Abstract
Background
Epidemiological studies in humans that have evaluated the association between fine particulate matter (PM2.5) and atherosclerosis have yielded mixed results.
Design
In order to further investigate this relationship, we conducted a comprehensive search for studies published through May 2014 and performed a meta-analysis of all available observational studies that investigated the association between PM2.5 and three noninvasive measures of clinical and subclinical atherosclerosis: carotid intima media thickness, arterial calcification, and ankle-brachial index.
Methods and results
Five reviewers selected studies based on predefined inclusion criteria. Pooled mean change estimates and 95% confidence intervals were calculated using random-effects models. Assessment of between-study heterogeneity was performed where the number of studies was adequate. Our pooled sample included 11,947 subjects for carotid intima media thickness estimates, 10,750 for arterial calcification estimates, and 6497 for ankle-brachial index estimates. Per 10 μg/m3 increase in PM2.5 exposure, carotid intima media thickness increased by 22.52 μm but this did not reach statistical significance (p = 0.06). We did not find similar associations for arterial calcification (p = 0.44) or ankle-brachial index (p = 0.85).
Conclusion
Our meta-analysis supports a relationship between PM2.5 and subclinical atherosclerosis measured by carotid intima media thickness. We did not find a similar relationship between PM2.5 and arterial calcification or ankle-brachial index, although the number of studies was small.
Keywords: Particulate matter, air pollution, tunica intima, vascular calcification, ankle-brachial index
Introduction
Exposure to fine particulate matter with aerodynamic diamete ≤2.5 μm (PM2.5) has been shown to have adverse health effects on multiple organ systems.1,2 Inhaled PM2.5 can be deposited deep in alveoli and is hypothesized to enhance inflammation and oxidative stress and alter cardiac autonomic activity.3–5 Though earlier studies primarily focused on respiratory health outcomes, there is evidence that PM2.5 is a risk factor for cardiovascular disease (CVD) events5,6 including hypertension,7,8 cardiovascular mortality,1,9 and increased hospital admissions for CVD.10
Experimental animal studies have reported more rapid progression of atherosclerosis with long-term ambient particulate matter exposure compared with filtered air.4,11 However, human studies, which cannot be performed in a controlled manner, are limited to observational cohorts that have yielded mixed results.12–14 In addition, these studies have assessed different measures of clinical and subclinical atherosclerosis, including carotid intima media thickness (CIMT), arterial calcification (coronary aortic calcification (CAC); abdominal aortic calcification (AAC); or thoracic aortic calcification (TAC)), and ankle-brachial index (ABI). In light of the prior inconclusive associations between PM2.5 and atherosclerosis, as well as the potential heterogeneity in study methodologies and outcomes, we therefore conducted a systematic review and meta-analysis of studies published to date.
Methods
Search strategy
We followed the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group in the design, implementation and reporting of our study.15 We conducted a comprehensive literature search of four databases –MEDLINE, EMBASE, Web of Science and Environmental Index – to identify relevant articles that were published through May 2014. Our search queries combined the exposure (PM2.5) with atherosclerosis and surrogate markers of atherosclerosis. Search terms for MEDLINE were (“Particulate Matter”[mesh] OR “Air Pollution”[mesh] OR air pollution[tiab] OR particulate*[ tiab] OR fine partic*[tiab] OR pm2.5[tiab] OR pm 2.5[tiab]) AND (“Arteriosclerosis”[mesh] OR “Tunica Intima”[Mesh] OR intima[tiab] OR “Vascular Calcification”[Mesh] OR “coronary artery calcification” OR “ankle brachial index” [Mesh] OR arterioscleros*[tiab] OR atheroscleros*[tiab] OR atherogen*[ tiab] OR arterial disease*[tiab] OR arterial occlus*[tiab]) NOT (“animals”[mesh] NOT “humans”[mesh]). Full details of our search strategies for the other databases are available in Supplementary Material online (Supplemental Table).
