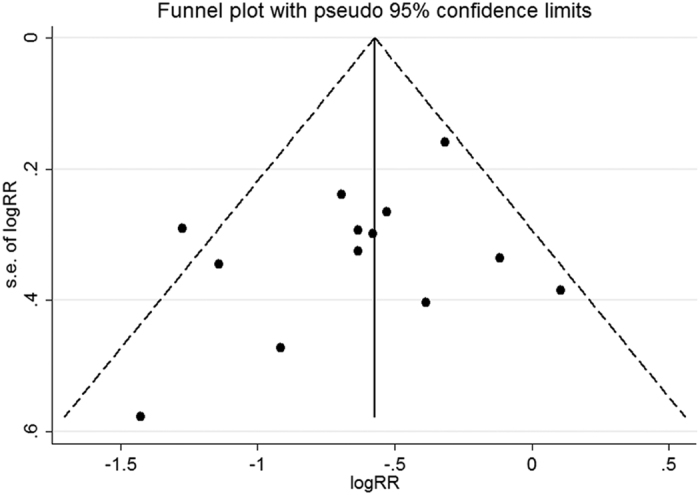
S.E. of logRR, Standard Error of logRR. The dashed lines represent the pseudo 95% CI. The sold line represents the effect estimate.
Subgroup analysis
Table 3 shows the results of subgroup analyses stratified by gender, geographic region, and adjusted factors (history of liver diseases, history of diabetes, BMI, education, and tea consumption). No differences were identified in the association between coffee intake and liver cancer risk by gender (Male: RR = 0.58, 95% CI: 0.40–0.83, I2 = 59.5%; Female: RR = 0.57, 95% CI: 0.42–0.79, I2 = 0.0%). In the subgroup analysis of the effects of confounding factors on liver cancer risk, the results were not significantly modified by history of liver diseases, history of diabetes, BMI, education, or tea consumption (Table 3). When we stratified the analysis according to geographic region, a slight, moderate, and strong reduction in liver cancer risk was observed for studies conducted in North America (RR = 0.75, 95% CI: 0.59–0.95, I2 = 0.0%), Asia (RR = 0.50, 95% CI: 0.38–0.66, I2 = 0.0%), and Europe (RR = 0.37, 95% CI: 0.25–0.54, I2 = 22.6%), respectively.
Table 3. Summary risk estimates of the association between coffee consumption and risk of liver cancer.
| Studies groups | References number | No. of cases/No. of total participants | RR (95% CI) | Heterogeneity | |
|---|---|---|---|---|---|
| I2(%) | P | ||||
| All studies | 3,24–32 | 3,389/2,248,500 | 0.55(0.44–0.67) | 38.0 | 0.081 |
| Study gender | |||||
| Male | 3,24–27,30 | 1,547/656,146 | 0.58(0.40–0.83) | 59.5 | 0.022 |
| Female | 3,24–27 | 604/879,632 | 0.57(0.42–0.79) | 0.0 | 0.588 |
| Study region | |||||
| Asia | 24–26,28,29 | 1,295/299,426 | 0.50(0.38–0.66) | 0.0 | 0.763 |
| Europe | 27,30,31 | 523/574,159 | 0.37(0.25–0.54) | 22.6 | 0.275 |
| North America | 3,32 | 1,571/1,374,915 | 0.75(0.59–0.95) | 0.0 | 0.543 |
| Adjustment for tea | |||||
| Yes | 24,29,30,32 | 1,341/342,768 | 0.53(0.39–0.72) | 0.0 | 0.557 |
| no | 3,25–28,31 | 2,041/1,905,732 | 0.55(0.41–0.74) | 53.0 | 0.030 |
| Adjustment for history of liver diseases | |||||
| Yes | 25–28 | 727/204,060 | 0.48(0.36–0.63) | 0.0 | 0.665 |
| No | 3,24, 29–32 | 2,662/2,044,440 | 0.58(0.43–0.78) | 53.5 | 0.035 |
| Adjustment for history of diabetes | |||||
| Yes | 24,27–32 | 1,894/890,534 | 0.43(0.33–0.56) | 9.9 | 0.353 |
| No | 3,25,26 | 1,495/1,357,966 | 0.69(0.56–0.84) | 0.0 | 0.453 |
| Adjustment for BMI | |||||
| Yes | 3,27–32 | 2,680/2,224,975 | 0.56(0.42–0.74) | 51.5 | 0.036 |
| No | 24–26 | 709/235,525 | 0.50(0.36–0.70) | 0.0 | 0.527 |
| Adjustment for education | |||||
| Yes | 24,27,29–32 | 1,670/889,899 | 0.43(0.32–0.58) | 24.6 | 0.249 |
| No | 3,25,26,28 | 1,719/1,358,601 | 0.67(0.54–0.82) | 0.0 | 0.430 |
No., Number.
