Abstract
Objectives
Hippocampal dysfunction has been proposed as a mechanism for memory deficits in schizophrenia. Available evidence suggests that the anterior and posterior hippocampus could be differentially affected. Accordingly, we used fMRI to test the hypothesis that activity in posterior hippocampus is disproportionately reduced in schizophrenia, particularly during spatial memory retrieval.
Methods
26 healthy participants and 24 patients with schizophrenia from the UC Davis Early Psychosis Program were studied while fMRI was acquired on a 3 Tesla Siemens scanner. During encoding, participants were oriented to critical items through questions about item features (e.g., “Does the lamp have a square shade?”) or spatial location (e.g., “Is the lamp on the table next to the couch?”). At test, participants determined whether scenes were changed or unchanged. fMRI analyses contrasted activation in a priori regions of interest (ROI) in anterior and posterior hippocampus during correct recognition of item changes and spatial changes.
Results
As predicted, patients with schizophrenia exhibited reduced activation in the posterior hippocampus during detection of spatial changes but not during detection of item changes. Unexpectedly, patients exhibited increased activation of anterior hippocampus during detection of item changes. Whole brain analyses revealed reduced fronto-parietal and striatal activation in patients for spatial but not for item change trials.
Conclusions
Results suggest a gradient of hippocampal dysfunction in which posterior hippocampus – which is necessary for processing fine-grained spatial relationships – is underactive, and anterior hippocampus – which may process context more globally - is overactive.
Keywords: Neurocognition, Episodic memory, Eye tracking, fMRI, Hippocampus, Schizophrenia
Highlights
-
•
Patients with schizophrenia show impaired memory for spatial relationships amongst objects in scenes.
-
•
Patients have less posterior hippocampal activation during spatial memory and more anterior hippocampal activation during item memory.
-
•
This gradient of hippocampal dysfunction in schizophrenia, suggests that it should not be examined as a unitary structure in future studies.
1. Introduction
Episodic memory impairment is a core symptom of psychotic disorders such as schizophrenia (Achim and Lepage, 2003, Achim and Lepage, 2005, Titone et al., 2004, Ranganath et al., 2008, Ragland et al., 2015), and this deficit is likely related to impaired hippocampal function. Basic behavioral and cognitive neuroscience research reveals important functional differences across the longitudinal extent of the anterior and posterior hippocampus. However, it is unclear whether the anterior and posterior hippocampus is differentially affected in schizophrenia.
Research in rodents demonstrates that the dorsal (corresponding to posterior in humans) and ventral hippocampus (corresponding to anterior in humans) differ in terms of anatomical connectivity and gene expression (Fanselow and Dong, 2010, Strange et al., 2014). Furthermore, lesions to the dorsal hippocampus in rats impair performance on spatial memory tasks, whereas ventral hippocampal lesions often have no effect on spatial learning (Moser and Moser, 1998, Bannerman et al., 2004). Studies of place cells in rats complement lesion findings by suggesting that place cells in dorsal hippocampus exhibit specific place fields, whereas place cells in ventral hippocampus are often large, sometimes extending across an entire spatial context (Kjelstrup et al., 2008, Keinath et al., 2014).
Dissociations between anterior and posterior hippocampal functions have also been observed in humans with fMRI (Strange et al., 2014). Functional dissociations between anterior and posterior hippocampus have been attributed to processing of; novel versus repeated stimuli (Ranganath and Rainer, 2003, Strange et al., 2005, Kumaran and Maguire, 2006), emotional versus non-emotional material (Gray and McNaughton, 1982, Murty et al., 2010) and encoding versus retrieval (Spaniol et al., 2009). Work has also linked posterior hippocampus to retrieving memories based on spatial context information, and anterior hippocampus to more global and less context-dependent relational memory processes (Hannula et al., 2013). Moreover, when processing spatial information, a distinction exists between retrieval of fine-grained spatial details (posterior hippocampus) versus more global gist-like information (anterior hippocampus). This has contributed to a “granularity gradient” model in which the head of the hippocampus retrieves course, large-scale representations; the hippocampal body retrieves medium sized spatial representations; and the tail of the hippocampus retrieves fine-grained local representations (Poppenk et al., 2013, Evensmoen et al., 2015). Based on this granularity model, we predict that manipulation of spatial locations during the current memory task will produce maximal activation in the posterior hippocampus, whereas the anterior hippocampus will be most sensitive to global changes in item identity.
This distinction between anterior and posterior hippocampal function has received minimal attention in the schizophrenia literature. The most consistent story comes from resting-state studies supporting a model of tonic hyperactivity of the anterior hippocampus in patients versus healthy controls (Grace, 2012) According to this view, increased activity of the ventral/anterior hippocampus leads to disinhibition of the ventral tegmental area, and this, in turn, contributes to increased dopaminergic activity and disordered cognition in schizophrenia (Lodge and Grace, 2011, Grace, 2012). Consistent with this idea, a series of resting-state studies of cerebral blood volume (CBV) (Schobel et al., 2009, Schobel et al., 2013, Talati et al., 2014), found that CBV was increased in patients relative to healthy controls specifically within the CA1 region of the anterior hippocampus [for review, see (Heckers and Konradi, 2015)]. It is unclear how this tonic hyperactivity would relate to specific episodic memory functions, although an early study suggested that a hyperactive baseline state could help explain the reduced responsivity of the hippocampus to memory demands (Weiss et al., 2003).
