Abstract
We recently reported that cluster determinant 36 (CD36), a fatty acid transporter, plays a pivotal role in glucotoxicity-induced β-cell dysfunction. However, little is known about how glucotoxicity influences CD36 expression. Emerging evidence suggests that the small GTPase Rac1 is involved in the pathogenesis of beta cell dysfunction in type 2 diabetes (T2D). The primary objective of the current study was to determine the role of Rac1 in CD36 activation and its impact on β-cell dysfunction in diabetes mellitus. To address this question, we subjected INS-1 cells and human beta cells (1.1B4) to high glucose conditions (30 mM) in the presence or absence of Rac1 inhibition either by NSC23766 (Rac1 GTPase inhibitor) or small interfering RNA. High glucose exposure in INS-1 and human beta cells (1.1b4) resulted in the activation of Rac1 and induced cell apoptosis. Rac1 activation mediates NADPH oxidase (NOX) activation leading to elevated ROS production in both cells. Activation of the Rac1-NOX complex by high glucose levels enhanced CD36 expression in INS-1 and human 1.1b4 beta cell membrane fractions. The inhibition of Rac1 by NSC23766 inhibited NADPH oxidase activity and ROS generation induced by high glucose concentrations in INS-1 & human 1.1b4 beta cells. Inhibition of Rac1-NOX complex activation by NSC23766 significantly reduced CD36 expression in INS-1 and human 1.1b4 beta cell membrane fractions. In addition, Rac1 inhibition by NSC23766 significantly reduced high glucose-induced mitochondrial dysfunction. Furthermore, NADPH oxidase inhibition by VAS2870 also attenuated high glucose-induced ROS generation and cell apoptosis. These results suggest that Rac1-NADPH oxidase dependent CD36 expression contributes to high glucose-induced beta cell dysfunction and cell death.
Keywords: Rac1, NADPH oxidase, Beta-cell dysfunction, CD36, Oxidative stress
Graphcal abstract
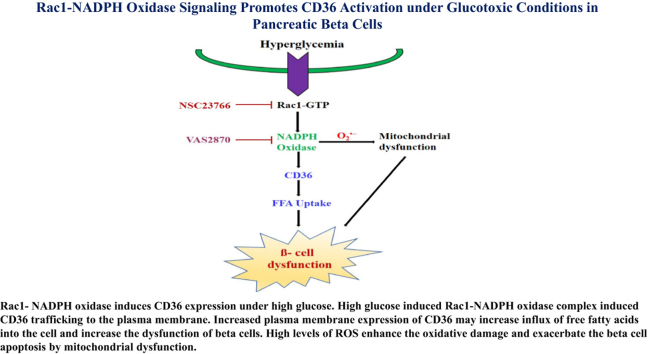
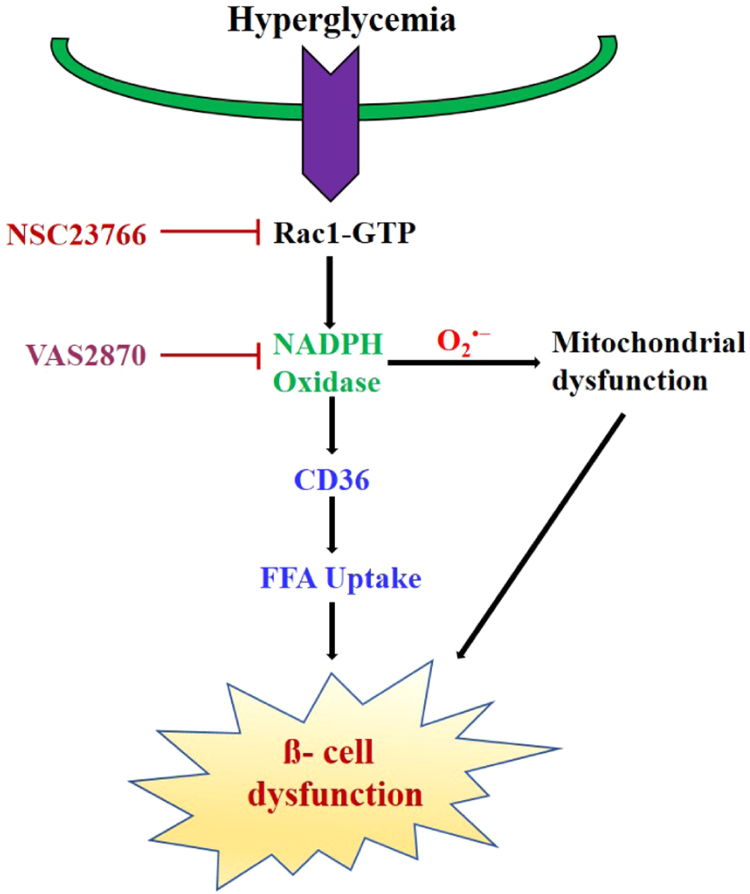
Rac1- NADPH oxidase induces CD36 expression under high glucose. High glucose induced Rac1-NADPH oxidase complex induced CD36 trafficking to the plasma membrane. Increased plasma membrane expression of CD36 may increase influx of free fatty acids into the cell and increase the dysfunction of beta cells. High levels of ROS enhance the oxidative damage and exacerbate the beta cell apoptosis by mitochondrial dysfunction.
Highlights
-
•
High glucose induce β-cell damage by Rac1 and NADPH oxidase activation.
-
•
High glucose induced Rac1-NADPH oxidase mediate CD36 expression and mitochondrial dysfunction.
-
•
Inhibition of Rac1 suppressed high glucose induced NADPH oxidase activity and downregulated CD36 expression.
-
•
Inhibition of Rac1 or NADPH oxidase prevent high glucose induced mitochondrial dysfunction and β-cell apoptosis.
1. Introduction
Type 2 diabetes is a chronic disease resulting from the progressive impairment of insulin resistance and insulin insensitivity in target tissues. However, at the core of type 2 diabetes pathology is the inability of β-cells to sustain a compensatory secretory response, leading to β-cell dysfunction and death [1], [2], [3]. Although, the molecular signals that trigger β-cell deterioration are unknown, recent experimental evidence suggests that it may involve an increased response to FFA arising from intra-abdominal fat stores [4], [5]. Importantly, studies suggest that dysfunction occurs in the presence of elevated glucose levels [6]. These chronic increases in circulating lipids and glucose levels contribute to impaired β-cell dysfunction and other forms of tissue damage. Studies in Zucker diabetic fatty (ZDF) rats showed that elevated lipid accumulation leads to β-cell destruction via apoptosis [7]. Several studies have shown that fatty acids can induce β-cell death via apoptosis, which was potentiated by glucose [8], [9]. The underlying molecular and cellular mechanisms by which glucotoxicty and lipotoxicity contributes to β-cell destruction and death in type 2 diabetes are still under debate. Recently, we reported that fatty acid translocase cluster determinant 36 (CD36), a membrane glycoprotein, influences glucotoxicity dysfunction by increasing the influx of free fatty acids into pancreatic β-cells [10]. Moreover, forced expression of CD36 in β-cells increases the uptake of fatty acids and leads to metabolic and functional alterations [11], [12]. However, the underlying molecular and cellular mechanisms by which glucotoxicity contributes to CD36 induction and cell death in type 2 diabetes are not completely understood.
