Abstract
Objectives:
To investigate the analgesic effect of nasal salmon calcitonin on the post-fracture period of distal radius fracture.
Methods:
In this prospective randomized double-blind study, forty-one postmenopausal women with a recent distal radius fracture treated conservatively were randomly assigned to receive either 200 IU of intranasal salmon calcitonin or placebo daily for 3 months following fracture. The assessment of the patient’s pain was recorded using the Visual Analogue Scale (VAS).
Results:
The average age of the calcitonin group was 67.11 (SD, ±8.68) years and 64.91 (SD, ±7.48) of the placebo group. In the calcitonin group, the mean VAS score improved from 4.05 to 0.53 while in the placebo group from 3.36 to 0.32. A higher decrease of VAS score during the first post-fracture period was observed in the calcitonin group.
Conclusions:
In the study, there is a statistically significant calcitonin mediated analgesic effect in the immediate post fracture period (at 10 days) when compared to placebo group. These results are in accordance with literature referring to the analgesic effect of calcitonin in the acute osteoporotic vertebral compression fracture. Thus calcitonin administration could be recommended to a short term course in acute osteoporotic conservatively treated distal radius fractures.
Keywords: Analgesia, Distal Radius Fracture, Nasal Calcitonin, Pain, Post-Fracture Period, Wrist Fracture
Introduction
Fractures of the distal radius are commonly encountered in the upper extremity particularly in the elderly and constitute a major public health concern[1]. Wrist fractures are associated with pain as with all fractures.
While immobilization reduce pain, local soft tissue swelling exaggerates aching, decreases patient satisfaction, and results in poor clinical outcome due to delayed rehabilitation[2,3]. Analgesic medications are usually prescribed in these patients, aiming at the relief of the symptoms and a better quality of life.
Although that salmon calcitonin has been widely investigated and used for the treatment of osteoporosis, there are limited clinical studies investigating the orthopaedic indications of nasal calcitonin in post-fracture[4,5] disease that leads to disability due to pain, resulting to a poorer quality of life. The purpose of the study is to investigate the effect of nasal salmon calcitonin in post-fracture pain in women with unilateral distal radius fracture.
Materials and methods
In this double-blind randomized prospective study, forty-one postmenopausal women aged 50 or above were included. These women were suffering from a fresh unilateral extra-articular distal radius fracture without ulnar styloid fracture based on AO classification with an indication of conservative treatment.
All patients were treated with close reduction and cast immobilization for a period of 35-45 days and followed-up for a period of 6 months after the fracture. The patients were randomly assigned to two groups. The first group consisted of 19 women received 200 IU of intranasal salmon calcitonin (Miacalcic®, Novartis Pharma AG, Basel, Switzerland) on a daily basis for 3 months whereas the second group composed of 22 women received intranasal daily dose of placebo for the same period of time. Additionally, all subjects received 1000 mg calcium carbonate per day. All subjects did not receive any analgesic or pain killer. All cases started treatment within the first 3 post-fracture days.
Approval by the Ιnstitutional Review Board (IRB) and written informed consent, as well as any necessary Health Insurance Portability and Accountability Act (HIPAA) consent, was obtained from each patient. Patients with known metabolic bone disease excluding osteoporosis, pathologic fracture in the examined body area or elsewhere, bilateral forearm fracture, poly-trauma, multiple-fracture patients, traffic-accident patients, unilateral distal forearm fracture with the indication of surgical treatment and re-fracture of the examined body area and any surgical procedure in the region of the studied extremity during the course of the study were excluded from the study.
The assessment of pain at the site of fracture was recorded using the Visual Analogue Scale (VAS)[6], which rates pain with the use of a 10 cm long horizontal line, with 0 rated as “no pain” and 10 as “very severe pain”. Pain severity was rated initially, on 10th day, the day that the cast was removed (35th-45th day), 90th and 180th.
Statistical analysis
Data is expressed as mean±standard deviation (S.D.) or median (in case of violation of normality) for continuous variables and as percentages for categorical data. The Kolmogorov-Smirnov test was utilized for normality analysis of the parameters.
The comparison of variables at each time point was performed using the Independent samples t-test or the Mann-Whitney test in case of violation of normality.