With one exception (Tamminga et al., 2012), fMRI studies of episodic memory in schizophrenia have not systematically examined anterior versus posterior hippocampus. In an attempt to detect a consistent anterior/posterior pattern of group differences we performed a qualitative review and found roughly an equal number of studies that; 1) employed tasks that failed to engage the hippocampus or detect group differences in either anterior or posterior hippocampus (Ragland et al., 2004, Bonner-Jackson et al., 2005, Ragland et al., 2005, Lepage et al., 2006), 2) utilized designs that were successful in detecting reduced activation in patients, relative to healthy controls, in the anterior hippocampus (Ongur et al., 2006, Tamminga et al., 2012, Ragland et al., 2015), or 3) utilized tasks that revealed evidence of reduced patient activation in the posterior hippocampus during episodic retrieval (Heckers et al., 1998, Weiss et al., 2003, Achim and Lepage, 2005). The one study that directly contrasted anterior and posterior hippocampus found that anterior hippocampal activity increased during novelty detection in healthy controls, and was reduced in unmedicated but not in medicated people with schizophrenia (Tamminga et al., 2012).
Based on findings indicating potentially different effects on anterior and posterior hippocampus, the goal of the current study was to test the hypothesis that patients with schizophrenia are specifically impaired at activating the posterior hippocampus during episodic retrieval. Participants were scanned while performing a memory paradigm that required detection of subtle changes to items or spatial configurations in complex visual scenes (Hannula et al., 2010a). In a previous eye-tracking study using a variant of the current task (Hannula et al., 2010a), we found that patients with schizophrenia spent less time viewing the portion of a scene that involved a spatial change in item location, but showed a normal increase in viewing when there was change in item identity, suggesting a disproportionate eye-movement memory deficit for spatial information. Based on findings indicating posterior hippocampal specialization for fine-grained spatial relationships and spatial context (Hannula and Ranganath, 2008, Poppenk et al., 2013, Evensmoen et al., 2015), we hypothesized that patients with schizophrenia would show relative reductions in posterior, but not anterior hippocampal activation during processing of spatial changes.
2. Methods and materials
2.1. Participants
Data were acquired on 30 patients with schizophrenia and 30 healthy controls. Data were excluded for excessive relative frame-to-frame movement (i.e., > 0.4 mm, exceeding 3 standard deviations from the mean) in 1 control and 1 patient; for below chance performance in 1 control and 2 patients; for 1 control and 2 patients who stopped participation; for 1 control with an incidental abnormal anatomical finding; and for 1 patient with a technical malfunction (faulty button box), leaving a final sample of 24 patients with schizophrenia and 26 controls. Groups were matched for age, gender, handedness and parental education (Table 1). Participant education was lower in patients. All patients were receiving medication (2 typical, 22 atypical), and clinically stable. Patients were early in their illness [Duration (mean ± standard deviation) = 3.02 ± 2.51 years], and overall symptom severity (BPRS total) was in the mild range (42.7 ± 13.3). Data collection began after participants provided written informed consent following Institutional Review Board approval.
Table 1.
Demographic characteristics of research participants.
| Healthy control group (n = 26) |
Patients with schizophrenia (n = 24) |
||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| Age (years) | 23.1 | 3.5 | 25.2 | 4.2 | ns |
| Gender (% male) | 4 F, 22 M | 6 F, 18 M | ns | ||
| Handedness | 1 left | 2 left | ns | ||
| Education (years) | 14.8 | 1.4 | 13.1 | 1.9 | < 0.1 |
| Parental education (years) | 15.6 | 2.9 | 14.8 | 3.4 | ns |
| BPRS (total) | 42.7 | 13.3 | |||
Note: SANS = Scale for the Assessment of Negative Symptoms; SAPS = Scale for the Assessment of Positive Symptoms; BPRS = Brief Psychiatric Rating Scale; SD = standard deviation; ns = no significant group difference at p < 0.05, two-tailed.
2.2. Materials
Stimuli included 128 rendered scenes created using Punch! Home Design software (Encore, Inc., El Segundo, CA). Three scene variants were created – the original, a version containing an item manipulation, and a version containing a spatial manipulation – producing a total of 384 scenes. One item embedded in each original scene was designated the critical item and, in manipulated versions, this item was either replaced with a different exemplar (item change) or displaced and moved to a new location (spatial change). Critical items were presented in the context of just one scene, and moved equally often from left (in the original scene) to right (in the changed scene) as right to left when the change was spatial.