Rac1 is a small guanosine triphosphate (GTP)–bound protein belonging to the Rho family. In particular, they are important regulators of cytoskeletal dynamics and of a variety of cellular processes, including cell polarization, morphogenesis, migration, apoptosis, vesicle trafficking, viral transport, and cellular transformation [13]. Moreover, Rac1 signaling induces localized actin polymerization at the membrane level, promoting the internalization of particles and microorganisms, and contributes to the assembly and activation of the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex involved in the elimination of bacteria [14]. Several pieces of evidence support a key role for Rac1 in various phenomena in the cytoskeleton, oxidative stress and insulin secretion. Because of its diverse functions, Rac1 has both positive and negative impacts on pancreatic beta cells [15]. One important effector of Rac1 activity is p67phox, which combines with other components of the NADPH oxidase (NOX) system when activated to generate a fully functional complex for producing ROS. Moreover, NOX has long been recognized as a signaling molecule in the inflammatory response to the induction of insulin resistance, both in vitro and in vivo [16], [17]. Based on the potential importance of NOX in the pathogenesis of type 2 diabetes, the role of Rac1-NOX in beta-cell dysfunction and apoptosis remains unknown.
In the present study, the effects of Rac1-NADPH oxidase signaling on CD36 activation were investigated in INS-1 and human pancreatic 1.1b4 beta cells. Our results showed that Rac1-NADPH oxidase activation mediates CD36 expression induced by high levels of glucose. Rac1-NADPH oxidase inhibition strongly blocked CD36 expression and protected beta cells from apoptosis induced by high glucose levels by suppressing mitochondrial dysfunction.
2. Materials and methods
2.1. Cell culture, chemicals
Rat insulinoma INS-1 cells and human pancreatic insulin-releasing 1.1b4 cells (passage 30–40; purchased from ECACC, European Collection of Cell Cultures, Sigma-Aldrich, St. Louis, MO, USA) were cultured in a humidified atmosphere containing 5% CO2 in RPMI 1640 (Life Technologies, Inc., Grand Island, NY) medium supplemented with 10% (v/v) heat-inactivated FBS (Gibco, Grand Island, NY, USA), 100 U/ml penicillin and 100 μg/ml streptomycin. Lipofectamine™ 2000 Transfection Reagent (Invitrogen, Carlsbad, CA) was used to transfect INS-1 cells. Cells were transfected with 100 nmol/l of Rac1-siRNA (Bioneer Corporation, Daejeon, Korea). After 24 h of transfection, the cells were exposed to 30 mM glucose for varying periods of time.
2.2. Rac1 activation assay
Activation of Rac1 was quantified using a Rac1 activation assay kit (Upstate Biotechnology). Briefly, cells were serum starved and then treated with NSC23766 (50 μM) for 2 h. Subsequently, cells were treated with a high concentration of glucose (30 mM) for the indicated time period. The plate was washed extensively. Protein samples were prepared according to the manufacturer's instructions. Samples were resolved using SDS-PAGE and transferred to a nitrocellulose membrane. GTP-bound Rac1 was identified using the anti-Rac1 antibody.
2.3. NADPH oxidase activity assay
NADPH oxidase activity in cell lysates was assayed using the lucigenin chemiluminescence assay according to methods described previously [18] with modifications. The cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high concentrations of glucose (30 mM) for the indicated period of time. After incubation, the cells were homogenized through sonication in PBS containing 1 mM MgCl2, 1 mM EGTA and protease inhibitors. The homogenates were centrifuged at 3000g for 10 min at 4 °C. The cleared lysates (250 μg/ml of protein) were then incubated with 20 μM lucigenin (Cayman Chemicals) and 100 μM NADPH (Sigma Aldrich) prepared in PBS. Chemiluminescence was measured every minute for 5 min using a luminometer. NADPH oxidase activity was expressed in relative light units (RLU) per μg protein. To detect the inhibitory effects of NADPH oxidase activity, cells were first incubated with VAS-2870 (10 μM) for 1 h. Subsequent steps followed the same procedures detailed above.
2.4. Apoptosis and mitochondrial functional assay
INS-1 cell apoptosis was assessed using the TUNEL staining kit (Roche, Basal, Switzerland). INS-1 cells were exposed to either vehicle or NSC23766 (50 μM) for 2 h or VAS-2870 (10 μM) for 1 h and then exposed to high concentrations of glucose (30 mM) for 24 h. Upon completion of the treatment, the cells were further processed, according to the manufacturer's instructions. The image was captured using fluorescence microscopy. Cell death was quantified using ImageJ software (National Institute of Health). The mitochondrial membrane potential was measured using DiOC6 (Sigma-Aldrich). Briefly, harvested cells were washed once with PBS and then labeled with 10 nM DiOC6 for 5 min at 37 °C. The cells were washed once and the cell fluorescence was analyzed using flow cytometry (BD Biosciences, San Jose, CA). Intracellular ROS generation was assessed using 2, 7-dichlorodihydrofluorescein diacetate (DCF-DA, Molecular Probes, Invitrogen, USA). INS-1 cells were washed and then incubated in the dark for 15 min with 10 μM/l DCF-DA at 37 °C and then visualized under a fluorescence microscope. The mean fluorescence intensity was used to quantify cellular ROS. Apoptosis and mitochondrial dysfunction were confirmed by assessing cytosolic cleaved caspase-3 and cytochrome c release using western blot analysis. Cytoplasmic extract were fractionated using the NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Scientific, Rockford, USA) according to the instructions of the supplier.
2.5. Cell viability and caspase-3 activity
Human pancreatic 1.1b4 cells were pretreated with or without NSC23766 (50 μM) for 2 h or VAS2870 (10 μM) for 1 h, followed by stimulation with 30 mM glucose. After 48 h, the percentage of viable cells were measured using the Cell Counting Kit-8 (CCK-8) (Dojindo Lab., Kumamoto, Japan). Caspase-3 activity in the cell extracts was determined using Caspase-Glo 3/7 Assay (Promega). The luminescence of each sample was measured using Flex station (Molecular Devices). Caspase 3/7 activity was expressed in terms of relative fluorescence units.
