Abstract
Objective:
We performed a meta-analysis to evaluate the effects of whole-body vibration on physiologic and functional measurements in children with cerebral palsy.
Design and methods:
We searched MEDLINE, Cochrane Controlled Trials Register, EMBASE, Scielo, CINAHL (from the earliest date available to November 2014) for randomized controlled trials, that aimed to investigate the effects of whole-body vibration versus exercise and/or versus control on physiologic and functional measurements in children with cerebral palsy. Two reviewers independently selected the studies. Weighted mean differences (WMDs) and 95% confidence intervals (CIs) were calculated.
Results:
Six studies with 176 patients comparing whole-body vibration to exercise and/or control were included. Whole-body vibration resulted in improvement in: gait speed WMDs (0.13 95% CI:0.05 to 0.20); gross motor function dimension E WMDs (2.97 95% CI:0.07 to 5.86) and femur bone density (1.32 95% CI:0.28 to 2.36). The meta-analysis also showed a nonsignificant difference in muscle strength and gross motor function dimension D for participants in the whole-body vibration compared with control group. No serious adverse events were reported.
Conclusions:
Whole-body vibration may improve gait speed and standing function in children with cerebral palsy and could be considered for inclusion in rehabilitation programs.
Keywords: Cerebral Palsy, Exercise, Whole-body Vibration, Mobility
Background
The development of alternative techniques and therapeutic resources to improve disability and quality of life in children with cerebral palsy is challenging[1]. The use of external devices such as treadmills and weight machines is increasing in treatment settings[2,3]. Whole-body vibration training was proposed as a new therapeutic modality for the treatment of the gross motor function, balance and functional performance[4-7].
Whole-body vibration is a neuromuscular training which uses oscillatory motion around an equilibrium point. This technique may be a effective stimulus to improve neuromuscular performance and balance in healthy individuals[8,9]. In patients with Parkinson disease has demonstrated limited, but beneficial effects on balance stability and mobility[10]. However, another review reported that whole-body vibration training only improves strength in neurological patients and balance/mobility in patients with musculoskeletal or metabolic disorders[11]. So, the effects of whole-body vibration are not consensual in the literature, what deserves some concern. No systematic review with meta-analysis has been performed to investigate the effects of whole-body vibration in children with cerebral palsy.
The aim of this systematic review was to analyze the published randomized controlled trials (RCTs) that investigated the effects of whole-body vibration on motor function and functional performance in children with cerebral palsy.
Methods
This review was planned and conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines[11].
Elegibility criteria
This systematic review included all RCTs that studied the effects of whole-body vibration on motor function and functional performance in children with cerebral palsy.
Studies were considered for inclusion regardless of their publication status, language or size. Trials enrolling children with cerebral palsy were included in this systematic review. To be eligible, the trial should have randomized cerebral palsy patients to, at least, one group of whole-body vibration. The main outcomes of interest were motor function and functional performance.
Search methods for identification of studies
We searched for references on MEDLINE, LILACS, EMBASE, SciELO, Cumulative Index to Nursing and Allied Health (CINAHL), PEDro, and the Cochrane Library up to November 2014 without language restrictions. A standard protocol for this search was developed and whenever possible, controlled vocabulary (Mesh term for MEDLINE and Cochrane and EMTREE for EMBASE) were used. Key words and their synonymous were used to sensitize the search.
For the identification of RCTs in PUBMED/MEDLINE the optimally sensitive strategy developed for the Cochrane Collaboration was used[12]. To identify the RCTs in EMBASE, a search strategy using similar terms was adopted. In the search strategy, there were four groups of keywords: study design, participants, interventions, and outcome measures as such: “randomized controlled trials”, “Cerebral palsy”, “exercise”, “whole-body vibration” and “mobility”, using Boolean operators and/or.
All eligible articles for this meta-analysis had their references lists analyzed in order to detect other potentially eligible studies. For ongoing studies or when the confirmation of any data or additional information was needed, the authors were contacted by e-mail.
Data collection and analysis
The previously described search strategy was used to obtain titles and abstracts of studies that might be relevant for this review. Each abstract identified in the search was independently evaluated by two authors. If at least one of the authors considered one reference eligible, the full text was obtained for complete assessment.
In a similar fashion, two authors independently evaluated full text articles for eligibility and filled inclusion and exclusion criteria in a standard form. A standardized data extraction form was used to inclusion and exclusion criteria. In case of any disagreement, the authors discussed the reasons for their decisions and a final decision was made by consensus.