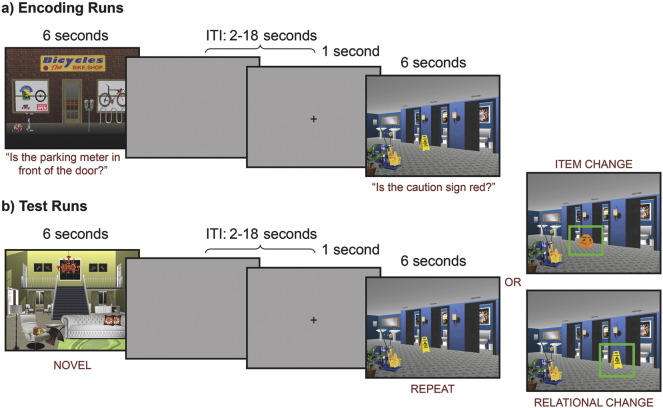
During encoding (Fig. 1a), participants were presented with orienting questions, directing their attention to the critical item, and were asked either about item features (e.g., “Does the lamp have a square shade?”) or spatial location (e.g., “Is the lamp on the table next to the couch?”). These questions ensured that participants attended to and encoded critical items that might subsequently change during the test phase (Hannula et al., 2010a, Hannula et al., 2006). The experiment was designed so that the type of change at test (item or spatial) was always consistent with processing required by the initial orienting question.
Fig. 1.
Experimental methods. (A) During encoding, participants viewed scenes for 6 seconds while they received an auditory presentation of a question orienting them to the critical item in the scene. One of two buttons were used to make a “yes/no” response. Following a variable ITI of 2-18 seconds, a visual fixation cross hair appeared for 1 second, alerting to upcoming presentation of the next scene. This figure illustrates both a spatial and item orienting question. (B) During retrieval, participants viewed scenes that had not been seen before (novel), had been repeated and unchanged, had a change in either the critical item (item change) or item location (spatial change), and made a button press to indicate if scenes were changed, unchanged or novel. Presentation timing was identical to the encoding condition.
2.3. Methods
After informed consent, each participant successfully completed a practice session before being situated in the fMRI scanner with head pads to minimize motion. Scanning consisted of four interleaved encoding and test runs.
Each encoding run consisted of 24 trials. Trials initiated with scene presentation, which remained in view for 6 s. Orienting questions were played via noise cancelling headphones, and participants were instructed to make “yes” or “no” button presses, and attempt to commit each picture to memory. Trials were separated by a variable duration inter-trial interval (ITI: 2–18 s), with a 1 s central fixation cross occurring during this ITI prior to onset of the next scene. Timing was optimized using Optseq (http://surfer.nmr.mgh.harvard.edu/optseq/). Each encoding run lasted 278 s and, across four runs, participants encoded 96 trial-unique pictures.
Each test run consisted of 32 trials. Presented scenes were either new and had not been seen during encoding (novel condition: 8 trials), or had been studied previously (24 trials), and were counterbalanced across conditions. Studied scenes were either unchanged (8 trials), contained a new item (item change: 8 trials), or contained a change in item location (spatial change: 8 trials). Each trial began with presentation of a scene for 6 s, and event timing was identical to encoding. Participants were instructed to indicate as quickly and accurately as possible whether each scene was new (i.e. had not been seen in the corresponding encoding run), unchanged (i.e. seen before and completely unchanged), or changed (i.e. seen before, but now contained an item or a spatial change). A variable duration ITI (2–18 s) culminated with a cross hair to encourage central fixation and alert participants to the next scene (see Fig. 1). Test runs lasted 366 s and, across four runs, participants completed 128 test trials. Eye movements were also recorded during the test phase to examine eye movement memory effects (Hannula et al., 2010b). However, because of technical difficulties, eye movement data were available for only a subset of participants. Although the pattern of results paralleled earlier findings (Hannula et al., 2010a), because of the limited sample size, and because they are not a focus of the current investigation, eye tracking methods and results are summarized in Supplementary Materials.
2.3.1. fMRI acquisition
Data were obtained at the UC Davis Imaging Research Center on a 3-T Siemens Tim Trio scanner (Erlangen, Germany) with a Siemens 8 channel phased array coil. After acquiring a rapid 3-plane localizer, trans-axial T2 weighted images were acquired with spatial resolution of 0.4 × 0.4 × 4.0 mm. A reference field-map image with spatial resolution of 3.4 × 3.4 × 4.0 mm was used for motion and distortion correction. Functional images were acquired with blood oxygenation level dependent imaging (BOLD) using a 34-slice whole-brain, single-shot gradient-echo echo-planar sequence (TR 2000 ms, TE 27 ms, flip angle 90°, FOV 220 × 220 mm, slice thickness 4.0 mm, no gap). Structural T1-weighted images were acquired using an MPRAGE sequence (TR = 2000 ms, TE ~ 3.0 ms, FA = 8°, FOV = 256 × 240 mm, matrix: 256 × 256, voxel size: 1 mm isotropic, 208 sagittal slices).