To indicate the trend in the first 180 min of intervention, the mean percentage changes after 45, 90 and 180 days respectively are calculated. Comparison of percentage change from baseline of parameters during the observation period between two groups was analyzed using the Mann-Whitney test because of violation of normality.
All tests are two-sided, statistical significance was set at p <0.05. All analyses were carried out using the statistical package SPSS vr 16.00 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Ill., USA).
Results
The main characteristics of the entire study population are shown in Table 1. The mean population age was 67.1 years (SD, 8.68 years) for calcitonin group and 64.9 years (SD, 7.48) for the placebo group. Nineteen patients received calcitonin and twenty-two patients had placebo therapy. Visual Analogue Scale (VAS) Score was assessed at study entry, 10th, 45th, 90th, 180th day. The follow-up period was 180 days for all participants. There is no statistical difference between the two groups in demographics (Table 1). Also, there is homogeneity between the two groups regarding demographic characteristics.
Table 1.
Demographic characteristics of study sample.
| N | Mean | SD | p-value | ||
|---|---|---|---|---|---|
| Age | Calcitonin | 19 | 67.11 | 8.68 | 0.389 |
| Placebo | 22 | 64.91 | 7.48 | ||
| Weight | Calcitonin | 19 | 69.72 | 7.33 | 0.613 |
| Placebo | 22 | 71.25 | 10.83 | ||
| Height | Calcitonin | 19 | 1.53 | 0.06 | 0.226 |
| Placebo | 22 | 1.55 | 0.06 | ||
| BMI | Calcitonin | 19 | 29.91 | 3.31 | 0.845 |
| Placebo | 22 | 29.67 | 4.35 | ||
The VAS Scores between the two groups at all time intervals are shown in Table 2.
Table 2.
VAS scores of all subjects at all time intervals.
| N | Mean | SD | p-value | ||
|---|---|---|---|---|---|
| VAS initial Score | Calcitonin | 19 | 4.05 | 2.37 | 0.371 |
| Placebo | 22 | 3.36 | 2.48 | ||
| VAS 10 days | Calcitonin | 19 | 2.50 | 1.86 | 0.826 |
| Placebo | 22 | 2.36 | 2.01 | ||
| VAS 45 days | Calcitonin | 19 | 2.16 | 1.38 | 0.079 |
| Placebo | 22 | 1.36 | 1.43 | ||
| VAS 90 days | Calcitonin | 19 | 1.16 | 1.12 | 0.605 |
| Placebo | 22 | 1.38 | 1.56 | ||
| VAS 180 days | Calcitonin | 19 | 0.53 | 0.90 | 0.376 |
| Placebo | 22 | 0.32 | 0.57 | ||
There is no statistical difference between the two groups at study entry, 10th, 90th, 180th day with VAS evaluation. The VAS Score variance between two groups of specific time intervals is shown in Table 3. Our findings demonstrate a higher decrease of pain during the first post-fracture period (10 days) between calcitonin and placebo group (Calcitonin: -50% vs Placebo: -16.7% p=0.028) (Figure 1).
Table 3.
VAS scores between groups and specific time intervals. A significant decrease of pain is observed during the first post-fracture period in the calcitonin group.
| % change | N | Median | p-value | |
|---|---|---|---|---|
| VAS Initial - 10 days | Calcitonin | 19 | -50.0 | 0.028 |
| Placebo | 22 | -16.7 | ||
| VAS Initial - 45 days | Calcitonin | 19 | -50.0 | 0.707 |
| Placebo | 22 | -50.0 | ||
| VAS Initial - 90 days | Calcitonin | 19 | -80.4 | 0.762 |
| Placebo | 22 | -75.0 | ||
| VAS Initial - 180 days | Calcitonin | 19 | -100.0 | 0.922 |
| Placebo | 22 | -100.0 | ||
Figure 1.
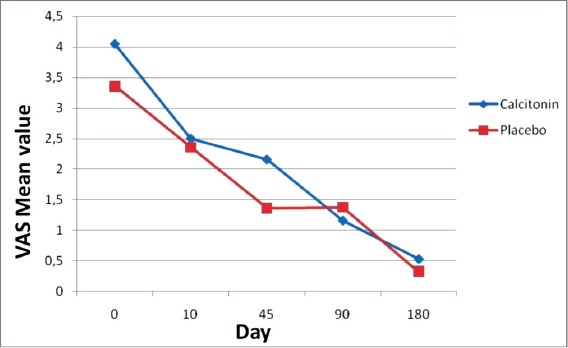
VAS Scores over time (VAS Initial – 10 Days: Calcitonin: -50% vs Placebo: -16.7% p=0.028).