2.6. Plasma membrane preparation
INS-1 and human pancreatic 1.1b4 cell plasma membrane extracts were prepared using a plasma membrane protein extraction kit (Biovision). The cells were washed once in cold PBS and plasma membrane protein extraction was performed according to the manufacturer's instructions using the reagents included in the kit. The protein concentration was obtained using the Bradford protein assay. NA+K+ATPASE was used as a loading control to show the same amounts of plasma membrane protein in each lane.
2.7. Western blotting
Cell protein lysates were resolved using NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to PVDF membranes (Millipore, Billerica, MA, USA). After blocking, the membranes were stored at 4 °C with the following primary antibodies: NA+K+ATPASE, phospho JNK, p38 MAPK, cleaved caspase 3 (Cell signaling Technology, Danvers, MA, USA), CD36 (Cayman Chemicals, Ann Arbor, MI, USA), Rac1, cytochrome c (BD Biosciences, San Jose, CA, USA) and actin (Abcam, Cambridge, UK). The membranes were then washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Immuno-reactive proteins were detected using ECL reagents (ECL Plus; Amersham, GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK). Band densities were measured using ImageJ software (NIH).
2.8. Statistical analysis
Statistical significance was determined using the one-way Tukey test or by the analysis of variance (ANOVA) as appropriate. P values <0.05 were considered statistically significant.
3. Results
3.1. Activation of Rac1, NADPH oxidase and induction of beta cell apoptosis by high glucose concentrations
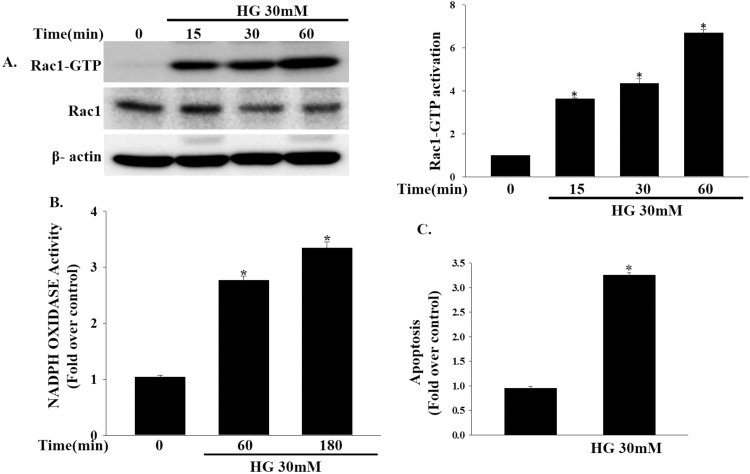
To determine whether high concentrations of glucose (30 mM) can induce Rac1 activation, we treated INS-1 cells with high concentrations of glucose for varying periods of time. As shown in Fig. 1a, a robust elevation of Rac1 activity was observed at 60 min. High glucose-induced Rac1 activity was associated with elevations in NADPH oxidase activity as measured by the chemiluminescence assay (Fig. 1b). Furthermore, treatment with high concentrations of glucose significantly induced INS-1 cell apoptosis (Fig. 1c).
Fig. 1.
Effects of high glucose concentrations on Rac1, NADPH oxidase activity and apoptosis. (a) INS-1 cells were treated with high concentrations of glucose (30 mM) for varying periods of time. Rac1 activity was measured by Rac1-Upstate assay kit. (*P<0.005 vs. Control). (b) INS-1 cells were treated with high concentrations of glucose (30 mM) for the indicated time periods. NADPH oxidase activity was measured by lucigenin based assay. Data represent the mean±SEM of three independent experiments. *P<0.001 vs Control. (c) INS-1 cells were treated with high concentrations of glucose (30 mM) for 24 h and apoptosis was assessed by TUNEL-assay using fluorescence microscopy. The results are expressed as the mean±SEM. *P<0.001 vs. Control.
3.2. NSC23766 inhibits Rac1, NADPH oxidase activation by high glucose concentrations
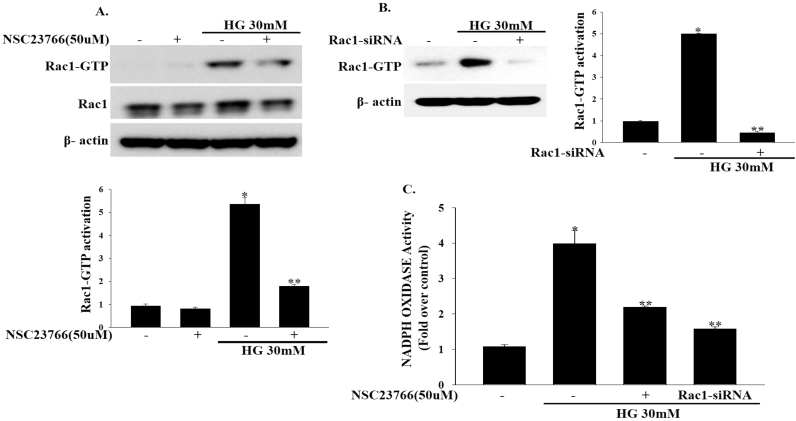
To test whether inhibition of Rac1 improves beta cell function, we treated INS-1 cells with the Rac1-GTPase inhibitor NSC23766 (50 μM) for 2 h and then exposed them to high concentrations of glucose (30 mM) for another 1 h. Treating INS-1 cells with NSC23766 (50 μM) effectively decreased Rac1 activity induced by high concentrations of glucose(30 mM) (Fig. 2a). To confirm whether the beta-cell dysfunction induced by high glucose concentrations is mediated by Rac1, we downregulated endogenous Rac1 expression by transfecting INS-1 cells with Rac1-siRNA and then incubating them in high concentrations of glucose (30 mM) for 1 h. Rac1-siRNA significantly inhibited the endogenous Rac1 expression at the high glucose concentrations. (Fig. 2b). Consistent with this finding, treatment of INS-1 cells with either NSC23766 (50 μM) or Rac1-siRNA significantly blocked high glucose-induced NADPH oxidase activity (Fig. 2c). Moreover, treatment of INS-1 cells with VAS-2870 (10 μM) reduced high glucose-induced NADPH oxidase activity (Supplementary Fig. 1).
Fig. 2.