2.3.2. fMRI processing
Pre-processing was accomplished with FMRI Expert Analysis Tool (FEAT) in the FMRIB Software Library (FSL version 4.1; www.fmrib.ox.ac.uk/fsl) using standard procedures, including fieldmap correction. Statistical analysis was performed using a general linear model (GLM) implemented in FEAT (FSL version 4.1). Test phase activity associated with responses for each scene type (unchanged, novel, item change, spatial change) was modeled for each 6 s presentation epoch by convolving a vector of expected neural activity with the 3 basis-function set of FMRIB's Linear Optimal Basis Sets (FLOBS), which adjusts the canonical hemodynamic response function to account for delay and dispersion in order to improve GLM fit. Although both correct and incorrect responses were modeled, only correct responses were examined at the group level. Temporal autocorrelation due to low frequency (“1/f”) noise was accounted for by pre-whitening (FSL's FILM). The 4 test runs were combined in a 2nd level fixed-effect analysis for each subject. These parameter estimates were used in a 3rd level mixed-effects analysis to derive group-level maps that separately contrasted item and spatial change trials with unchanged trials for all subjects (FSL's FLAME). Because relative motion (mean ± standard deviation, in mm) was greater in patients with schizophrenia (0.11 ± 0.06 mm) than in healthy controls (0.07 ± 0.03 mm; t(48) = − 2.92, p < 0.01), relative motion was included as a covariate of no-interest.
To test hypotheses about the effects of memory for item versus spatial information on hippocampal activation, we used previously established methods (Hannula et al., 2013), utilizing anatomical landmarks to define a single set of structural regions of interest (ROIs) in the head, body, and tail of the hippocampus that were examined for task effects and group differences for each contrast (i.e., item change minus unchanged, spatial change minus unchanged). Given our hypotheses, results are summarized here only for the anterior (head) and posterior (tail) hippocampus, and body results are summarized in Supplementary Materials. The MNI coordinates marking the anterior/posterior, medial/lateral and superior/inferior bounds of the hippocampus were y = − 12/− 39, x = ± 18/±36, and z = 6/− 24. The hippocampus was subdivided into three sections of equal length – an anterior segment corresponding to the head, a middle segment corresponding to body, and a posterior segment corresponding to the tail (for a similar approach see (Litman et al., 2009, Staresina et al., 2009)). ROI analysis was followed by exploratory whole-brain analyses to identify additional task effects and group differences.
For each ROI, voxelwise one-sample t-tests identified activated voxel clusters for two contrasts (item change minus unchanged, spatial change minus unchanged), across all participants, with a voxelwise threshold of z > 2.3, and a corrected cluster mass significance threshold of p < 0.05 based on Gaussian Random Field theory (Worsley, 2001) as implemented in FEAT. Resulting parameter estimates (beta weights) were extracted from anterior and posterior ROIs and averaged across hemispheres for each participant. Differences in parameter estimates as a function of group (patients, controls) and change condition (item, spatial) was evaluated using repeated measures analysis of variance (ANOVA) in SAS (version 9.3) separately for anterior and posterior hippocampus. Effect sizes for any significant group differences were estimated using Cohen's d. For the exploratory whole-brain analyses, two-sample t-tests were used to identify any group differences for each contrast, using the FSL whole brain gray matter mask, with the same thresholding and cluster-correction procedures as the ROI analysis.
3. Results
3.1. Performance
Examination of recognition accuracy revealed a main effect of group on percent correct performance [F(1,48) = 8.59, p < 0.01]. Performance (mean ± standard deviation) was less accurate in patients with schizophrenia for unchanged (72.5 ± 13.5 patients, 82.4 ± 9.1 controls), item change (63.5 ± 17.7 patients, 73.7 ± 12.2 controls), and spatial change scenes (72.3 ± 14.7 patients, 80.0 ± 12.0 controls). There was also a main effect of scene type [F(2,47) = 9.10, p < 0.0005], with all participants performing better on unchanged versus item change (77.6 ± 12.4 and 68.8 ± 15.8 respectively; F(1,49) = 14.8, p < 0.001), and spatial change versus item change scenes (75.7 ± 13.6 and 68.8 ± 15.8 respectively; F(1,49) = 15.9, p < 0.0005). However, there was no group by scene type interaction [F(2,47) = 0.77, p = 0.47]. Because of these performance differences, fMRI contrasts were restricted to correct trials only.
3.2. fMRI results
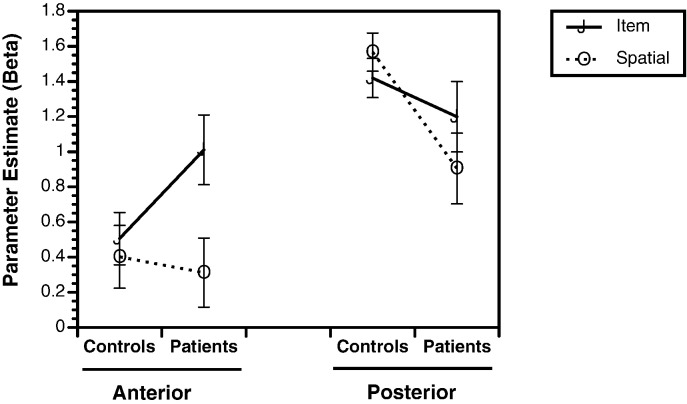
When hippocampal ROIs were examined for item and relational change trials, there were no hemisphere by group [F(1,48) = 0.05, p = 0.83], hemisphere by change trial [F(1,48) = 0.04, p = 0.84], or hemisphere by group by change trial interactions [F(1,48) = 2.0, p = 0.16]. We, therefore, examined bilateral parameter estimates in subsequent analyses. In the anterior hippocampus, there was a main effect of change trial [F(1,48) = 9.87, p < 0.005], and a change trial by group interaction [F(1,48) = 5.41, p < 0.05]. As seen in Fig. 2, this interaction was due to greater anterior hippocampal activation in patients with schizophrenia versus healthy participants for the item change condition [F(1,48) = 4.23, p < 0.05; Cohen's d = 0.51], with no group differences when there was a spatial change [F(1,48) = 0.12, p = 0.73; Cohen's d = 0.10]. Examination of the posterior hippocampus revealed the hypothesized reduction in task-related activation in patients. There was a main effect of group [F(1,48) = 4.86, p < 0.05], and a change trial by group interaction [F(1,48) = 4.79, p < 0.05]. This interaction was due to reduced activation in the posterior hippocampus in patients relative to healthy controls for correct spatial change trials [F(1,48) = 8.76, p < 0.005; Cohen's d = 0.83]. Conversely, there was no group difference in posterior hippocampal activation when participants correctly recognized item changes [F(1,48) = 0.96, p = 0.33; Cohen's d = 0.27].