Two authors independently extracted data from the published reports using standard data extraction forms adapted from the Cochrane Collaboration’s[12] model for data extraction, considering: 1) aspects of the study population, such as the average age and sex; 2) aspects of the intervention performed (sample size, frequency, and duration of each session); 3) follow-up; 4) loss to follow-up; 5) outcome measures; and 6) presented results. Disagreements were resolved by one of the authors. Any further information required from the original author was requested by e-mail.
Risk of bias of included studies
The risk of bias of included studies was assessed independently by two authors using The Cochrane Collaboration’s ‘Risk of bias’ tool[12]. The following criteria were assessed: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, intention-to-treat analysis and completeness of follow-up.
Quality of meta-analysis evidence
The quality of evidence generated by this meta-analysis was classified using the PEDro scale. There are several scales for assessing the quality of RCTs. The PEDro scale assesses the methodological quality of a study based on important criteria, such as concealed allocation, intention-to-treat analysis, and the adequacy of follow-up. These characteristics make the PEDro scale a useful tool for assessing the quality of physical therapy and rehabilitation trials[13].
Methodological quality was independently assessed by two researchers. Studies were scored on the PEDro scale based on a Delphi list[14] that consisted of 11 items. One item on the PEDro scale (eligibility criteria) is related to external validity and is generally not used to calculate the method score, leaving a score range of 0 to 10[15]. Any disagreements were resolved by a third reviewer.
Statistical assessment
Pooled-effect estimates were obtained by comparing the least square mean percentage change from baseline to study end for each group, and were expressed as the weighted mean difference between groups. When the SD of change was not available, the SD of the baseline measure was used for the meta-analysis. Calculations were done using a random-effects model. One comparison was made: whole-body vibration versus control group. An α value of 0.05 was considered significant. Statistical heterogeneity of the treatment effect among studies was assessed using Cochran’s Q-test and the inconsistency I2 test, in which values above 25 and 50% were considered indicative of moderate and high heterogeneity, respectively[16]. If a meta-analysis was not possible due to clinical heterogeneity, data were analysed descriptively. All analyses were conducted using Review Manager Version 5.0 (Cochrane Collaboration)[17].
Results
Description of selected studies
The initial search led to the identification of 12 abstracts, from which six studies were considered as potentially relevant and were retrieved for detailed analysis. Only six papers met the eligibility criteria. Figure 1 shows the PRISMA flow diagram of studies in this review.
Figure 1.
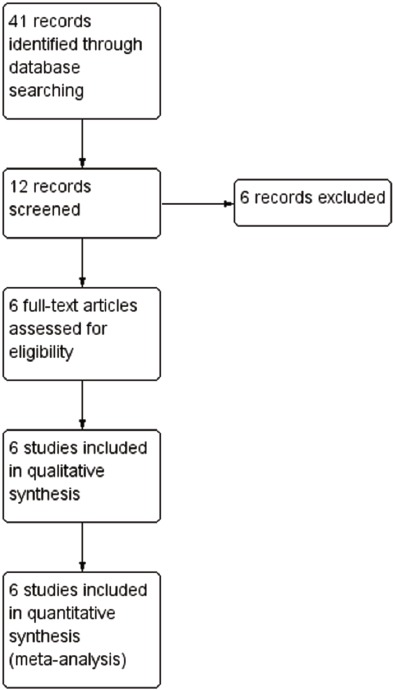
Search and selection of studies for systematic review according PRISMA.
The remaining six articles were fully analyzed and approved by both reviewers and had the extraction of data from each RCT. Each of the papers was assessed using the PEDro scale methodology by both reviewers. The results of the assessment of the PEDro scale are presented individually in Table 1.
Table 1.
Study quality on the PEDro scale.
| Study | 1* | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ibrahim et al, 2014[18] | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| 2 | El-Shamy et al, 2014[19] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| 3 | Lee & Chon, 2013[20] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| 4 | El-Shamy et al, 2012[21] | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| 5 | Ruck et al, 2010[22] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| 6 | Wren et al, 2010[23] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 |
1: eligibility criteria and source of participants; 2: random allocation; 3: concealed allocation; 4: baseline comparability; 5: blinded participants; 6: blinded therapists;7: blind assessors; 8: adequate follow-up; 9: intention-to-treat analysis; 10: between-group comparisons; 11: point estimates and variability.