Dose-response analysis
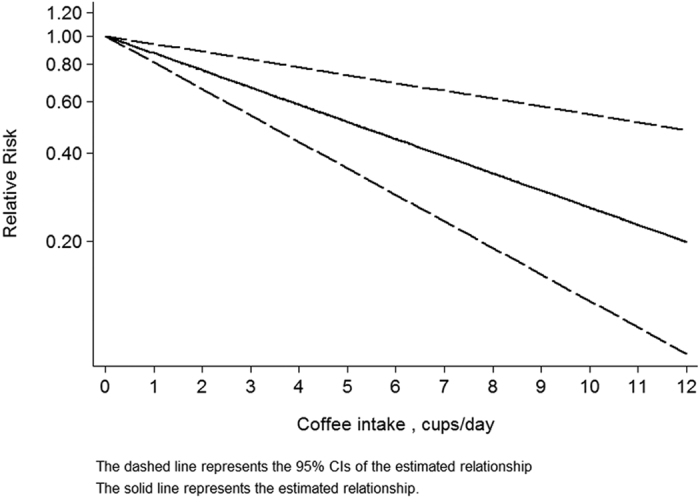
Eight publications providing necessary data were included in the dose-response analysis3,24,25,26,27,29,31,32. As shown in Fig. 3, a linear association between coffee consumption (cups/day) and risk of liver cancer was observed (P = 0.36). Compared with non-drinkers of coffee, the pooled RRs with 95% CIs were 0.87 (0.81–0.94), 0.76 (0.66–0.88), 0.67 (0.54–0.83), 0.58 (0.44–0.78), 0.51 (0.35–0.74), 0.44 (0.29–0.69) for consumption of 1 cup/day, 2 cups/day, 3 cups/day, 4 cups/day, 5 cups/day, and 6 cups/day, respectively.
Figure 3. Dose-response relationships for the association between coffee consumption and liver cancer risk.
Discussion
Main Findings
The current meta-analysis summarized evidence from twenty prospective cohort studies described in ten publications assessing the effect of coffee consumption on liver cancer risk. The results suggested that higher coffee intake was inversely associated with liver cancer risk. Analyses stratified by gender indicated that coffee intake was associated with a similar reduction in liver cancer risk in men and women. When the analysis was stratified by geographic region, a slight, moderate, and strong reduction in liver cancer risk was observed for observed in North American, Asian, and Europe an, respectively. Subgroup analyses performed to clarify the effects of confounding factors on liver cancer showed that our results were less susceptible to biases resulting from history of liver disease, history of diabetes, BMI, education, or tea consumption.
Issues to further discuss and limitations
First, the types of coffee (caffeinated vs. decaffeinated coffee) associated with liver cancer risk were not differentiated. Only three studies provided separate data for caffeinated and decaffeinated coffee3,31,32. Findings from these three studies suggested a decreasing trend in HCC risk with increased caffeinated coffee consumption, however, no significant association was observed between decaffeinated coffee consumption and risk of liver cancer3,31,32. The significance of this discrepancy is unknown. Notably, limited numbers of subjects reporting decaffeinated coffee consumption were included in these studies. The null results were possibly due to limited statistical power. Moreover, only two studies quantified the effect of the amount of caffeine intake on liver cancer risk29,32. The Singapore Chinese Health Study reported that the RRs with 95% CIs for the 2nd (72–115 mg/day), 3rd (115–224 mg/day), and 4th (224–1,055 mg/day) quartile of caffeine intake were 1.09 (0.82–1.46), 0.82 (0.61–1.11), and 0.73 (0.54–1.00), respectively (p for trend = 0.02), when compared with the lowest quartile (0–722 mg/day)29. In the US Multiethnic Cohort, some interesting results were identified. Caffeine was associated with a decreased risk of HCC in models adjusting for age, sex, and ethnicity adjusted; however, when regular coffee was included as a confounding factor, the results were no longer statistically significant32. In consideration of the aforementioned findings, it’s possible that the inverse association between coffee consumption and liver cancer risk may not be largely a result of caffeine intake, and thus more studies are warranted to better explore the possibility of differential associations between caffeinated and decaffeinated coffee and liver cancer risk.