Effects of NSC23766 on Rac1 and NADPH oxidase activity. (a) INS-1 cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high concentrations of glucose (30 mM) for 1 h to measure Rac1 activity. (*P<0.0001 vs. Control, **P<0.005 vs. HG). (b) INS-1 cells were transfected with 100 nmol/l of Rac1-siRNA and then exposed to high concentrations of glucose (30 mM) for 1 h. (*P<0.0001 vs. Control, **P<0.0001 vs. HG). (c) Cells were treated with NSC23766 (50 μM) for 2 h or transfected with Rac1-siRNA and then exposed to high concentrations of glucose (30 mM) for 3 h. NADPH oxidase activity was measured by lucigenin based assay. Values represent the means±SEM. (*P<0.001 vs. Control, **P<0.001 vs. HG).
3.3. Induction of CD36 expression by high concentrations of glucose and inhibition by NSC23766
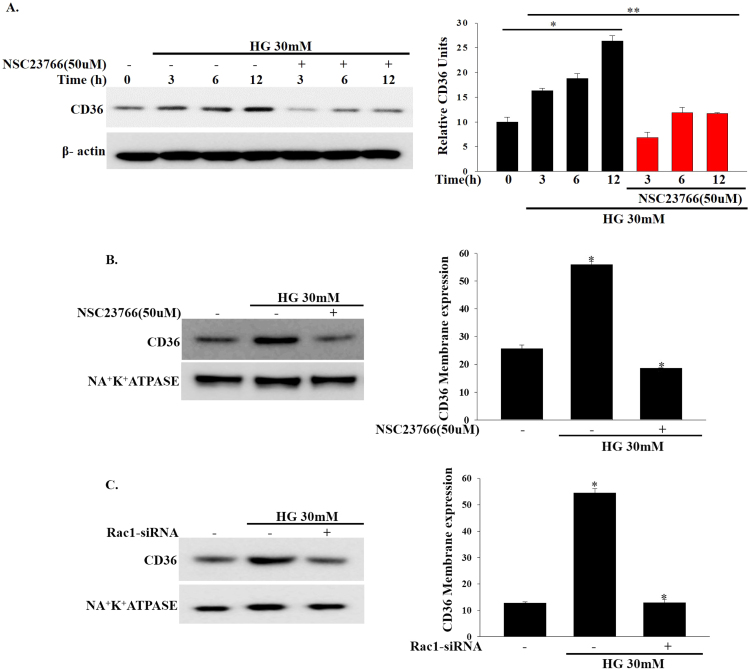
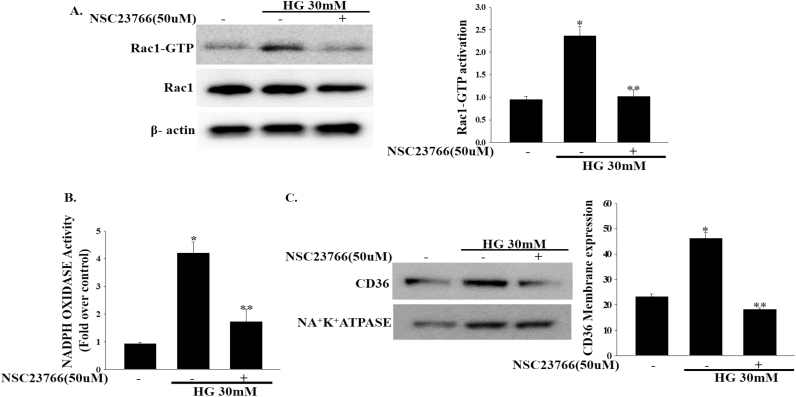
In addition to the role of CD36 as a master transporter of fatty acid uptake, evidence also suggests that CD36 plays a role in the regulation of beta cell dysfunction [19]. Based on this finding, we speculated that high glucose-induced upregulation of Rac1-NADPH oxidase activity may induce CD36 expression. Consistent with this assumption, we found that CD36 expression increased in a time dependent manner in INS-1 cells after treatment with high concentrations of glucose (Fig. 3a). We then examined the membrane translocation of CD36. As shown in Fig. 3b, at 12 h the CD36 protein levels in the plasma membranes were significantly upregulated in high glucose-treated INS-1 cells. Indeed, INS-1 cells treated with NSC23766 (50 μM) exhibited time dependent decreases in CD36 expression (Fig. 3a). Additionally, NSC23766 (50 μM) treatment significantly decreased CD36 protein expression in the plasma membrane fractionation (Fig. 3b). To determine whether CD36 expression induced by high glucose concentrations (30 mM) is mediated by Rac1, we downregulated endogenous Rac1 expression by transfecting INS-1 cells with Rac1-siRNA and then incubated them with high concentrations of glucose (30 mM). Rac1-siRNA significantly inhibited high glucose-induced upregulation of CD36 expression in the plasma membrane (Fig. 3c). These observations suggest that high glucose-induced CD36 expression is mediated by Rac1 activation.
Fig. 3.
Effects of NSC23766 on high glucose-induced CD36 expression. (a) Cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high concentrations of glucose (30 mM) for the indicated periods of time. Total cell lysates was used to measure CD36 expression by immunoblotting. *P<0.001 vs. control, **P<0.005 vs. HG. (b, c) The effect of NSC237669 (50 μM) or Rac1-siRNA on high glucose induced CD36 expression at plasma membrane fractionation was analyzed by immunoblotting. Cellular plasma membrane were prepared using a plasma membrane protein extraction kit. NA+K+ATPASE was used as the loading control. *P<0.0001 vs. control, *P<0.001 vs. HG.
