Abstract
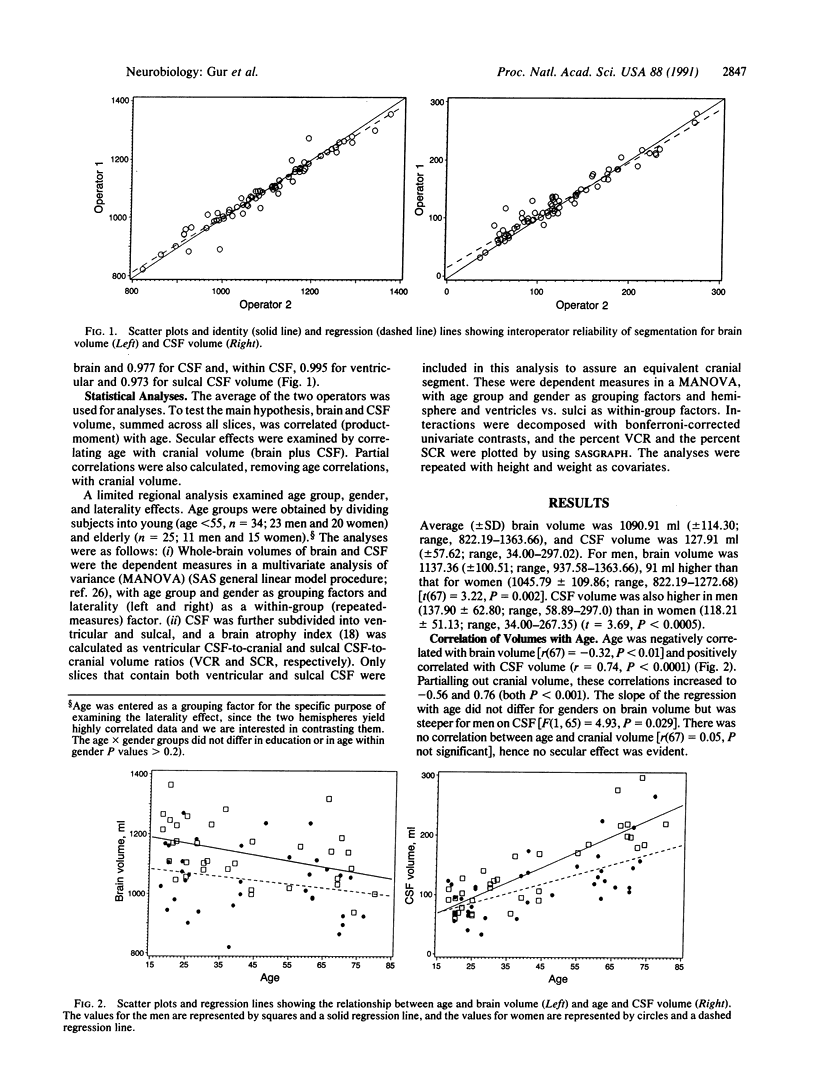
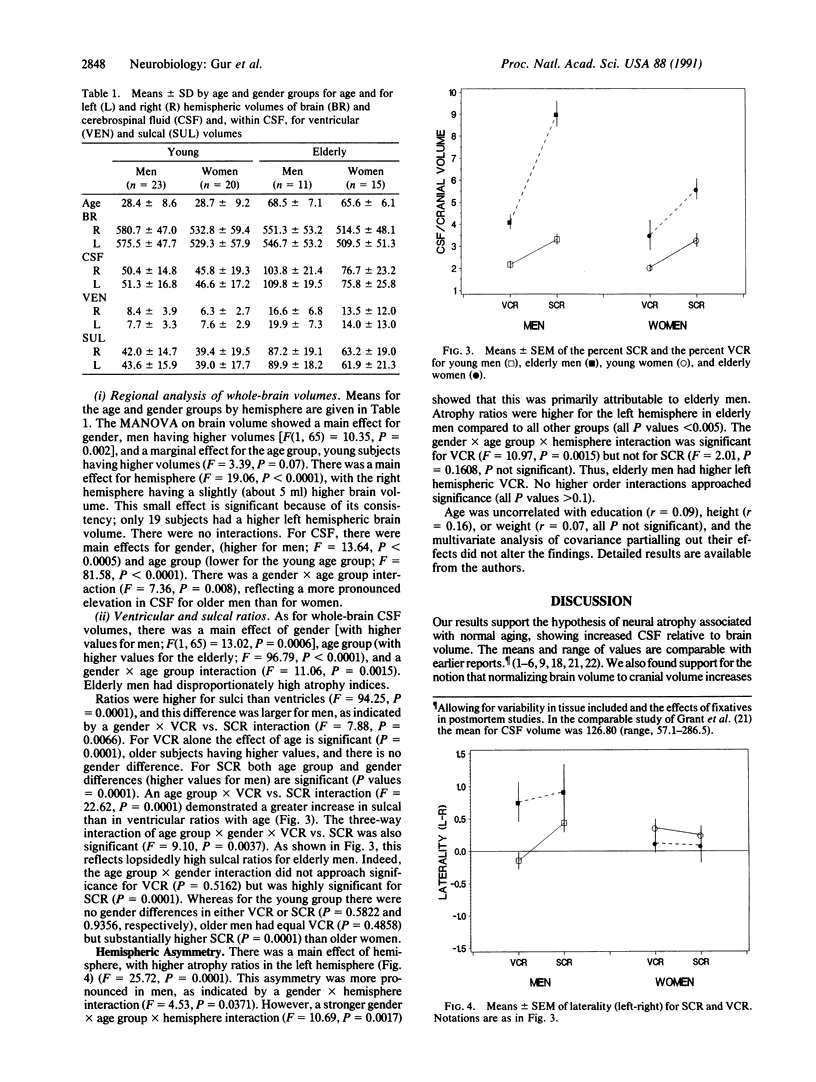
A prospective sample of 69 healthy adults, age range 18-80 years, was studied with magnetic resonance imaging scans (T2 weighted, 5 mm thick) of the entire cranium. Volumes were obtained by a segmentation algorithm that uses proton density and T2 pixel values to correct field inhomogeneities ("shading"). Average (+/- SD) brain volume, excluding cerebellum, was 1090.91 ml (+/- 114.30; range, 822.19-1363.66), and cerebrospinal fluid (CSF) volume was 127.91 ml (+/- 57.62; range, 34.00-297.02). Brain volume was higher (by 5 ml) in the right hemisphere (P less than 0.0001). Men (n = 34) had 91 ml higher brain and 20 ml higher CSF volume than women (n = 35). Age was negatively correlated with brain volume [r(67) = -0.32, P less than 0.01] and positively correlated with CSF volume (r = 0.74, P less than 0.0001). The slope of the regression line with age for CSF was steeper for men than women (P = 0.03). This difference in slopes was significant for sulcal (P less than 0.0001), but not ventricular, CSF. The greatest amount of atrophy in elderly men was in the left hemisphere, whereas in women age effects were symmetric. The findings may point to neuroanatomic substrates of hemispheric specialization and gender differences in age-related changes in brain function. They suggest that women are less vulnerable to age-related changes in mental abilities, whereas men are particularly susceptible to aging effects on left hemispheric functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Condon B., Grant R., Hadley D., Lawrence A. Brain and intracranial cavity volumes: in vivo determination by MRI. Acta Neurol Scand. 1988 Nov;78(5):387–393. doi: 10.1111/j.1600-0404.1988.tb03674.x. [DOI] [PubMed] [Google Scholar]
- Condon B., Patterson J., Jenkins A., Wyper D., Hadley D., Grant R., Rowan J., Teasdale G. MR relaxation times of cerebrospinal fluid. J Comput Assist Tomogr. 1987 Mar-Apr;11(2):203–207. doi: 10.1097/00004728-198703000-00001. [DOI] [PubMed] [Google Scholar]