Item 1 does not contribute to the total score.
Study characteristics
The final sample ranged from 17[21 to 30[18,19,20,22], and mean age of participants ranged from 8.2 to 9.8 years. All studies included patients of both genders, but there was a predominance of male. Table 2 presents summary data from the two RCTs eligible for this systematic review.
Table 2.
Characteristics of the included studies.
| Intervention groups | |||||||
|---|---|---|---|---|---|---|---|
| Study | Patients (diagnosis, N analysed, age, gender) | Treatment | Control | Outcome measures | Results | ||
| 1 | Ibrahim et al[18] | Spastic Diplegia, 30 children; 9.93 years. | Conventional Therapy + WBV | Conventional Therapy | Knee extensor strength Walking Speed Walking Balance GMFM | Knee extensor strength, Walking Speed and GMFM (E) was significantly increase only WBV group | |
| 2 | El-Shamy et al[19] | Spastic Diplegia, 30 children; 9.93 years; 76,6% male. | Conventional Therapy + WBV | Conventional Therapy | Knee extensor strength Balance and postural stability | Increase the gains in muscle strength and balance | |
| 3 | Lee & Chon20 | Cerebral Palsy, 30 children; 10 years; 50% male. | Conventional Therapy + WBV | Conventional Therapy | Gross motor function Leg muscle thickness Three-dimensional gait analyses | Improve mobility in children with cerebral palsy Positive effect on the leg muscles | |
| 4 | El-Shamay et al[21] | Spastic Diplegia; 30 children; 10-13 years; GMFCS = I, II. | Conventional Therapy + WBV | Conventional Therapy | Bone densitometry Anthropometry | Improvements in Bone densitometry | |
| 5 | Ruck et al[22] | Cerebral Palsy, 20 children 18 analysed, 6.2 to 12.3 years, 70% male; GMFCS = II-IV. | Conventional Therapy + WBV | Conventional Therapy | Walking ability Gross motor function Bone densitometry | Improve mobility function Without detect a positive treatment effect on bone | |
| 6 | Wren et al[23] | Cerebral Palsy; 36 children; 9.4 years; 42% male; GMFCS = I-IV. | WBV | Stand up without WBV | Bone densitometry and Muscle strength | Did not result from increases in muscle mass or strength. No effect was seen on bone | |
GMFM=Gross motor function; GMFCS= Gross Motor Function Classification System.
Characteristics of intervention programs
The parameters used in the application of whole-body vibration have been reported in the searched studies, and the progressive nature of the programs was described.
The duration of whole-body vibration programs ranged from 8[20 to 24[21-23] weeks. Regarding the session duration, there was a variation from 10[21,23] to 60[18,20] minutes. The frequency ranged from 3[18,20] to 7[23] times per week. The characteristics of the intervention programs are in Table 3.
Table 3.
Characteristics of the experimental intervention in the trials included in the review.
| Study | Modality | Intensity/duration (wk) | Volume | Frequency (x per Wk) | Time (min) | Length (wk) | Supervision | |
|---|---|---|---|---|---|---|---|---|
| 1 | Ibrahim et al[18] | Whole Body Vibration | 12-18 Hz 2-6 mm |
3 min of WBV 3 min of rest 3 min of WBV 3 min of rest 3 min of WBV |
3 | 60 | 12 | Yes |
| 2 | El-Shamy et al[19] | Whole Body Vibration | 12-18 Hz | 3 min of WBV 3 min of rest 3 min of WBV 3 min of rest 3 min of WBV |
5 | 18 | 12 | Yes |
| 3 | Lee & Chon20 | Whole Body Vibration Witchout shoes | 5-25 Hz | 3 min of 5-8 Hz 3 min of 10-15 Hz 3 min of 15-20 Hz 3 min of 20-25 H 3 min of 15-20 Hz 3 min of 10-15 Hz |
3 | 60 | 8 | Yes |
| 4 | El Shamay et al[21] | Whole Body Vibration | 0,3 g 25 Hz 1,7 mm |
5 min of warming up 10 min of WBV |
5 | 10 | 24 | Yes |
| 5 | Ruck et al[22] | Whole Body Vibration With shoes | 12-18 Hz | 3 minutes of WBV 3 minutes rest 3 minutes of WBV 3 minutes rest 3 minutes of WBV |
5 | 18 | 24 | Yes |
| 6 | Wren et al[23] | Whole Body Vibration | 30 Hz | 10 minutes of WBV | 7 | 10 | 24 | No |
WBV= Whole Body Vibration.