Fig. 2.
Parameter estimates (Beta values) illustrating group differences (controls versus patients) on bilateral fMRI activation during correct detection of item changes (solid lines) and spatial changes (dotted lines) for regions of interest in the anterior hippocampus and posterior hippocampus. Statistical analyses revealed significant group by condition interactions for the anterior hippocampus (greater activation for patients than controls for item but not spatial memory), and posterior hippocampus (greater activation for controls than for patients for spatial but not item).
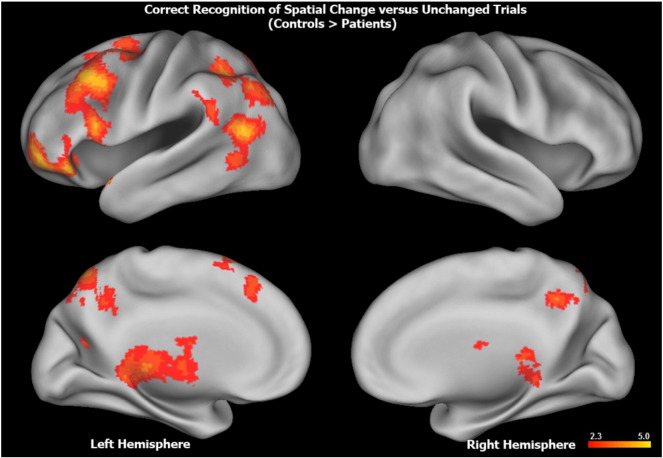
Exploratory whole brain analyses revealed additional group differences for spatial change but not for item change conditions. For the contrast of spatial change with unchanged trials, patients with schizophrenia, relative to healthy controls, had reduced bilateral activation in the precuneus and reduced left hemisphere activation in the lateral and medial prefrontal cortex, inferior and superior parietal cortex and cerebellum (Fig. 3; Supplementary Materials Table 1). There was no evidence of greater activation in patients with schizophrenia relative to healthy controls for either contrast.
Fig. 3.
Surface rendering of greater whole brain activation in healthy controls versus patients with schizophrenia during correct responses to spatial change trials minus unchanged trials. Top row illustrates lateral, and bottom row illustrates medial surface (left hemisphere on left, right hemisphere on right). Hotter colors reflect larger group differences in fronto-parietal and striatal activation (range, z = 2.3–5.0). Anatomical labels, Montreal Neurological Institute coordinates, and z-values for significant clusters of within and between groups effects can be found in Table 1 (Supplementary Materials).
4. Discussion
The goal of the present study was to utilize a previously validated scene memory paradigm (Hannula et al., 2010a) to contrast memory for spatial versus item changes in complex scenes, and test the effects of schizophrenia on anterior and posterior hippocampal activation during successful change detection. Based on evidence that the posterior hippocampus mediates memory for fine-grained spatial information (Evensmoen et al., 2013, Poppenk et al., 2013), and prior evidence that patients show unimpaired eye-movement memory effects for item but not for spatial changes (Hannula et al., 2010a), we predicted that patients would be specifically impaired in their ability to recruit the posterior hippocampus to detect spatial changes, but that there would be no group difference during detection of item changes. fMRI results supported the prediction of reduced posterior hippocampal activation in patients with schizophrenia during recognition of spatial changes, but, unexpectedly, also showed a group difference in the anterior hippocampus, in which patients demonstrated greater activation than healthy controls during correct recognition of item changes.