Study selection
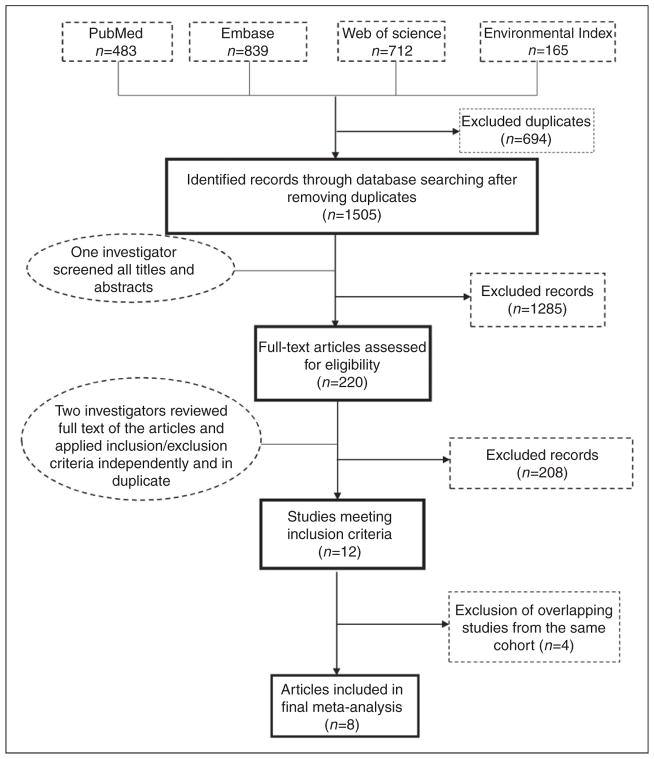
We included cross-sectional and longitudinal cohort studies evaluating associations between PM2.5 and clinical or subclinical atherosclerosis as assessed by CIMT, arterial calcification, or ABI. All languages were included in our search. For the meta-analysis, we excluded non-human studies, studies reporting environmental exposures other than PM2.5, and studies reporting estimates other than absolute change in outcome per change in the level of PM2.5. In addition, for overlapping studies from the same cohort, we included only the most comprehensive or updated study with the most extensive method of covariate adjustment. Five authors (EA, LS, IO, OM, JAD) independently evaluated non-duplicate abstracts found in the four databases (N = 1505) using our search algorithm. Articles deemed relevant to our study (N = 220) were then selected for independent review of full text and references by two separate authors (Figure 1). We applied our inclusion and exclusion criteria to determine articles for final inclusion; disagreements between two authors were resolved by a third author. Reference lists of all relevant articles (including review articles) were scanned to identify publications that were potentially missed by our initial literature search.
Figure 1.
Screening and selection process. Of 1505 non-duplicate articles found, 220 were retained after screening titles and abstracts. These articles underwent full-text review by two separate investigators; there were 12 studies that met our predefined inclusion criteria; after removing overlapping cohorts, our final sample included eight studies.
Data extraction
Data from the final selected manuscripts were independently extracted by two authors and compared to ensure accuracy. Information extracted included citation data, authors’ names, publication year, data source, country, sample size, age distribution, sex distribution, year of data collection, study design, baseline exposure level, outcome measure, effect estimate, and standard error of effect estimate. For studies that reported multiple effect estimates, we extracted the estimate from the main model or model that reflected the greatest degree of control for potential confounders. For each included manuscript, we extracted mean change in CIMT, relative risk for arterial calcification, or mean change in ABI, as applicable.
Statistical analysis
In order to ensure uniformity of exposure across studies, all estimates were standardized to per 10 μg/m3 increase in PM2.5. Effect measures were pooled using the random effect model of DerSimonian and Laird to account for between-study variation.16
Heterogeneity between studies was explored by visual inspection of the forest plot, Cochran Q statistic (p<0.05), and I-squared (I2) statistic. Consistent with prior thresholds we considered an I2 statistic ≥50% to represent substantial heterogeneity and ≥75% to represent considerable heterogeneity.17 We assessed potential sources of heterogeneity such as year of publication, country, study design, sample size, and baseline level of exposure by using meta-regression. We did not perform an assessment for publication bias given the small number of studies for each endpoint.18
All statistical tests were two-sided and p values less than 0.05 were considered to be statistically significant. Analyses were conducted with STATA Version 13.