Second, the association between coffee consumption and liver cancer risk may vary by the method used for brewing coffee. Studies have shown that the presence of various compounds retained in coffee is dependent upon the preparation method of preparation. For example, compared with drip-filtered, percolated, or instant coffee, the amounts of cafestol and kahweol were found to be much higher in Scandinavian-styled boiled and Turkish coffee33,34. To our knowledge, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBCCPS) has been the first prospective analysis of the relationship between liver cancer risk and coffee intake by coffee preparation method30. In the ATBCCPS of 20,737 men, boiling (about 21%) and filtering (about 71%) were the two most common methods of coffee preparation. Similar results were observed for consumption of boiled and filtered coffee.
Third, the dose effect of coffee intake duration or cumulative coffee intake prior to liver cancer diagnosis on liver cancer risk requires further elucidation. Some of included studies only reported data at baseline, which may not reflect changes in consumption habits following the date of study initiation. It is possible that non-coffee consuming individuals became coffee drinkers or individuals who consumed coffee at baseline may have stopped this consumption behavior after questionnaire administration. Thus, an over- or under-estimation of association between liver cancer risk and the true amount of coffee intake may have resulted.
Fourth, as a meta-analysis of published studies, potential publication biases may distort the results in either direction, however, no evidence for publication bias was observed.
Finally, the presence of unmeasured confounding inherited from original studies may be a matter of major concern. All included studies provided risk estimates derived from multivariable models (Table 1). However, only two studies provided risk estimates adjusted for age, sex, smoking, alcohol intake, history of liver disease, diabetes, and BMI. Thus, our findings may have been influenced if included studies failed to consider potential confounding factors.
Comparison with previous meta-analyses and strengths
To elucidate this issue, five systematic reviews or meta-analyses have been published7,8,9,10,35. In a meta-analysis of four cohort and 5 case-control studies, Larsson et al. found that increased consumption of coffee may reduce the risk of liver cancer7. Three latter meta-analyses of cohort and case-control studies also found an inverse association between coffee consumption and liver cancer risk8,9,10. In 2016, Bravi et al. performed an updated meta-analysis of cohort studies and observed a 15% decreased risk in liver cancer for an increment of 1 cup of coffee per day35. In a model comparing regular, low, and high consumption with no or occasional coffee consumption, the pooled RRs with 95 CIs were 0.66 (0.55–0.78) for regular, 0.78 (0.66–0.91) for low, and 0.50 (0.43–0.58) for high coffee consumption, respectively.35 Compared with previous meta-analyses, our study had two major strengths. First, no retrospective case-control studies were included. This meta-analysis included twenty prospective cohort studies, which enhanced statistical power to detect significant associations. Additionally, potential biases related to recall, interviewers, and selection were greatly minimized. Second, an analysis of dose–response, which is considered to be an important criterion for the determination of causal relationships between exposure and disease in epidemiologic studies, was performed to quantify the relationship between coffee intake and liver cancer risk. We observed no evidence of statistically significant departure from linearity (P = 0.36). The pooled RRs with 95% CIs were 0.87 (0.81–0.94), 0.76 (0.66–0.88), 0.67 (0.54–0.83), 0.58 (0.44–0.78), 0.51 (0.35–0.74), and 0.44 (0.29–0.69) for 1 cup/day, 2 cups/day, 3 cups/day, 4 cups/day, 5 cups/day, and 6 cups/day, respectively.
Conclusions
In conclusion, the results of our study provide evidence of a significant inverse linear dose-response association between coffee consumption and liver cancer risk.
Additional Information
How to cite this article: Yu, C. et al. An updated dose–response meta-analysis of coffee consumption and liver cancer risk. Sci. Rep. 6, 37488; doi: 10.1038/srep37488 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The work was supported by grants from the National Scientific and Technological Major Project of China (No. 2013ZX10004904).