3.4. NSC23766 blocks mitochondrial dysfunction in beta cells by suppressing Rac1-NADPH oxidase activity
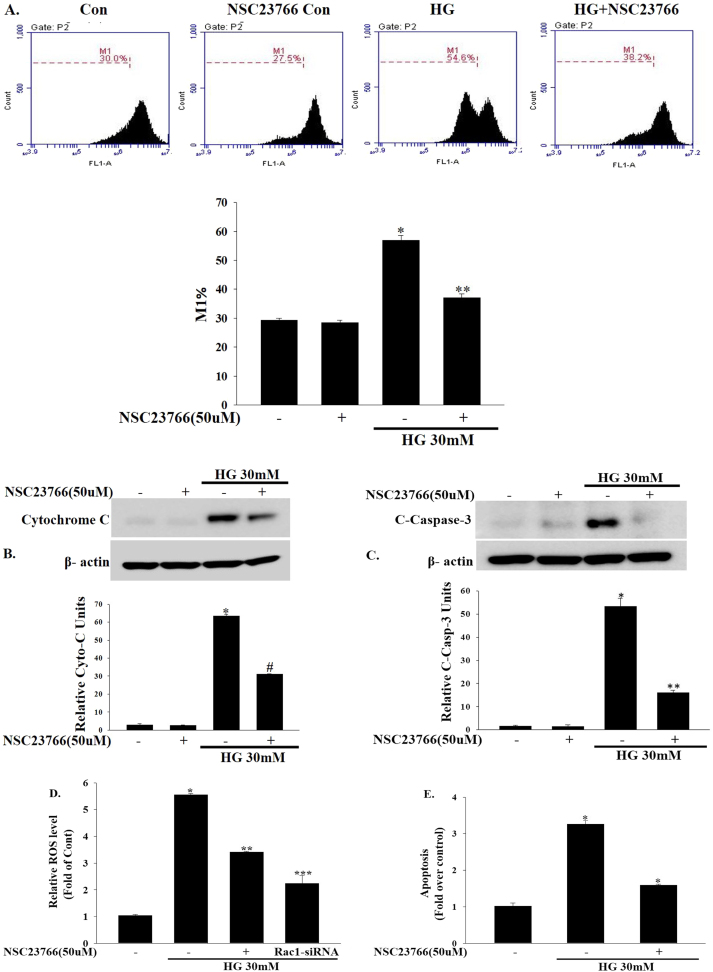
Next, we determined whether Rac1 inhibition blocked high glucose-induced mitochondrial dysfunction. The mitochondrial membrane potential was assessed using flow cytometry. Interestingly, Rac1 inhibition by NSC23766 (50 μM) abolished high glucose-induced mitochondrial membrane potential loss followed by a release of cytochrome c into the cytosol and caspase-3 activation, reflecting the activation of the permeability transition pore (PTP), a key event in cell death (Fig. 4a–c). High glucose-induced caspase-3 activation was also blocked by the NADPH oxidase inhibitor VAS-2870 (10 μM) (Supplementary Fig. 2). Intracellular ROS production plays a critical role in mediating mitochondrial dysfunction and cell death [20], [21], [22]. As shown in Fig. 4d, intracellular ROS levels were significantly increased by high glucose (30 mM). Interestingly, Rac1 inhibition by NSC23766 (50 μM) or Rac1-siRNA suppressed high glucose-induced intracellular ROS in INS-1 cells (Fig. 4d). In addition, intracellular ROS production was also decreased by the NADPH oxidase inhibitor VAS-2870(10 μM) (Supplementary Fig. 3). Consistent with the changes in ROS levels, high glucose-induced cell apoptosis was inhibited by Rac1 inhibition (Fig. 4e) or NADPH oxidase inhibition (Supplementary Fig. 4).
Fig. 4.
Inhibitory effects of NSC23766 on high glucose-induced mitochondrial dysfunction. (a) INS-1 cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high concentrations of glucose (30 mM) for 24 h and then mitochondrial membrane potentials was analyzed by FACs using DiO6 dye; a representative FACs data shown in the upper panel and the quantification of the M1 ratio from three independent experiments shown in the lower panel. *P<0.0001 vs. control, **P<0.0001 vs. HG. (b, c) INS-1 cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high concentrations of glucose (30 mM) for 24 h. Cytoplasmic extract were fractionated using the NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit. Cytosolic cytochrome c and cleaved caspase-3 protein expression were analyzed by immunoblotting. *P<0.0001 vs. control, #P<0.001 vs. HG, **P<0.005 vs. HG. Data represents three independent experiments and equal loading was confirmed with β-actin. (d) Inhibition of Rac1 activity blocks high glucose induced ROS production. INS-1 cells were treated with NSC23766 (50 μM) for 2 h or transfected with 100 nmol/L Rac1-siRNA and then exposed to high concentrations of glucose (30 mM) for 24 h. Cellular ROS production was analyzed by fluorescence microscopy using 10 μM/L DCF-DA. *P<0.0001 vs. control, **P<0.005 vs. HG, ***P<0.001 vs. HG. (e) Inhibition of Rac1 activity blocks high glucose induced INS-1 cell apoptosis. INS-1 cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high concentrations of glucose (30 mM) for 24 h. INS-1 cell apoptosis was assessed by TUNEL-assay using fluorescence microscopy. *P<0.001 vs. control, *P<0.001 vs. HG.
3.5. High glucose induced Rac1 activation in human 1.1b4 beta cells
In an attempt to establish the effects of high glucose concentrations on Rac1 activation in human pancreatic beta cells, we treated human pancreatic 1.1b4 beta cells with high levels of glucose (30 mM) for 24 h. As expected, treatment with high concentrations of glucose resulted in Rac1 activation. In addition, pretreatment with NSC23766 (50 μM) resulted in marked Rac1 inhibition (Fig. 5a). We further detected that treatment with high concentrations of glucose (30 mM) induces NADPH oxidase activity. These results indicated that the Rac1 activity in 1.1b4 cells was also increased by high glucose concentrations and that Rac1-induced activation of NADPH oxidase activity was inhibited by NSC23766 (50 μM) (Fig. 5b). Consequently, Rac1 activity mediated by high glucose concentrations increased CD36 expression in the membrane fraction of 1.1b4 cells. In addition, NSC23766-mediated Rac1 inhibition decreased CD36 accumulation (Fig. 5c). Combined, these results indicate that Rac1 inhibition blocks CD36 expression in human pancreatic 1.1b4 cells.
Fig. 5.
Inhibitory effects of NSC23766 on high glucose-induced Rac1, NADPH oxidase and CD36 activation in human 1.1b4 cells. (a, b) Human pancreatic 1.1b4 cells were incubated with NSC23766 (50 μM) for 2 h and then exposed to high glucose concentrations (30 mM) for 24 h. Rac1 (*P<0.05 vs. control, **P<0.05 vs. HG) and NADPH oxidase (*P<0.001 vs. control, **P<0.001 vs. HG) activity were measured as described in the methods section. The results represent the mean±SEM of three independent experiments. (c) Human pancreatic 1.1b4 cells were treated with NSC23766 (50 μM) for 2 h and then exposed to high glucose concentrations (30 mM) for 24 h. Cellular plasma membrane extracts were prepared using a plasma membrane protein extraction kit. Plasma membrane fractionation cell lysates were analyzed by western blotting with CD36 or NA+K+ATPASE antibodies. *P<0.001 vs. control, **P<0.001 vs. HG. Data represents three independent experiments.