Results
Of the 12 manuscripts considered for data extraction, four were from the Multi-ethnic Study of Atherosclerosis (MESA; providing three CIMT, three arterial calcification, and one ABI estimate), four were from the German Heinz Nixdorf Recall Study (HNRS; providing one CIMT, two arterial calcification, and one ABI estimate), and two (both CIMT) were from the same author (Kunzli et al.). After retaining only one article per cohort for each endpoint, our final sample included eight manuscripts, from which we extracted five CIMT, two CAC, and two ABI estimates. We analyzed a total of 18,590 subjects (mean age 58 years, 52% female). As some studies reported more than one type of atherosclerotic marker (e.g. all markers were evaluated in the MESA cohort), 11,947 subjects contributed to the CIMT estimates, 10,750 contributed to the arterial calcification estimate and 6497 contributed to the ABI estimates. The mean level of PM2.5 exposure among studies ranged from 13.66 μg/m3 to 22.8 μg/m3. Other study characteristics are summarized in Table 1.12–14,19–27 Although there were some differences in exposure assessment, overall the methods of assessment were comparable across studies and within each endpoint (Table 2).
Table 1.
Demographic characteristics of the 12 studies that evaluated the association between PM2.5 and atherosclerosis.
| First author and year | Outcome | Country | Study design | Data collection | N | Age, years Mean (SD/range) | % female | Data source | Baseline exposure level (mg/m3) |
|---|---|---|---|---|---|---|---|---|---|
| Adar 201312 a | CIMT | USA | Longitudinal | 2000–2005 | 5362 | 62 (10) | 52 | MESA | 16.6 |
| Bauer 201013 | CIMT | Germany | Cross-sectional | 2000–2003 | 3380 | 60 (7.7) | 48 | HNRS | 16.8 |
| Breton 201214 | CIMT | USA | Cross-sectional | 2007–2009 | 768 | 20 (1.5) | 59 | TROY | 15.7 |
| Sun 201319 | CIMT | USA | Cross-sectional | 2000–2002 | 6256 | 62 (45–84) | 52 | MESA | 13.66 |
| Diez Roux 2008 20a | CIMT | USA | Longitudinal | 2000–2002 | 5037 | 62 (45–84) | 53 | MESA | 16.7 |
| Künzli 200521 | CIMT | USA | Cross-sectional | 1998–2003 | 798 | 59 (9.8) | 45 | VEAPS & BVAIT | 20.3 |
| Lenters 201022 | CIMT | Netherlands | Longitudinal | 1999–2000 | 745 | 28 (0.9) | 53 | Not reported | 20.7 |
| Künzli 201023a | CIMT | USA | Longitudinal | 1995–2007 | 1438 | 59 (9.6) | 63 | Five trialsb | 20.79 |
| Allen 200924 a | AAC | USA | Cross-sectional | 2000–2002 | 1147 | 66 (9.4) | 50 | MESA | 15.8 |
| Kälsch 201425 a | TAC | Germany | Cross-sectional | 2000–2003 | 4238 | 60 (7.8) | 50 | HNRS | 16.62 |
| Sun 201319 | CAC | USA | Cross-sectional | 2000–2002 | 6256 | 62 (45–84) | 52 | MESA | 13.66 |
| Diez Roux 2008 20a | CAC | USA | Cross-sectional | 200–2002 | 2149 | 62 (45–84) | 53 | MESA | 16.7 |
| Hoffmann 200726 | CAC | Germany | Cross-sectional | 200–2003 | 4494 | 60 (7.8) | 51 | HNRS | 22.8 |
| Hoffmann 200927 | ABI | Germany | Cross-sectional | 200–2003 | 4348 | 60 (7.8) | 51 | HNRS | 22.8 |
| Diez Roux 2008 20 | ABI | USA | Cross-Sectional | 2000–2002 | 2149 | 62 (45–84) | 52 | MESA | 16.7 |
Study was excluded from the meta-analysis due to cohort overlap.
The five trials included in Kunzli 2010 are: B-vitamin Atherosclerosis Intervention Trial (BVAIT), Vitamin E Atherosclerosis Prevention Study (VEAPS), Estrogen in the Prevention of Atherosclerosis Trial (EPAT), Troglitazone Atherosclerosis Regression Trial (TART), Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELL-HART). PM2.5: particulate matter with aerodynamic diameter ≤ 2.5 μm; CIMT: carotid intima media thickness; CAC: coronary artery calcification; ABI: ankle-brachial index; HNRS: Heinz Nixdorf Recall Study; MESA: Multi-Ethnic Study of Atherosclerosis; TROY: Testing Responses On Youth study
Table 2.