As noted in the introduction, the dorsal hippocampus in rats, which is homologous to the posterior hippocampus in humans, is known to support fine-grained representation of spatial configurations, and dorsal hippocampal lesions impair spatial memory (Evensmoen et al., 2013). The present paradigm was designed to place heavy demands on these processes by assessing memory for subtle changes in the positions of objects in a complex scene. Consistent with this, fMRI results showed that healthy controls relied disproportionately on the posterior hippocampus to detect item and spatial changes. The notion that the posterior hippocampus may be particularly vulnerable to the pathophysiology of schizophrenia was first suggested by post-mortem studies (Benes et al., 1998). More recently, a resting state fMRI study used dynamic causal modeling (DCM) to demonstrate that connectivity from the hippocampus to inferior frontal gyrus was reduced in patients with schizophrenia and in individuals at high risk for psychosis in the posterior but not in the anterior hippocampus (Benetti et al., 2009). Moreover, a structural MRI study demonstrated regionally specific volumetric reductions in the tail of the hippocampus that were more prominent in patients with schizophrenia than in patients with major depressive disorder (Maller et al., 2012). The present study provides further support to the notion of a dysfunctional posterior hippocampus in people with schizophrenia and suggests that future fMRI studies may wish to separately examine anterior and posterior portions of the hippocampus rather than treating it as a unitary structure.
An unexpected finding was that, during detection of item changes, activity in the anterior hippocampus was abnormally increased in patients with schizophrenia relative to healthy controls. Although this finding was not predicted, it fits well with theories proposing that portions of the anterior hippocampus are “hyperactive” in schizophrenia (Tamminga et al., 2010, Heckers and Konradi, 2015). According to this view, either abnormal modulation of phasic dopamine activity (Lodge and Grace, 2011, Grace, 2012), or disrupted glutamatergic or GABAergic signaling (Tamminga et al., 2010, Heckers and Konradi, 2015) may lead to hyperexcitability in the anterior hippocampus. A possible explanation for patient hyperactivity in the current study is that patients may have disproportionately relied on the anterior hippocampus to successfully detect item changes. If the model of Evensmoen et al. (2015) is correct, patients may have been relying on a coarse-grained representation of the studied scene, whereas controls relied on a more fine-grained, detailed representation.
An alternative explanation is that patients may have relied upon relatively spared function in an anterior temporal memory network in order to compensate for dysfunctional posterior medial network activity. The anterior temporal network has preferential connectivity with the anterior hippocampus, and includes the lateral entorhinal, perirhinal, and lateral orbitofrontal cortex (Kahn et al., 2008, Libby et al., 2012b). This anterior temporal network has been implicated in familiarity-based item recognition and semantic processes that are less severely impaired in patients with schizophrenia (Ranganath et al., 2008, van Erp et al., 2008, Libby et al., 2012a). In contrast, the posterior medial network includes the parahippocampal, retrosplenial, medial prefrontal, and posterior cingulate cortex, and shows preferential connectivity with the posterior hippocampus. Activation in this posterior medial network is enhanced during recollection-based recognition and spatial memory retrieval (Ranganath and Ritchey, 2012) - processes that are known to be impaired in patients with schizophrenia. This explanation is admittedly speculative, and whole-brain results did not reveal a pattern of regional impairments during spatial memory that could clearly be assigned to either network. A possible way forward may be to examine resting-state functional connectivity in these anterior temporal and posterior medial networks in relation to validated measures of specific encoding and retrieval processes.
People with schizophrenia also had memory impairments across item change and spatial change conditions. Although this might seem surprising, it parallels results from our previous study (Hannula et al., 2010a), which also found that patients showed a generalized deficit in detection of both item and spatial changes, but showed specific eye movement memory deficits only for spatial memory trials (see current Supplementary Materials). Participants learned each item in relation to the item's location in a complex visual scene, which provided a very strong learning context. It is well established that when items are initially associated with a strong context, recognition of the items is context dependent (see, for example, (Light and Carter-Sobell, 1970)). Given well-known deficits in item-context binding in schizophrenia (see, (Ranganath et al., 2008, Libby et al., 2013 Lepage et al., 2015) for review), it is not surprising that patients showed item memory as well as spatial memory deficits.
To facilitate study compliance, we studied medicated and clinically stable patients with relatively mild symptoms, raising the issue of a potential medication confound. Our decision to study medicated patients was partially driven by the complexity of our task design, which would be difficult to successfully administer to more symptomatic individuals. Either resting-state studies (Kraguljac et al., 2016), or studies with less complex designs, such as novelty detection (Tamminga et al., 2012), tend to be more successful at studying unmedicated patients. Examination of these memory processes with the current task paradigm in individuals who are at clinical or genetic high risk for psychosis, but are not medicated, is another approach that may help to identify any role that medication plays in this differential pattern of anterior and posterior hippocampal functioning in patients with schizophrenia. Nevertheless, the observed group by condition interactions for both the anterior and posterior hippocampus are difficult to attribute to a medication confound, which might be expected to have a less regionally and functionally specific impact on hippocampal processing. The current study was not designed to identify group differences in encoding-related activity (e.g., subsequent memory effects), which is a promising area for future research.
4.1. Conclusions
In summary, there was strong evidence that patients with schizophrenia have a regionally specific impairment in posterior hippocampal activation during retrieval of fine-grained spatial information, which parallels previous findings of an absence of eye-movement memory effects for spatial but not for item information. Although the unexpected finding of abnormally increased anterior hippocampal activation during detection of item changes may relate to consistent evidence of anterior hippocampal hyperactivity during resting-state, this is not a fully satisfactory explanation, and more work needs to be done to integrate resting-state with task-based fMRI data to better understand the mneumonic and other functional consequences of what appears to be a differential impact of schizophrenia across the long axis of the hippocampus.