- Dekaban A. S. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978 Oct;4(4):345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Geschwind N., Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968 Jul 12;161(3837):186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Grant R., Condon B., Lawrence A., Hadley D. M., Patterson J., Bone I., Teasdale G. M. Human cranial CSF volumes measured by MRI: sex and age influences. Magn Reson Imaging. 1987;5(6):465–468. doi: 10.1016/0730-725x(87)90380-8. [DOI] [PubMed] [Google Scholar]
- Gur R. C., Gur R. E., Obrist W. D., Hungerbuhler J. P., Younkin D., Rosen A. D., Skolnick B. E., Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982 Aug 13;217(4560):659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Gur R. C., Gur R. E., Obrist W. D., Skolnick B. E., Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Arch Gen Psychiatry. 1987 Jul;44(7):617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- Gur R. C., Packer I. K., Hungerbuhler J. P., Reivich M., Obrist W. D., Amarnek W. S., Sackeim H. A. Differences in the distribution of gray and white matter in human cerebral hemispheres. Science. 1980 Mar 14;207(4436):1226–1228. doi: 10.1126/science.7355287. [DOI] [PubMed] [Google Scholar]
- Gur R. E. Motoric laterality imbalance in schizophrenia. A possible concomitant of left hemisphere dysfunction. Arch Gen Psychiatry. 1977 Jan;34(1):33–37. doi: 10.1001/archpsyc.1977.01770130035003. [DOI] [PubMed] [Google Scholar]
- Harper C., Kril J., Raven D., Jones N. Intracranial cavity volumes: a new method and its potential applications. Neuropathol Appl Neurobiol. 1984 Jan-Feb;10(1):25–32. doi: 10.1111/j.1365-2990.1984.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Herscovitch P., Auchus A. P., Gado M., Chi D., Raichle M. E. Correction of positron emission tomography data for cerebral atrophy. J Cereb Blood Flow Metab. 1986 Feb;6(1):120–124. doi: 10.1038/jcbfm.1986.14. [DOI] [PubMed] [Google Scholar]
- Jack C. R., Jr, Gehring D. G., Sharbrough F. W., Felmlee J. P., Forbes G., Hench V. S., Zinsmeister A. R. Temporal lobe volume measurement from MR images: accuracy and left-right asymmetry in normal persons. J Comput Assist Tomogr. 1988 Jan-Feb;12(1):21–29. doi: 10.1097/00004728-198801000-00003. [DOI] [PubMed] [Google Scholar]
- Jernigan T. L., Press G. A., Hesselink J. R. Methods for measuring brain morphologic features on magnetic resonance images. Validation and normal aging. Arch Neurol. 1990 Jan;47(1):27–32. doi: 10.1001/archneur.1990.00530010035015. [DOI] [PubMed] [Google Scholar]