Methodological details in eight studies included in the meta-analysis.
| First author and year | Location | Exposure | Method of exposure measurement | Outcome | Method of outcome measurement | Confounders adjusted for |
|---|---|---|---|---|---|---|
| Bauer 201013 | Essen, Mulheim and Bochum | PM2.5 | Average of the previous 365 days of daily surface concentrations of PM2.5 were taken for each participant. A chemistry transport model was used with input data from emission inventories, meteorology, and regional topography. | CIMT | B-mode ultrasound. The mean of all 10 manual measurements on both was used as the outcome variable. | City, area of residence, age, sex, education, economic activity, smoking variables, environmental tobacco smoke, alcohol consumption, physical activity, BMI, diabetes, LDL-C, HDL-C, intake of statins. |
| Breton 201214 | Southern CA | PM2.5 | Residential addresses geocoded. Spatial interpolation of ambient air quality data from four stations used along with geocoded residential address of participants. Air quality data was interpolated using inverse distance squared weighting. Air pollutant estimates were from EPA’s Air Quality System database. | CIMT | The mean of 70–100 measurements of the right common carotid artery was used. Instrument was a high resolution B-mode ultrasound attached to a 10MHz linear array transducer. | Age, sex, race/ethnicity, BMI, systolic blood pressure, secondhand smoke in childhood, current secondhand smoke, hsCRP, LDL-C, HDL-C. No effect modification by any variable was found on analysis |
| Lenters 201022 | Utrecht, Netherlands | PM2.5 | Overall concentrations of PM2.5 for the year 2000 at the residential address were assessed regionally via interpolation of regional background concentrations. The urban component was assessed with regression models using data on 10 categories of land use in 100-m grids, population density and land use predictors. | CIMT | High resolution B-mode ultrasound of the right and left common carotid arteries using a 7.5MHz linear array transducer. Both the mean and the maximum CIMT were assessed. | Age, sex, BMI, pack years of active smoking, exposure to secondhand smoke in childhood, alcohol intake, highest education, highest profession, diabetes mellitus, neighborhood income, hypertension, HDL-C, LDL-C, family history of CVD. Non-significance evidence of effect modification by sex (stronger association in women), smoking status (stronger association with smokers), and education (stronger association in the less educated). |
| Künzli 200521 | Los Angeles, CA | PM2.5 | Geostatistical model derived for mean home outdoor PM2.5 level, data source was year 2000 data obtained from 23 state and local district monitoring stations. Residential geocoding used. | CIMT | High-resolution far wall B-mode ultrasound images of the right common carotid artery. | Age, sex, education, income, current secondhand smoke, current personal smoking, former personal smoking, blood pressure, LDL-C, antihypertensive medications, lipid lowering medications. Effect modification by age and sex was found, with the association strongest among elderly women aged ≥60 years. |
| Sun 201319 | Los Angeles County, CA; Chicago, IL; Baltimore MD; St Paul, MN; Forsyth County, NC; and New York, NY | PM2.5 | Residential addresses geocoded. Three different approaches were used: the annual average concentration of the two week measurement at the monitor nearest to each study participant’s residence, inverse distance weighting of all annual average monitor concentrations in each area relative to each subject’s residence, and city wide average concentrations based on all monitors within each area. | CIMT CAC |
High resolution B-mode ultrasound. Mean far wall thickness of the right common carotid retrospectively gated to end-diastole was used. Two chest CT scans per participant. Mean Agatston score34 of the scans used for analysis. |
Age, gender, race and ethnicity, total cholesterol, LDL-C, smoking status, hypertension, lipid lowering medications, level of education, waist circumference, family income, body surface area, BMI, squared BMI, diabetes, HDL-C, triglycerides. Effect modification by variables not assessed. |