3.6. Inhibition of Rac1 prevents high glucose-induced ROS, caspase-3 activity and cell viability loss
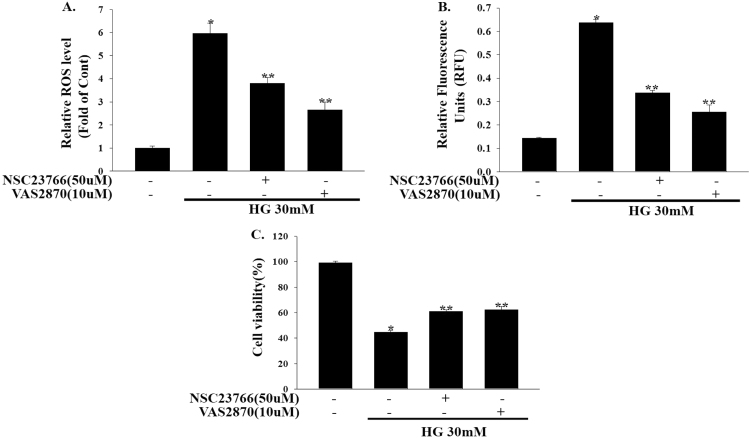
Given that Rac1 is a key regulator of NADPH oxidase activation and superoxide O2•− production, we determined whether intracellular ROS production was affected by high glucose concentrations and Rac1-NADPH oxidase inhibition. As shown in Fig. 6a, when compared to the control, high levels of glucose (30 mM) strongly increased intracellular ROS production in 1.1b4 cells. This high glucose-induced ROS production was abolished by either NSC23766 (50 μM) or VAS-2870 (10 μM) treatment. Rac1 or NADPH oxidase inhibition also inhibited high glucose-induced caspase-3 activity in 1.1b4 cells (Fig. 6b). Furthermore, we also examined the possibility that high glucose-induced 1.1b4 cell viability loss would be prevented by Rac1-NADPH oxidase inhibition. To a similar extent, inhibition of Rac1-NADPH oxidase with NSC23766 (50 μM) or VAS-2870 (10 μM) significantly reduced high glucose-induced 1.1b4 cell viability loss (Fig. 6c). Taken together, these results demonstrate that high glucose-mediated Rac1-NADPH oxidase activation resulted in mitochondrial dysfunction and the loss of cell viability.
Fig. 6.
NSC23766 inhibits high glucose induced ROS, Caspase-3 activity and cell viability loss in Human 1.1b4 cells. (a) Human pancreatic 1.1b4 cells were treated with NSC23766 (50 µM) for 2 h and VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 48 h. Cellular ROS production was analyzed by fluorescence microscopy using 10 µM/l DCF-DA. *P<0.001 vs. control, **P<0.001 vs. HG. (b) Human 1.1b4 cells were treated as mentioned above and caspase-3 activity was measured by Caspase-Glo 3/7 assay kit. Values expressed as relative fluorescence units. *P<0.0001 vs. control, **P<0.001 vs. HG. (c) Human pancreatic 1.1b4 cells were treated with NSC23766 (50 µM) for 2 h and VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 48 h. Cell viability were measured using Cell Counting Kit-8 after experimental conditions. *P<0.0001 vs. control, **P<0.005 vs. HG. The results are expressed as the means±SEM of three independent experiments.
4. Discussion
In the present study, we demonstrated high glucose-induced toxicity in pancreatic beta cells by means of Rac1-NADPH oxidase activation. Importantly, we showed that exposure of pancreatic beta cells to high glucose concentrations induced CD36 expression through Rac1-NADPH oxidase activation.
An accumulation of evidence suggests that hyperglycemia contributes to pancreatic beta cell failure and the development of diabetes [1], [2], [3], [4]. Our previous study demonstrated that CD36 activation by high glucose concentrations mediates beta cell dysfunction [10]. However, the underlying mechanisms of CD36 activation remain unclear. To identify the signaling pathway involved in CD36 activation of glucose, we established a potential link between Rac1-NADPH oxidase and CD36 activation during beta cell dysfunction. Although Rac1-NADPH oxidase signaling has been implicated in beta cell damage [16], [17], [18], [23], its role in CD36 activation provides a link to the completely distinct process of beta cell dysfunction. Overwhelming evidence suggests that Rac1 inhibition may have protective effects in beta cells exposed to high concentrations of glucose [24]. In agreement with what has been previously found, we observed that Rac1 activation led to increased cell apoptosis after exposure to high concentrations of glucose (30 mM). Pharmacological inhibition of Rac1 by NSC23766 inhibited increases in cell apoptosis. Moreover, inhibition of Rac1 activity using siRNAs confirmed these results. In addition to increased Rac1 activation, exposure to high concentrations of glucose was also associated with increased ROS production [25]. Interestingly, Rac1 plays a crucial role in the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a membrane associated protein complex that catalyzes the one electron reduction of oxygen to a superoxide anion [26], [27]. With this functional characterization, we also tested the effects of high glucose concentrations on NADPH oxidase activity in INS-1 as well as in human 1.1b4 cells. A significant increase in NADPH oxidase activity was observed in cells after exposure to high concentrations of glucose; whereas Rac1 inhibition by NSC23766 strongly inhibited increases in NADPH oxidase activity. NADPH oxidase activity had similar inhibitory effects in INS-1 cells after VAS-2870 treatment. Our findings suggest that Rac1-NADPH oxidase is involved in the metabolic dysfunction of beta cells under hyperglycemic conditions.
Several studies have demonstrated that hyperglycemia-induced CD36 expression mediates cellular dysfunction in various tissues [28], [29]]. This upregulation was found to coincide with increased oxidative stress. With this functional characterization, we also tested the effects of high glucose concentrations on CD36 expression as well as its trafficking to the plasma membrane. Of note, CD36 expression was significantly increased in INS-1 and human 1.1b4 pancreatic beta cells, suggesting the pronounced cytotoxic effects of high levels of glucose. Moreover, increases in membrane CD36 were observed after treatment with high concentrations of glucose reflect its function in cellular fatty acid uptake. Recently, it has been shown that CD36 deficiency attenuates obesity associated oxidative stress in the heart through reduced NADPH oxidase activity [30]. Interestingly, pharmacological inhibition of Rac1 by NSC23766 or siRNA attenuated high glucose induced NADPH oxidase activity, suggesting the critical involvement of NADPH oxidase in the activation of CD36 expression.
On the other hand, pharmacological and genetic inhibition of NADPH oxidase prevent oleate-induced beta cell dysfunction [31]. These findings support the hypothesis that Rac1-NADPH oxidase contributes to CD36 activation under hyperglycemic conditions.