- Kohn M. I., Tanna N. K., Herman G. T., Resnick S. M., Mozley P. D., Gur R. E., Alavi A., Zimmerman R. A., Gur R. C. Analysis of brain and cerebrospinal fluid volumes with MR imaging. Part I. Methods, reliability, and validation. Radiology. 1991 Jan;178(1):115–122. doi: 10.1148/radiology.178.1.1984289. [DOI] [PubMed] [Google Scholar]
- Marshall J. Relations between the Weight of the Brain and its Parts, and the Stature and Mass of the Body, in Man. J Anat Physiol. 1892 Jul;26(Pt 4):445–500. [PMC free article] [PubMed] [Google Scholar]
- Miller A. K., Alston R. L., Corsellis J. A. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol Appl Neurobiol. 1980 Mar-Apr;6(2):119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Schlenska G. Messungen der Oberfläche und der Volumenanteile des Gehirnes menschlicher Erwachsener mit neuen Methoden. Z Anat Entwicklungsgesch. 1969;128(1):47–59. [PubMed] [Google Scholar]
- Steiner I., Gomori J. M., Melamed E. Progressive brain atrophy during normal aging in man: a quantitative computerized tomography study. Isr J Med Sci. 1985 Mar;21(3):279–282. [PubMed] [Google Scholar]
- Takeda S., Matsuzawa T. Age-related brain atrophy: a study with computed tomography. J Gerontol. 1985 Mar;40(2):159–163. doi: 10.1093/geronj/40.2.159. [DOI] [PubMed] [Google Scholar]
- Takeda S., Matsuzawa T., Matsui H. Age-related changes in regional cerebral blood flow and brain volume in healthy subjects. J Am Geriatr Soc. 1988 Apr;36(4):293–297. doi: 10.1111/j.1532-5415.1988.tb02353.x. [DOI] [PubMed] [Google Scholar]
- Yoshii F., Barker W. W., Chang J. Y., Loewenstein D., Apicella A., Smith D., Boothe T., Ginsberg M. D., Pascal S., Duara R. Sensitivity of cerebral glucose metabolism to age, gender, brain volume, brain atrophy, and cerebrovascular risk factors. J Cereb Blood Flow Metab. 1988 Oct;8(5):654–661. doi: 10.1038/jcbfm.1988.112. [DOI] [PubMed] [Google Scholar]
- de Leon M. J., George A. E., Ferris S. H., Christman D. R., Fowler J. S., Gentes C. I., Brodie J., Reisberg B., Wolf A. P. Positron emission tomography and computed tomography assessments of the aging human brain. J Comput Assist Tomogr. 1984 Feb;8(1):88–94. doi: 10.1097/00004728-198402000-00017. [DOI] [PubMed] [Google Scholar]