| Hoffmann 200726 | The three cities: Essen, Mulheim and Bochum (Germany) of the Ruhr area in Germany | PM2.5 | Residential geocoding. EURAD modeling of daily mean values for PM2.5 for the year 2002 with input data from official emission inventories, meteorological information, and regional topographical data. Annual average calculated for each grid and assigned to each participant living in that grid. | CAC | Use of chest CTs for each participant. CAC score calculated by the Agatston score. Final CAC score was summation of CAC scores of all foci in the epicardial coronary system. | Age, sex, city of residence, area of residence, education, smoking, physical inactivity, waist to hip ratio, diabetes, blood pressure and lipids. No effect modification was carried out although a subgroup analysis of elderly patients was carried out. |
| Hoffmann 200927 | The three cities: Essen, Mulheim and Bochum (Germany) of the Ruhr area in Germany | PM2.5 | Residential geocoding. EURAD dispersion and chemistry transport modeling of daily mean values for PM2.5 for the year 2002 with input data from official emission inventories, meteorological information, and regional topographical data. Annual average calculated for each grid and assigned to each participant living in that grid. | ABI | 8MHz Doppler transducer used. The index was calculated as the ratio of: highest ankle artery pressures measured either in the posterior tibial or the dorsalis pedis artery and the highest systolic brachial pressure measured in the right and left arm. | Age, sex, city of residence, area of residence, education, smoking, physical inactivity, waist to hip ratio, diabetes, BMI, socioeconomic status, lipid lowering medication, antihypertensive medication, blood pressure and lipids. Non-significant effect modification by age was found with a stronger association among enrolled subjects ≥60 years of age. |
| Diez Roux 200820 | Los Angeles County, CA; Chicago, IL; Baltimore MD; St Paul, MN; Forsyth County, NC; and New York, NY | PM2.5 | Spatiotemporal modeling of the monthly mean PM2.5 measures for the prior 20 years with data obtained from the US EPA’s aerometric information retrieval service database. Residential geocoding used. | ABI | 5 MHz probe on a hand held Doppler instrument. For each lower extremity, ABI numerator was the highest pressure (dorsalis pedis or posterior tibial from that leg) obtained. ABI denominator was the averaged brachial artery blood pressure except if there was a difference of 10mmHg or more, in which case the highest systolic blood pressure was used. Ratios were calculated separately for the left and right sides and the minimum value was used for analyses. | Age, Sex, race, socioeconomic factors, BMI, hypertension, HDL-C, LDL-C, smoking, diabetes, diet and physical activities. The following variables were explored for effect modification: age, sex, lipid levels, site, education, race/ethnicity, diabetes, BMI, smoking. No effect modification by these variables was found. |
PM2.5: particulate matter with aerodynamic diameter ≤ 2.5 μm; CIMT: carotid intima media thickness; BMI: body mass index; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; hsCRP: high-sensitivity C-reactive protein; EPA: Environmental Protection Agency; CVD: cardiovascular disease; CAC: coronary aortic calcification; CT: computed tomography; EURAD: European Air Pollution Dispersion; ABI: ankle-brachial index
CIMT
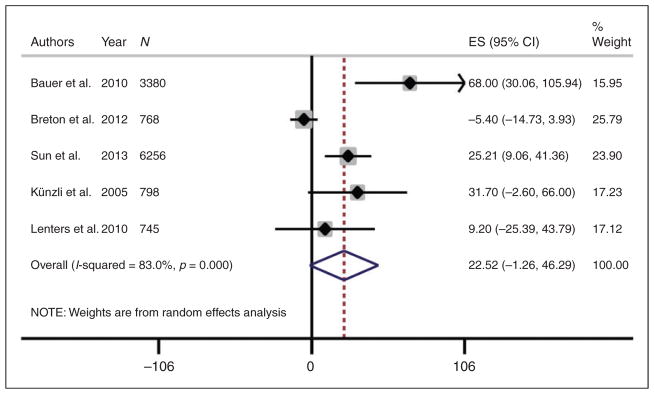
Meta-analysis of the five studies evaluating the outcome of CIMT demonstrated that CIMT increased by 22.52 μm for every 10 μg/m3 increase in PM2.5 but this association did not reach statistical significance (p = 0.06) (Figure 2). There was considerable heterogeneity between studies (I2 = 83%, p<0.01), although exploration using meta-regression showed that year of publication (p = 0.61), country (p = 0.23), study design (p = 0.52), sample size (p = 0.50), and baseline level of exposure (p = 0.97) did not explain this heterogeneity.
Figure 2.
Meta-analysis of mean change in carotid intima media thickness (CIMT) per 10 μg/m3 increase in PM2.5. Mean change in CIMT from each study was standardized to per 10 μg/m3 increase in PM2.5.