Most strikingly, it is closely integrated with mitochondria, which play an important role in high glucose-induced beta cell apoptosis [32], [33]. In support of this finding, altered mitochondrial membrane potentials were found to be associated with the release of cytochrome c leading to the activation of caspase-3, which reflects perturbations in mitochondrial function imposed by high levels of glucose. Under the same conditions, ROS generation in response to high levels of glucose was increased. However, we show that Rac1 inhibition could counteract high glucose-induced mitochondrial dysfunction by inhibiting mitochondrial membrane potential loss linked to the reduction of cytochrome c release and caspase-3 activation. In addition, Rac1 and NADPH oxidase inhibition depleted cellular ROS generation induced by high concentrations of glucose. Moreover, it should be noted that changing the balance of mitochondrial enzymes and increasing ROS production can alter susceptibility to dysfunction by the activation of stress kinases [34], [35]. A recent study conducted by Sidarala et al. [36] reported that Rac1-NADPH oxidase was associated with increases in MAPK activation by glucotoxicity. Considering this, we found that high concentrations of glucose induced MAPK activation while Rac1 inhibition blocked this activation (Supplementary Fig. 5).
Similar to our findings in INS-1 cells, we demonstrated that prolonged exposure of human pancreatic 1.1b4 cell lines to high concentrations of glucose resulted in Rac1 activation and increased NADPH oxidase activity. Furthermore, we found that CD36 membrane expression was significantly increased by treatment with high concentrations of glucose in human 1.1b4 cells. Interestingly, similar inhibitory effects by NSC23766 on Rac1 activation were observed in human 1.1b4 cells. Moreover, Rac1 inhibition would have been sufficient to inhibit high glucose-induced NADPH oxidase activity as well as CD36 membrane translocation. Finally, treatment of human 1.1b4 cells with Rac1 or NADPH oxidase inhibitor prevented high glucose-induced ROS production, caspase-3 activity and 1.1b4 cell viability loss. Our results present the first evidence that Rac1 inhibition protects human 1.1b4 cells from high glucose-induced apoptosis by blocking NADPH oxidase-CD36 activation.
In conclusion, we clearly demonstrated that Rac1-NADPH oxidase activation by high glucose-induced CD36 expression. The membrane expression of CD36 may increase beta cell dysfunction by increasing fatty acid uptake. Pharmacological or siRNA-mediated inhibition of Rac1 prevents high glucose-induced pancreatic beta cell apoptosis by blocking the NADPH oxidase-CD36-mitochondrial pathway (Fig. 7). Future studies examining the precise relationship between Rac1 and CD36 should address their potential interplay in the pancreatic beta cell dysfunction and failure.
Fig. 7.
Rac1-NADPH oxidase induces CD36 expression under high glucose. High glucose induced Rac1-NADPH oxidase complex induced CD36 trafficking to the plasma membrane. Increased plasma membrane expression of CD36 may increase influx of free fatty acids into the cell and increase the dysfunction of beta cells. High levels of ROS enhance the oxidative damage and exacerbate the beta cell apoptosis by mitochondrial dysfunction.
Funding
This study was supported by 2015 Yeungnam University Research Grant (Kyu Chang Won) and Grant of the Korea Health Technology R&D project, Ministry of Health and Welfare, Republic of Korea (In Kyu Lee HI16C1501).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.11.009.
Appendix A. Supplementary material
Supplementary material Supplemental Fig. 1. INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 3 h. NADPH oxidase activity was measured by lucigenin based assay. Results represent the means±SEM. (*P<0.001 vs. Control, *P<0.005 vs. HG). Supplemental Fig. 2. INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 24 h. Cleaved caspase-3 was analyzed by immunoblotting. Supplemental Fig. 3. Inhibition of NADPH oxidase activity using VAS2870 (10 µM) reduced intracellular ROS production by high glucose (30 mM). INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 24 h. Cellular ROS production was analyzed by fluorescence microscopy using 10 µM/L DCF-DA. (*P<0.001 vs. Control, *P<0.005 vs. HG). Supplemental Fig. 4. VAS2870 inhibit high glucose induced INS-1 cell apoptosis. INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 24 h. (*P<0.001 vs. Control, *P<0.05 vs. HG). Apoptosis was analyzed by TUNEL-assay. Supplemental Fig. 5. Inhibition of Rac1 using NSC23766 blocked high glucose induced JNK and p38 MAPK activation. INS-1 cells were incubated with NSC23766 (50 µM) for 2 h and then exposed to high glucose concentrations (30 mM) for 24 h. Phosphorylation of JNK and p38 MAPK was analyzed by immunoblotting using specific antibodies.
.
References
- 1.Lee S.C., Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int. J. Biochem. Cell Biol. 2007;39:497–504. doi: 10.1016/j.biocel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Robertson R.P., Harmon J.S. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet ß cell. Free Radic. Biol. Med. 2006;41(2):177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Leahy J.L. Pathogenesis of type 2 diabetes mellitus. Arch. Med. Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Robertson R.P., Harmon J., Tran P.O., Poitout V. ß cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 5.Goh T.T., Mason T.M., Gupta N., So A., Lam T.K., Lam L., Lewis G.F., Mari A., Giacca A. Lipid-induced ß-cell dysfunction in vivo in models of progressive ß-cell failure. Am. J. Physiol. Endocrinol. Metab. 2007;292:E549–E560. doi: 10.1152/ajpendo.00255.2006. [DOI] [PubMed] [Google Scholar]
- 6.Dubois M., Kerr-Conte J., Gmyr V., Bouckenooghe T., Muharram G., D’Herbomez M., Martin-Ponthieu A., Vantyghem M.C., Vandewalle B., Pattou F. Non-esterified fatty acids are deleterious for human pancreatic islet function at physiological glucose concentration. Diabetologia. 2004;47:463–469. doi: 10.1007/s00125-004-1347-1. [DOI] [PubMed] [Google Scholar]
- 7.Shimabukuro M., Zhou Y.T., Levi M., Unger R.H. Fatty acid-induced ß cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. USA. 1998;3:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maedler K., Oberholzer J., Bucher P., Spinas G.A., Donath M.Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic ß-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 9.Maedler K., Spinas G.A., Dyntar D., Moritz W., Kaiser N., Donath M.Y. Distinct effects of saturated and monounsaturated fatty acids on ß-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.W., Moon J.S., Seo Y.J., Park S.Y., Kim J.Y., Yoon J.S., Lee I.K., Lee H.W., Won K.C. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem. Biophys. Res. Commun. 2012;420:462–466. doi: 10.1016/j.bbrc.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Noushmehr H., D'Amico E., Farilla L. Fatty acid translocase (FAT/CD36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes. 2005;54:472–481. doi: 10.2337/diabetes.54.2.472. [DOI] [PubMed] [Google Scholar]