Gait speed
Two studies[20,22] assessed gait speed as an outcome. A significant improvement in gait speed of 0.13 m/s (95% CI: 0.05, 0.2, N=46) was found for participants in the whole-body vibration group compared with control group (Figure 2).
Figure 2.
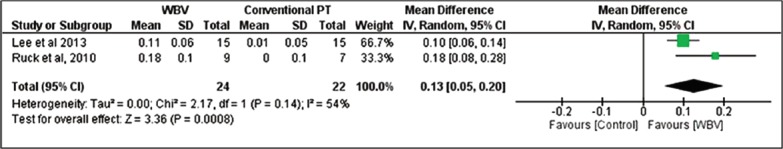
WBV versus Control: Gait Speed. Review Manager (RevMan). Version 5.2 The Cochrane Collaboration, 2013.
Muscle strength
Three studies[18,19,23] assessed muscle strength as an outcome. Due to the difference between the instruments used in the measurement of muscle strength, a meta-analysis using standardized mean difference was performed. A nonsignificant improvement in muscle strength of 2.59 (95% CI: -0.08, 5.26, N=90) was found for participants in the whole-body vibration group compared with control group (Figure 3).
Figure 3.
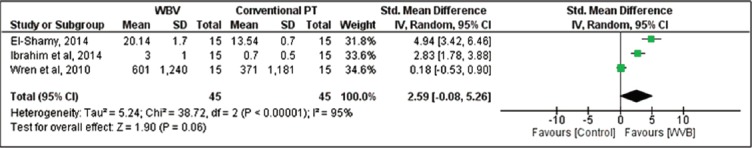
WBV versus control: Muscle Strength. Review Manager (RevMan). Version 5.2 The Cochrane Collaboration, 2013.
Gross motor function
Two studies[18,22] assessed gross motor function D and gross motor function E as outcomes. A nonsignificant improvement in gross motor function D of 6.34 (95% CI: -1.37, 14.06, N=46) was found for participants in the whole-body vibration group compared with control group. Considering gross motor function E, a significant improvement of 2.97 (95% CI: 0.07, 5.86, N=46) was found for participants in the whole-body vibration group compared with control group (Figure 4).
Figure 4.

WBV versus control: (A) GMFM D and (B) GMFM E. Review Manager (RevMan). Version 5.2 The Cochrane Collaboration, 2013.
Bone density
Due to the difference between the instruments used in the measurement of bone density, it was performed a meta-analysis with standardized mean difference. Three studies[21-23] assessed lumbar spine bone density as an outcome. A nonsignificant improvement in lumbar spine bone density of 0.41 (95% CI: -0.42, 1.25, N=77) was found for participants in the whole-body vibration group compared with control group. Two studies[21,22] assessed femur bone density as an outcome. A significant improvement in femur bone density of 1.32 (95% CI: 0.28, 2.36, N=47) was found for participants in the whole-body vibration group compared with control group (Figure 5).
Figure 5.
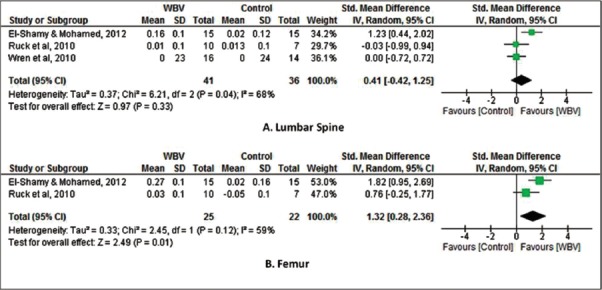
WBV versus control: (A) Lumbar Spine bone density and (B) Femur bone density. Review Manager (RevMan). Version 5.2 The Cochrane Collaboration, 2013.
Discussion
In the present systematic review, a meta-analysis of four studies demonstrated a significant difference in gait speed, Gross motor function and femur bone density in cerebral palsy children submitted to whole-body vibration. Moreover, whole-body vibration did not show improvements in muscle strength and lumbar spine bone density.
Whole-body vibration is a potential tool in the rehabilitation of elderly and patients with chronic diseases[9,10,24]. However, no systematic review has been performed concerning whole-body vibration in children with cerebral palsy. This systematic review is important because it analyzes the whole-body vibration as a potential co-adjuvant modality in rehabilitation.