ES: standardized estimate; CI: confidence interval; PM2.5: particulate matter with aerodynamic diameter ≤2.5 μm
Arterial calcification
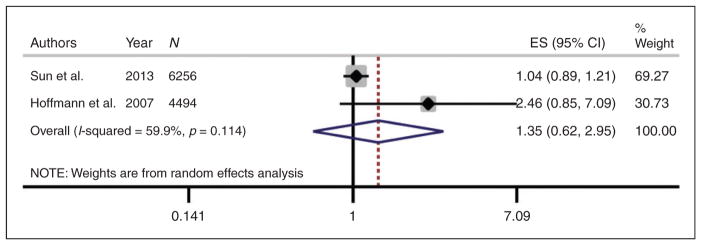
We found five manuscripts reporting on the association between PM2.5 exposure and three subtypes of arterial calcification: CAC (three), AAC (one), and TAC (one), but three were from the MESA cohort while the remaining two were from the HNRS cohort. After excluding overlapping studies from the same cohort, only two manuscripts reporting on CAC were retained for the final analysis, which yielded a non-significant positive association (relative risk = 1.35 per 10 μg/m3 increase in PM2.5, p = 0.44) (Figure 3). Given the small number of studies we did not test for heterogeneity.
Figure 3.
Meta-analysis of relative risk of arterial calcification per 10 μg/m3 increase in PM2.5. Relative risk estimates from each study were standardized to per 10μg/m3 increase in PM2.5.
ES: standardized estimate; CI: confidence interval; PM2.5: particulate matter with aerodynamic diameter ≤2.5 μm
ABI
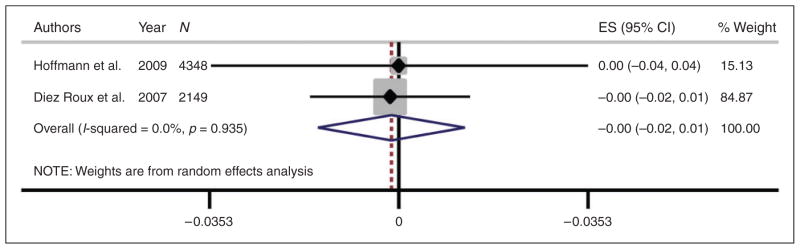
Only two manuscripts reported on the association between PM2.5 exposure and peripheral arterial disease by ABI. Our meta-analysis of these two studies yielded a non-significant association between PM2.5 and ABI (change in ABI per 10 μg/m3 increase in PM2.5 = – 0.001, p = 0.85) (Figure 4). As with arterial calcification, we did not consider a test for heterogeneity given the low number of studies.
Figure 4.
Meta-analysis of mean change in ankle-brachial index (ABI) per 10μg/m3 increase in PM2.5. Mean change in ABI from each study was standardized to per 10 μg/m3 increase in PM2.5.
ES: standardized estimate; CI: confidence interval; PM2.5: particulate matter with aerodynamic diameter ≤2.5 μm
Discussion
Human studies that have investigated the association between PM2.5 and clinical and subclinical atherosclerosis have yielded mixed results. In addition, a variety of different outcome measures have been used. In order to summarize the available evidence in the literature, we conducted a meta-analysis among eight studies comprising 18,590 subjects. We found marginal evidence to support the association between PM2.5 exposure and CIMT. While there was considerable heterogeneity (I2 = 83%) among CIMT studies, the positive association between PM2.5 and CIMT appeared to be consistent across all but one study.
Though not statistically significant, our findings demonstrated that for every 10 μg/m3 increase in PM2.5, CIMT increased by 22.52 μm (95% confidence interval (CI) –1.26, 46.29 μm). This estimate is within the range of change in CIMT that has been associated with CVD events.28 While the magnitude of effect of PM2.5 exposure may appear relatively small, PM2.5 exposure is common with a wide range in world-wide exposures. It can vary from as low as a US Environmental Protection Agency recommended level of ≤35 μg/m3 to as high as over 200 μg/m3 in countries such as China,29,30 with levels that may exceed 500 μg/ m3. Therefore, a 200 μg/m3 increase in PM2.5 would be expected to translate into roughly a 450 μm increase in CIMT, an estimation that would be of significant clinical impact given that average CIMT in the general population is around 800 μm.31 Also of note, potential interactions were evaluated in three of the five CIMT studies, and two of these (Lenters et al.,22 Kunzli et al.21) suggested that the association between PM2.5 and CIMT was stronger in females than in males. Kunzli et al. also reported a significant interaction with age, indicating a stronger association in participants ≥60 years compared with participants<60 years. Accordingly, there may be subgroups that are at particularly high risk of adverse effects from PM2.5.