- 12.Wallin T., Ma Z., Ogata H. Facilitation of fatty acid uptake by CD36 in insulin-producing cells reduces fatty-acid-induced insulin secretion and glucose regulation of fatty acid oxidation. Biochim. Biophys. Acta. 2010;1801:191–197. doi: 10.1016/j.bbalip.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Chi X., Wang S., Huang Y. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 2013;14:7089–7108. doi: 10.3390/ijms14047089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinauer M.C. Regulation of neutrophil function by Rac GTPases. Curr. Opin. Hematol. 2003;10:8–15. doi: 10.1097/00062752-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kowluru A. Friendly, and not so friendly, roles of Rac1 in islet beta-cell function: lessons learnt from pharmacological and molecular biological approaches. Biochem. Pharmacol. 2011;81:965–975. doi: 10.1016/j.bcp.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newsholme P., Morgan D., Rebelato E., Oliveira-Emilio H.C., Procopio J., Curi R. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia. 2009;52:2489–2498. doi: 10.1007/s00125-009-1536-z. [DOI] [PubMed] [Google Scholar]
- 17.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2014;12(1):5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed A.M., Kowluru A. Activation of apocynin sensitive NADPH oxidase (Nox2) activity in INS-1 832/13 cells under glucotoxic conditions. Islets. 2013;5(3):129–131. doi: 10.4161/isl.25058. [DOI] [PubMed] [Google Scholar]
- 19.Karunakaran U., Moon J.S., Lee H.W., Won K.C. CD36 initiated signaling mediates ceramide-induced TXNIP expression in pancreatic beta-cells. Biochim. Biophys. Acta. 2015;1852(11):2414–2422. doi: 10.1016/j.bbadis.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Karunakaran U., Park K.G. A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes Metab. J. 2013;37(2):106–112. doi: 10.4093/dmj.2013.37.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aharoni-Simon M., Shumiatcher R., Yeung A., Shih A.Z., Dolinsky V.W., Doucette C.A., Luciani D.S. Bcl-2 regulates reactive oxygen species signaling and a redox-sensitive mitochondrial proton leak in mouse pancreatic beta-cells. Endocrinology. 2016;157(6):2270–2281. doi: 10.1210/en.2015-1964. [DOI] [PubMed] [Google Scholar]
- 22.Wali J.A., Rondas D., McKenzie M.D., Zhao Y., Elkerbout L., Fynch S. The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell Death Dis. 2014;5:e1124. doi: 10.1038/cddis.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan D., Oliver-Emilio H.R., Keane D., Hirata A.E., Santos da Rocha M., Bordin S. Glucose, palmitic and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal cell line. Diabetologia. 2007;50:359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 24.Kowluru A. Small G proteins in islet β-cell function. Endocr. Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Hordijk P.L. Regulation of NADPH oxidases: the role of Rac proteins. Circ. Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 27.Abo A., Pick E., Hall A., Totty N., Teahan C.G., Segal A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 28.Chen M., Yang Y.K., Loux T.J., Georgeson K.E., Harmon C.M. The role of hyperglycemia in FAT/CD36 expression and function. Pediatr. Surg. Int. 2006;22:647–654. doi: 10.1007/s00383-006-1704-x. [DOI] [PubMed] [Google Scholar]
- 29.Xue J.H., Yuan Z., Wu Y., Liu Y., Zhao Y., Zhang W.P., Tian Y.L., Liu W.M., Liu Y., Kishimoto C. High glucose promotes intracellular lipid accumulation in vascular smooth muscle cells by impairing cholesterol influx and efflux balance. Cardiovasc. Res. 2010;86(1):141–150. doi: 10.1093/cvr/cvp388. [DOI] [PubMed] [Google Scholar]
- 30.Gharib M., Tao H., Fungwe T.V., Hajri T. Cluster Differentiating 36 (CD36) deficiency attenuates obesity-associated oxidative stress in the heart. PLoS One. 2016;11(5):e0155611. doi: 10.1371/journal.pone.0155611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koulajian K., Desai T., Liu G.C., Ivovic A., Patterson J.N., Tang C. NADPH oxidase inhibition prevents beta cell dysfunction induced by prolonged elevation of oleate in rodents. Diabetologia. 2013;56:1078–1087. doi: 10.1007/s00125-013-2858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newsholme P., Haber E.P., Hirabara S.M., Rebelato E.L., Procopio J., Morgan D. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Men X., Wang H., Li M. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int. J. Biochem. Cell Biol. 2009;41:879–890. doi: 10.1016/j.biocel.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Mokhtari D., Myers J.W., Welsh N. The MAPK kinase kinase-1 is essential for stress-induced pancreatic islet cell death. Endocrinology. 2008;149:3046–3053. doi: 10.1210/en.2007-0438. [DOI] [PubMed] [Google Scholar]
- 35.Hou N., Torii S., Saito N., Hosaka M., Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654–1665. doi: 10.1210/en.2007-0988. [DOI] [PubMed] [Google Scholar]
- 36.Sidarala V., Veluthakal R., Syeda K., Vlaar C., Newsholme P., Kowluru A. Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic β-cells under glucotoxic conditions: Evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1) Biochem. Pharmacol. 2015;95(4):301–310. doi: 10.1016/j.bcp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplemental Fig. 1. INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 3 h. NADPH oxidase activity was measured by lucigenin based assay. Results represent the means±SEM. (*P<0.001 vs. Control, *P<0.005 vs. HG). Supplemental Fig. 2. INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 24 h. Cleaved caspase-3 was analyzed by immunoblotting. Supplemental Fig. 3. Inhibition of NADPH oxidase activity using VAS2870 (10 µM) reduced intracellular ROS production by high glucose (30 mM). INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 24 h. Cellular ROS production was analyzed by fluorescence microscopy using 10 µM/L DCF-DA. (*P<0.001 vs. Control, *P<0.005 vs. HG). Supplemental Fig. 4. VAS2870 inhibit high glucose induced INS-1 cell apoptosis. INS-1 cells were treated with VAS2870 (10 µM) for 1 h and then exposed to high glucose (30 mM) for 24 h. (*P<0.001 vs. Control, *P<0.05 vs. HG). Apoptosis was analyzed by TUNEL-assay. Supplemental Fig. 5. Inhibition of Rac1 using NSC23766 blocked high glucose induced JNK and p38 MAPK activation. INS-1 cells were incubated with NSC23766 (50 µM) for 2 h and then exposed to high glucose concentrations (30 mM) for 24 h. Phosphorylation of JNK and p38 MAPK was analyzed by immunoblotting using specific antibodies.