Funding sources
This research was supported by the National Institute of Health [grants: R01MH084895 and R01MH105411].
Financial disclosures
Dr. Ragland has received research grants from the NIH and NARSAD.
Mr. Layher has no currently active grant or contract support from private or public sources.
Dr. Hannula has received research grants from the NIH and NSF.
Dr. Niendam has received research grants from the NIH, NARSAD, the Robert Wood Johnson Foundation, and the UC Davis Behavioral Health Center of Excellence.
Dr. Lesh has no currently active grant or contract support from private or public sources.
Dr. Solomon has received research grants from the NIH and the UC Davis Behavioral Health Center of Excellence.
Dr. Carter has received research grants from the NIMH, NIDA, and the Robert Wood Johnson Foundation.
Dr. Ranganath has received research grants from the NIH, the Department of Defense, and has been an external consultant for Helicon Pharmaceuticals.
Acknowledgments
We thank Dennis Thompson for his technical assistance and the staff at the Imaging Research Center for their hard work, and our participants for their time, energy and cooperation.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.11.017.
Appendix A. Supplementary data
Supplementary material
References
- Achim A.M., Lepage M. Is associative recognition more impaired than item recognition memory in schizophrenia? A meta-analysis. Brain Cogn. 2003;53(2):121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Achim A.M., Lepage M. Neural correlates of memory for items and for associations: an event-related functional magnetic resonance imaging study. J. Cogn. Neurosci. 2005;17(4):652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Bannerman D.M., Rawlins J.N., McHugh S.B., Deacon R.M., Yee B.K., Bast T., Zhang W.N., Pothuizen H.H., Feldon J. Regional dissociations within the hippocampus–memory and anxiety. Neurosci. Biobehav. Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Benes F.M., Kwok E.W., Vincent S.L., Todtenkopf M.S. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol. Psychiatry. 1998;44(2):88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Benetti S., Mechelli A., Picchioni M., Broome M., Williams S., McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132(Pt 9):2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A., Haut K., Csernansky J.G., Barch D.M. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol. Psychiatry. 2005;58(1):47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evensmoen H.R., Lehn H., Xu J., Witter M.P., Nadel L., Haberg A.K. The anterior hippocampus supports a coarse, global environmental representation and the posterior hippocampus supports fine-grained, local environmental representations. J. Cogn. Neurosci. 2013;25(11):1908–1925. doi: 10.1162/jocn_a_00436. [DOI] [PubMed] [Google Scholar]
- Evensmoen H.R., Ladstein J., Hansen T.I., Moller J.A., Witter M.P., Nadel L., Haberg A.K. From details to large scale: the representation of environmental positions follows a granularity gradient along the human hippocampal and entorhinal anterior-posterior axis. Hippocampus. 2015;25(1):119–135. doi: 10.1002/hipo.22357. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62(3):1342–1348. doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.A., McNaughton N. Oxford University Press; 1982. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. [Google Scholar]
- Hannula D.E., Tranel D., Cohen N.J. The long and the short of it: relational memory impairments in amnesia, even at short lags. J. Neurosci. 2006;26(32):8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. (Aug 9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula D.E., Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J. Neurosci. 2008;28(1):116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula D.E., Ranganath C., Ramsay I.S., Solomon M., Yoon J., Niendam T.A., Carter C.S., Ragland J.D. Use of eye movement monitoring to examine item and relational memory in schizophrenia. Biol. Psychiatry. 2010;68(7):610–616. doi: 10.1016/j.biopsych.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula D.E., Althoff R.R., Warren D.E., Riggs L., Cohen N.J., Ryan J.D. Worth a glance: using eye movements to investigate the cognitive neuroscience of memory. Front. Hum. Neurosci. 2010;4:166. doi: 10.3389/fnhum.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula D.E., Libby L.A., Yonelinas A.P., Ranganath C. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia. 2013;51(12):2322–2332. doi: 10.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S., Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr. Res. 2015;167(1–3):4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S., Rauch S.L., Goff D., Savage C.R., Schacter D.L., Fischman A.J., Alpert N.M. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat. Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Kahn I., Andrews-Hanna J.R., Vincent J.L., Snyder A.Z., Buckner R.L. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(1):129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath A.T., Wang M.E., Wann E.G., Yuan R.K., Dudman J.T., Muzzio I.A. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus. 2014;24(12):1533–1548. doi: 10.1002/hipo.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup K.B., Solstad T., Brun V.H., Hafting T., Leutgeb S., Witter M.P., Moser E.I., Moser M.B. Finite scale of spatial representation in the hippocampus. Science. 2008;321(5885):140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kraguljac N.V., White D.M., Hadley N., Hadley J.A., VerHoef L., Davis E., Lahti A.C. Abberant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr. Bull. 2016;42(4):1046–1055. doi: 10.1093/schbul/sbv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Maguire E.A. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49(4):617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Lepage M., Montoya A., Pelletier M., Achim A.M., Menear M., Lal S. Associative memory encoding and recognition in schizophrenia: an event-related fMRI study. Biol. Psychiatry. 2006;60(11):1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Lepage M., Hawco C., Bodnar M. Relational memory as a possible neurocognitive marker of schizophrenia. JAMA Psychiat. 2015;72(9):946–947. doi: 10.1001/jamapsychiatry.2015.0488. [DOI] [PubMed] [Google Scholar]