Though the exact mechanism of the association between PM2.5 and cardiovascular disease remains uncertain, experimental animal studies have suggested some mechanistic links between PM2.5 and increased CIMT. PM2.5 may provoke an inflammatory response and cytokine release from the pulmonary vascular bed, altering vasomotor tone and lipid peroxidation.4,11,32,33 These studies have emphasized the relationship between PM2.5 exposure and pro-oxidant and pro-inflammatory mediators important in the pathogenesis of atherosclerosis. However, most of these experiments involve concurrent administration of a high fat diet in order to accelerate atheroma formation, thereby indicating that dietary factors may be an important modifier of the effect of PM2.5.
We did not find evidence of an association between PM2.5 and either CAC (estimated based on Agatston score34) or ABI (measured by Doppler ultrasound). For both measures we found a low number of studies and thereforewemay have been underpowered to find a meaningful association. Alternatively, it is possible that CIMT may identify areas of increased thickness and nonocclusive atherosclerotic plaque, which may represent earlier stages of arterial injury or atherosclerosis than measures of arterial calcification orABI.28 It is also possible that the atherosclerotic mechanism of PM2.5 directly alters CIMT with little or no influence on arterial calcification or ABI.
Our meta-analysis has several strengths, including a protocol-driven approach in order to limit bias in study selection, as well as a broad population represented, including a wide age range that was studied across three different countries (United States, Germany, and Netherlands). However, there are potential limitations that deserve consideration. First, the number of studies, particularly for arterial calcification and ABI, was small, limiting our ability to derive strong conclusions from these analyses and to explore for potential sources of heterogeneity. Second, we found evidence of significant heterogeneity among CIMT estimates, thereby limiting the generalizability of our results. Third, most studies were cross-sectional, which limits estimations of causality given the lack of temporality between exposure and outcome. However, these cross-sectional studies did adjust for major cardiovascular risk factors, demographic information, as well as socio-economic status, which could potentially confound the association between PM2.5 and atherosclerosis. Lastly, despite a rigorous methodology within each study, there is the potential for measurement error in the assessment of exposure and/or outcome that may have biased study estimates towards no-association.
In conclusion, we found a positive association across multiple studies between PM2.5 and subclinical atherosclerosis as measured by CIMT, although this did not reach statistical significance. There was considerable heterogeneity among studies, which may limit the generalizability of this finding. We did not find similar associations between PM2.5 and other surrogate markers of atherosclerosis (arterial calcification or ABI), which may be due to lower sensitivity of these indices or lack of a sufficient number of studies. More studies may be needed to explore potential sources of heterogeneity among CIMT estimates, and to further assess the association between PM2.5 and the other surrogate markers.
Acknowledgments
We thank Paul Bain PhD at Harvard University’s Countway Library for providing guidance for our literature search. We also thank Chung-Cheng Hsieh ScD and Julie Goodman PhD at Harvard School of Public Health for providing guidance in our methods and statistical analysis.