In clinical practice, interventions are rarely applied in isolation and are commonly combined with other therapies for maximum therapeutic benefit. Moreover, the eligibility of gait speed and gross motor function as outcomes in this meta-analysis is important because are complementary measurements in the functional assessment of children with cerebral palsy[25]. Gait speed and gross motor function are related to daily-life mobility[26] and improving the ability to walk is often the essential therapeutic goal for such children[27].
Considering gait speed, our meta-analysis showed an improvement of 0.14 m/s (34%) in the whole-body vibration group. We found no studies that estimated the minimum detectable change in children with cerebral palsy. However, Lam et al[28], estimate the minimum detectable change in patients with spinal cord injury of 0.13 m/s and 0.1 m/s for patients with stroke[29].
The assessment of gross motor function is essential in a cerebral palsy rehabilitation program. The gross motor function for dimension (D), which is related to the standing ability increased in whole-body vibration groups, but showed no significant difference when compared to conventional physical therapy. For dimension (E), which is related to the walking, running and jumping, the results were similar to the gross motor function for dimension (D).
Considering the bone density a significant improvement in femur bone density was found in the whole-body vibration group. The mechanisms involved in this improvement might be related to a greater muscle and bone blood circulation and the offer of nutrients[30,31]. In spite of the positive results for increasing bone density, our meta-analysis found no significant effect in relation to muscle strength. Our results differ from previous systematic reviews[32,33] in aging adults who have identified increased muscle strength after whole-body vibration training.
Overall, whole-body vibration seems to be well tolerated among children with cerebral palsy, although the incidence of long-term hazards requires more research.
It is difficult to make a pragmatic recommendation about whole-body vibration in children with cerebral palsy. Our search strategy found six studies and they used different protocols of whole-body vibration. Different variables must be influencing the effects of the whole-body vibration, such as frequency, intensity and volume. The study by El-Shamy et al[19], for example, used a frequency of the 12- 18 Hz for 18 minutes and the study by Wren et al[23] used a frequency of the 30 Hz for 10 minutes.
Caution is warranted when interpreting the present results given the small amount of studies and the significant heterogeneity in the primary analyses. Further research is required to investigate how to sustain positive effects of WBV over time and to determine essential attributes of whole-body vibration training (mode, rhythm, intensity, frequency and duration).
Considering the available data, our meta-analysis showed that the whole-body vibration should be considered as an alternative method in addition to conventional physical therapy in children with cerebral palsy. Well controlled RCTs are needed to a clear understand of the effects of whole-body vibration in rehabilitation.
Footnotes
Edited by: F. Rauch
References
- 1.Richards CL, Malouin F. Cerebral palsy: definition, assessment and rehabilitation. Handb Clin Neurol. 2013;111:183–95. doi: 10.1016/B978-0-444-52891-9.00018-X. [DOI] [PubMed] [Google Scholar]
- 2.Franki I, Desloovere K, De Cat J, Feys H, Molenaers G, Calders P. The evidence-base for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the International Classification of Functioning, Disability and Health as a conceptual framework. J Rehabil Med. 2012;44:385–95. doi: 10.2340/16501977-0983. [DOI] [PubMed] [Google Scholar]
- 3.Damiano DL, Alter KE, Chambers H. New Clinical and Research Trends in Lower Extremity Management for Ambulatory Children with Cerebral Palsy. Phys Med Rehabil Clin N Am. 2009;20:469–491. doi: 10.1016/j.pmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickin DC, Faust KA, Wang H, Frame J. The acute effects of whole-body vibration on gait parameters in adults with cerebral palsy. J Musculoskelet Neuronal Interact. 2013;13:19–26. [PubMed] [Google Scholar]
- 5.Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006;38(5):302–8. doi: 10.1080/16501970600680262. [DOI] [PubMed] [Google Scholar]
- 6.Stark C, Nikopoulou-Smyrni P, Stabrey A, Semler O, Schoenau E. Effect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsy. J Musculoskelet Neuronal Interact. 2010;10:151–8. [PubMed] [Google Scholar]
- 7.Semler O, Fricke O, Vezyroglou K, Stark C, Schoenau E. Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. J Musculoskelet Neuronal Interact. 2007;7(1):77–81. [PubMed] [Google Scholar]