- Libby L.A., Yonelinas A.P., Ranganath C., Ragland J.D. Recollection and familiarity in schizophrenia: a quantitative review. Biol. Psychiatry. 2012;73:944–950. doi: 10.1016/j.biopsych.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby L.A., Ekstrom A.D., Ragland J.D., Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J. Neurosci. 2012;32(19):6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby L.A., Yonelinas A.P., Ranganath C., Ragland J.D. Recollection and familiarity in schizophrenia: a quantitative review. Biol. Psychiatry. 2013;73(10):944–950. doi: 10.1016/j.biopsych.2012.10.027. (May 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light L., Carter-Sobell L. Effects of changed semantic context on recognition memory. J. Verbal Learn. Verbal Behav. 1970;9:1–11. [Google Scholar]
- Litman L., Awipi T., Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19(3):308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D.J., Grace A.A. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol. Sci. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J.J., Daskalakis Z.J., Thomson R.H., Daigle M., Barr M.S., Fitzgerald P.B. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil's in de-tail. Hippocampus. 2012;22(1):9–16. doi: 10.1002/hipo.20873. [DOI] [PubMed] [Google Scholar]
- Moser M.B., Moser E.I. Distributed encoding and retrieval of spatial memory in the hippocampus. J. Neurosci. 1998;18(18):7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Ritchey M., Adcock R.A., LaBar K.S. fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia. 2010;48(12):3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D., Cullen T.J., Wolf D.H., Rohan M., Barreira P., Zalesak M., Heckers S. The neural basis of relational memory deficits in schizophrenia. Arch. Gen. Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Ragland J.D., Gur R.C., Valdez J., Turetsky B.I., Elliott M., Kohler C., Siegel S., Kanes S., Gur R.E. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am. J. Psychiatry. 2004;161(6):1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Gur R.C., Valdez J.N., Loughead J., Elliott M., Kohler C., Kanes S., Siegel S.J., Moelter S.T., Gur R.E. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am. J. Psychiatry. 2005;162(10):1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Ranganath C., Harms M.P., Barch D.M., Gold J.M., Layher E., Lesh T.A., MacDonald A.W., III, Niendam T.A., Phillips J., Silverstein S.M., Yonelinas A.P., Carter C.S. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA Psychiat. 2015;72(9):909–916. doi: 10.1001/jamapsychiatry.2015.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Rainer G. Neural mechanisms for detecting and remembering novel events. Nat. Rev. Neurosci. 2003;4(3):193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Minzenberg M.J., Ragland J.D. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol. Psychiatry. 2008;64(1):18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel S.A., Lewandowski N.M., Corcoran C.M., Moore H., Brown T., Malaspina D., Small S.A. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel S.A., Chaudhury N.H., Khan U.A., Paniaqua B., Styner M.A., Asllani I., Inbar B.P., Corcoran C.M., Lieberman J.A., Moore H., Small S.A. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S., Kim A.S., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8–9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Staresina B.P., Gray J.C., Davachi L. Event congruency enhances episodic memory encoding through semantic elaboration and relational binding. Cereb. Cortex. 2009;19(5):1198–1207. doi: 10.1093/cercor/bhn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B.A., Hurlemann R., Duggins A., Heinze H.J., Dolan R.J. Dissociating intentional learning from relative novelty responses in the medial temporal lobe. NeuroImage. 2005;25(1):51–62. doi: 10.1016/j.neuroimage.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Talati P., Rane S., Kose S., Blackford J.U., Gore J., Donahue M.J., Heckers S. Increased hippocampal CA1 cerebral blood volume in schizophrenia. NeuroImage Clin. 2014;5:359–364. doi: 10.1016/j.nicl.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga C.A., Stan A.D., Wagner A.D. The hippocampal formation in schizophrenia. Am. J. Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Thomas B.P., Chin R., Mihalakos P., Youens K., Wagner A.D., Preston A.R. Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophr. Res. 2012;138(2–3):157–163. doi: 10.1016/j.schres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Titone D., Ditman T., Holzman P.S., Eichenbaum H., Levy D.L. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr. Res. 2004;68(2–3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- van Erp T.G., Lesh T.A., Knowlton B.J., Bearden C.E., Hardt M., Karlsgodt K.H., Shirinyan D., Rao V., Green M.F., Subotnik K.L., Nuechterlein K., Cannon T.D. Remember and know judgments during recognition in chronic schizophrenia. Schizophr. Res. 2008;100(1–3):181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A.P., Schacter D.L., Goff D.C., Rauch S.L., Alpert N.M., Fischman A.J., Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol. Psychiatry. 2003;53(1):48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation images. In: Jezzard P., Matthews P.M., Smith S.M., editors. Functional MRI: An Introduction to Methods. OUP; New York: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material