Footnotes
Author Contributions Guarantor of integrity of the entire study: L.L., C.Y. Study concepts and study design: C.Y., L.L. Literature research and data acquisition: C.Y., Q.C. Data analysis: C.Y., Q.C., P.C., S.Y., M.D. Manuscript preparation: Q.C., Y.W. Manuscript editing: C.Y., Y.W., Q.C. Manuscript review: C.Y., Y.W., L.L.
References
- McGlynn K. A., Petrick J. L. & London W. T. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 19, 223–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- An N. Oral Contraceptives Use and Liver Cancer Risk: A Dose-Response Meta-Analysis of Observational Studies. Medicine (Baltimore). 94, e1619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick J. L. et al. Coffee Consumption and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma by Sex: The Liver Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 24, 1398–1406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 147, 1031–1042 (2014). [DOI] [PubMed] [Google Scholar]
- Nkondjock A. Coffee consumption and the risk of cancer: an overview. Cancer Lett. 277, 121–125 (2009). [DOI] [PubMed] [Google Scholar]
- Bode A. M. & Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 247, 26–39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S. C. & Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 132, 1740–1745 (2007). [DOI] [PubMed] [Google Scholar]
- Bravi F. et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 46, 430–435 (2007). [DOI] [PubMed] [Google Scholar]
- Sang L. X., Chang B., Li X. H. & Jiang M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol. 13, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravi F., Bosetti C., Tavani A., Gallus S. & La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 11, 1413–11421 (2013). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson S., Tatt I. D. & Higgins J. P. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 36, 666–676 (2007). [DOI] [PubMed] [Google Scholar]
- Lo C. K., Mertz D. & Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 14, 45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials. 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med. 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey, Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 135, 1301–1309 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N., Bellocco R. & Greenland S. Generalized least squares for trend estimation of summarized doseresponse data. Stata J. 6, 46–57 (2006). [Google Scholar]
- Harrell F. E. Jr., Lee K. L. & Pollock B. G. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 80, 1198–1202 (1988). [DOI] [PubMed] [Google Scholar]
- Wakai K. et al. Liver cancer risk, coffee, and hepatitis C virus infection: a nested case-control study in Japan. Br J Cancer. 97, 426–428 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M. et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev. 18, 1746–1753 (2009). [DOI] [PubMed] [Google Scholar]
- Aleksandrova K. et al. The association of coffee intake with liver cancer risk is mediated by biomarkers of inflammation and hepatocellular injury: Data from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 102, 1498–1508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan V. W., Wilkens L. R., Hernandez B. Y., Marchand L. L. & Henderson B. E. Coffee intake reduces hepatocellular carcinoma risk: The Multiethnic Cohort. Cancer Res. 74, 281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Yoshimi I., Sobue T. & Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst. 97, 293–300 (2005). [DOI] [PubMed] [Google Scholar]
- Kurozawa Y. et al. Coffee and risk of death from hepatocellular carcinoma in a large cohort study in Japan. Br J Cancer. 93, 607–610 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T. et al. Coffee consumption and the risk of primary liver cancer: pooled analysis of two prospective studies in Japan. International journal of cancer. Int J Cancer. 116, 150–154 (2005). [DOI] [PubMed] [Google Scholar]
- Hu G. et al. Joint effects of coffee consumption and serum gamma-glutamyltransferase on the risk of liver cancer. Hepatology. 48, 129–136 (2008). [DOI] [PubMed] [Google Scholar]
- Ohishi W. et al. Risk factors for hepatocellular carcinoma in a Japanese population: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 17, 846–854 (2008). [DOI] [PubMed] [Google Scholar]
- Johnson S. et al. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes Control. 22, 503–510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai G. Y. et al. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. Br J Cancer. 109, 1344–1351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamia C. et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int J Cancer. 136, 1899–1908 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan V. W. et al. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 148, 118–125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgert R. et al. Levels of the Cholesterol-Elevating Diterpenes Cafestol and Kahweol in Various Coffee Brews. J Agric Food Chem. 43, 2167–2172 (1995). [Google Scholar]
- Gross G., Jaccaud E. & Huggett A. C. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem Toxicol. 35, 547–554 (1997). [DOI] [PubMed] [Google Scholar]
- Bravi F., Tavani A., Bosetti C., Boffetta P. & La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev., doi: 10.1097/CEJ.0000000000000252 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.