- 8.Cardinale M, Wakeling J. Whole body vibration exercise: are vibrations good for you? Br J Sports Med. 2005;39:585–9. doi: 10.1136/bjsm.2005.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehn B, Lidström J, Skoglund J, Lindström B. Effects on leg muscular performance from whole-body vibration exercise: a systematic review. Scand J Med Sci Sports. 2007;17:2–11. doi: 10.1111/j.1600-0838.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharififar S, Coronado RA, Romero S, Azari H, Thigpen M. The effects of whole body vibration on mobility and balance in Parkinson disease: a systematic review. Iran J Med Sci. 2014;39:318–26. [PMC free article] [PubMed] [Google Scholar]
- 11.Chanou K, Gerodimos V, Karatrantou K, Jamurtas A. Whole-body vibration and rehabilitation of chronic diseases: a review of the literature. J Sports Sci Med. 2012;11:187–200. [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S Wiley & Sons. The Cochrane Library. Issue 4. Chichester: John. Cochrane handbook for Systematic Reviews of Interventions 4.2.6; 2006. [update September 2006] [Google Scholar]
- 14.Olivo SA, Macedo LG, Gadotti IN, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–75. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 15.Verhagen AP, de Vet HCW, de Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The Delphi List: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi Consensus. J Clin Epidemiol. 1998;51:1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 16.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating of quality randomized controlled trials. Phys Ther. 2003;83:713–21. [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaboration TC. [Accessed 3 Feb 2008]; Available at: www.cochrane.org . [Google Scholar]
- 19.Ibrahim MM, Eid MA, Moawd SA. Effect of whole-body vibration on muscle strength, spasticity, and motor performance in spastic diplegic cerebral palsy children. Egyptian Journal of Medical Human Genetics. 2014;15:173–179. [Google Scholar]
- 20.El-Shamy SM. Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93:114–21. doi: 10.1097/PHM.0b013e3182a541a4. [DOI] [PubMed] [Google Scholar]
- 21.Lee BK, Chon SC. Effect of whole body vibration training on mobility in children with cerebral palsy: a randomized controlled experimenter-blinded study. Clin Rehabil. 2013;27:599–607. doi: 10.1177/0269215512470673. [DOI] [PubMed] [Google Scholar]
- 22.El-Shamy SM, Mohamed MSE. Effect of whole body vibration training on bone mineral density in cerebral palsy children. Indian J Phys Occup Ther. 2012;6:139–141. [Google Scholar]
- 23.Ruck J, Chabot G, Rauch F. Vibration treatment in cerebral palsy: A randomized controlled pilot study. J Musculoskelet Neuronal Interact. 2010;10:77–83. [PubMed] [Google Scholar]
- 24.Wren TAL, Lee DC, Hara MA R, Rethlefsen SA, Kay RM, Dorey FJ. Effect of High-frequency, Low-magnitude Vibration on Bone and Muscle in Children With Cerebral Palsy. J Pediatr Orthop. 2010;30:732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogan S, Hilfiker R, Herren K, Radlinger L, de Bruin ED. Effects of whole-body vibration on postural control in elderly: a systematic review and meta-analysis. BMC Geriatr. 2011;11:1–18. doi: 10.1186/1471-2318-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damiano DL, Abel MF. Relation of gait analysis to gross motor function in cerebral palsy. Dev Med Child Neurol. 1996;38:389–96. doi: 10.1111/j.1469-8749.1996.tb15097.x. [DOI] [PubMed] [Google Scholar]
- 27.Ketelaar M, Van Schie PE, Dallmeijer AJ, Lindeman E, et al. Relationship between gross motor capacity and daily-life mobility in children with cerebral palsy. Dev Med Child Neurol. 2010;52:e60–6. doi: 10.1111/j.1469-8749.2009.03525.x. [DOI] [PubMed] [Google Scholar]
- 28.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–1631. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 29.Lam T, Noonan V, Eng JJ. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2007;46:246–254. doi: 10.1038/sj.sc.3102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera S, Mody S, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 31.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 32.Kerschan-Schindl K, Grampp S, Henk C, et al. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol. 2001;21:377–82. doi: 10.1046/j.1365-2281.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 33.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25:975–88. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 34.Sitjà-Rabert M, Rigau D, Fort Vanmeerghaeghe A, Romero-Rodríguez D, Bonastre Subirana M, Bonfill X. Efficacy of whole body vibration exercise in older people: a systematic review. Disabil Rehabil. 2012;34:883–93. doi: 10.3109/09638288.2011.626486. [DOI] [PubMed] [Google Scholar]