Funding
Dr. Newman is supported by a grant from the National Institute for Diabetes and Digestive and Kidney Diseases (U24DK076169-09, subcontract 25732-60). Dr. Dodson is supported by a grant from the National Institute of Aging (R03AG045067) and a T Franklin Williams Scholarship Award (funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American College of Cardiology). None of the remaining authors receive any specific grant from any funding agency in the public, commercial, or not for profit sectors.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Laden F, Schwartz J, Speizer F, et al. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloog I, Melly SJ, Ridgway WL, et al. Using new satellite based exposure methods to study the association between pregnancy pm2. 5 exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012;11:1–8. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelin TD, Joseph AM, Gorr MW, et al. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett. 2012;208:293–299. doi: 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suwa T, Hogg JC, Quinlan KB, et al. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002;39:935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 6.Newby DE, Mannucci PM, Tell GS, et al. on behalf of ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation and ESC Heart Failure Association. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83b–93b. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubesch N, De Nazelle A, Guerra S, et al. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur J Prev Cardiol. 2015;22:548–557. doi: 10.1177/2047487314555602. [DOI] [PubMed] [Google Scholar]
- 8.Bilenko N, van Rossem L, Brunekreef B, et al. Trafficrelated air pollution and noise and children’s blood pressure: Results from the PIAMA birth cohort study. Eur J Prev Cardiol. 2015;22:4–12. doi: 10.1177/2047487313505821. [DOI] [PubMed] [Google Scholar]
- 9.Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–35. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- 11.Soares SR, Carvalho-Oliveira R, Ramos-Sanchez E, et al. Air pollution and antibodies against modified lipoproteins are associated with atherosclerosis and vascular remodeling in hyperlipemic mice. Atherosclerosis. 2009;207:368–373. doi: 10.1016/j.atherosclerosis.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Adar SD, Sheppard L, Vedal S, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: A prospective cohort study from the multiethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer M, Moebus S, Möhlenkamp S, et al. Study Investigative Group HNR Urban particulate matter air pollution is associated with subclinical atherosclerosis: Results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56:1803–1808. doi: 10.1016/j.jacc.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 14.Breton CV, Wang X, Mack WJ, et al. Childhood air pollutant exposure and carotid artery intima-media thickness in young adults. Circulation. 2012;126:1614–1620. doi: 10.1161/CIRCULATIONAHA.112.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [accessed 1 October 2014]. Chapter 9: Analysing data and undertaking meta-analyses. Version 5.1.0 (updated March 2011) www.cochrane-handbook.org. [Google Scholar]
- 18.Lau J, Ioannidis JP, Terrin N, et al. The case of the mis-leading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun M, Kaufman JD, Kim SY, et al. Particulate matter components and subclinical atherosclerosis: Common approaches to estimating exposure in a Multi-Ethnic Study of Atherosclerosis cross-sectional study. Environ Health. 2013;12:39. doi: 10.1186/1476-069X-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez Roux AV, Auchincloss AH, Franklin TG, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi- Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:667–675. doi: 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- 21.Künzli N, Jerrett M, Mack W, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenters V, Uiterwaal CS, Beelen R, et al. Long-term exposure to air pollution and vascular damage in young adults. Epidemiology. 2010;21:512–520. doi: 10.1097/EDE.0b013e3181dec3a7. [DOI] [PubMed] [Google Scholar]
- 23.Künzli N, Jerrett M, Garcia-Esteban R, et al. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One. 2010;5:e9096. doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20:254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kälsch H, Hennig F, Moebus S, et al. Are air pollution and traffic noise independently associated with atherosclerosis: The Heinz Nixdorf Recall Study; Heinz Nixdorf Recall Study Investigative Group. Eur Heart J. 2014;35:853–860. doi: 10.1093/eurheartj/eht426. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann B, Moebus S, Möhlenkamp S, et al. Heinz Nixdorf Recall Study Investigative Group Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann B, Moebus S, Kröger K, et al. Residential exposure to urban air pollution, ankle-brachial index, and peripheral arterial disease. Epidemiology. 2009;20:280–288. doi: 10.1097/EDE.0b013e3181961ac2. [DOI] [PubMed] [Google Scholar]
- 28.Stein JH, Korcarz CE, Hurst RT, et al. American Society of Echocardiography Carotid Intima-Media Thickness Task Force Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 29.United States Environmental Protection Agency. Office of Air and Radiation, Office of Air Quality Planning and Standards, Fact Sheet. Revised air quality standards for particle pollution and updates to the Air Quality Index (AQI) US EPA; North Carolina, USA: [Google Scholar]
- 30.Wang X, Bi X, Sheng G, et al. Hospital indoor PM10/ PM2. 5 and associated trace elements in Guangzhou, China. Sci Total Environ. 2006;366:124–135. doi: 10.1016/j.scitotenv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 32.Chen T, Jia G, Wei Y, et al. Beijing ambient particle exposure accelerates atherosclerosis in ApoE knockout mice. Toxicol Lett. 2013;223:146–153. doi: 10.1016/j.toxlet.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Navab M, Pakbin P, et al. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDRL-null mice. J Lipid Res. 2013;54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]