Looking at the remaining time intervals and the percentage change of pain, no statistical difference is observed (Table 3).
Discussion
According to our results, the analgesic effect of calcitonin starts earlier than 10 days post-fracture and is maintained until 45 days have elapsed. The maximum analgesic effect in the treatment group takes place at 10 days post fracture (50% decrease in the VAS system in pain in the calcitonin group, compared to 16.7% in the placebo group) (Table 3, Figure 1).
Apart from acute osteoporotic vertebral compression fractures, calcitonin has been used as an analgesic in the short term treatment of acute traumatic coccigeal fractures[4], rib fractures[5], post total hip arthroplasty (for hip fractures)[7] and for the treatment of pain from bone metastases[8]. The proposed mechanism of action is calcitonin inducing an increase of the level of endorphins in the central nervous system. Though its prolonged use might lead to a decrease of therapeutic effect, due to formation of antibodies targeted against it[4].
In a review and meta-analysis by Knopp-Sihota et al. in 2012[9] concerning analgesia from calcitonin in acute and chronic osteoporotic vertebral compression fractures, calcitonin significantly reduces the severity of acute pain in recent fractures, while having no effect on chronic ones. Pain at rest was reduced by the 1st week, though improvement continued by 4 weeks post fracture. Maximum analgesia was achieved at 4 weeks.
Our results are in accordance with those by Knopp-Sihota et al.[9] and other papers[10-17] dealing with the same subject. Only that, in distal radius fracture pain treated with calcitonin, the maximum analgesic effect is achieved much earlier, in the 10th day post fracture. This leads to the hypothesis that an even shorter course of calcitonin might be effective in such fractures.
Calcitonin is a potent osteoclast inhibitor through receptors, and has been used in the treatment of established post-menopausal osteoporosis. Nevertheless high dose and long term use might increase the risk for liver cancer and decrease the risk for breast cancer in females being treated for post-menopausal osteoporosis[18]. This concern, has led the committee for medicinal products for human use (CHMP) of the European medicine agency (EMA), to restrict the indications for calcitonin use and recommend only its short term use[19].
Thus benefits from using a short course of calcitonin in the treatment of distal radius fractures include adequate and timely pain relief, as well as earlier functional rehabilitation of the wrist joint. These specific effects of calcitonin have not been previously presented in medical literature.
Limitations to our study include the small sample size which nonetheless led to a homogenous patient sample with all the advantages that this could carry. Further studies are required to establish the optimal, minimal duration of calcitonin treatment in osteoporotic low energy distal radius fractures.
Conclusion
In the study, there is a statistically significant calcitonin mediated analgesic effect in the immediate post fracture period (at 10 days) when compared to placebo group. These results are in accordance with literature referring to the analgesic effect of calcitonin in the acute osteoporotic vertebral compression fracture. Thus calcitonin administration could be recommended to a short term course in acute osteoporotic conservatively treated distal radius fractures.
Acknowledgements
This study was supported by Novartis Pharma AG, Basel, Switzerland.
Footnotes
Edited by: G.P. Lyritis
References
- 1.Chung KC, Shauver MJ, Birkmeyer J.D. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg. 2009;91A:1868–1873. doi: 10.2106/JBJS.H.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slutsky DJ, Herman M. Rehabilitation of distal radius fractures: a biomechanical guide. Hand Clin. 2005;21:455–468. doi: 10.1016/j.hcl.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cheing GL, Wan JW, Lo S. Ice and pulsed electromagnetic field to reduce pain and swelling after distal radius fractures. J Rehabil Med. 2005;37:372–377. doi: 10.1080/16501970510041055. [DOI] [PubMed] [Google Scholar]
- 4.Patrick MF. Coccyx fractures treated with intranasal calcitonin. Pain physician. 2014;17:E229–E233. [PubMed] [Google Scholar]
- 5.Jones AM, Dodd ME, Webb AK, Selby PL. Acute rib fracture pain in CF. Thorax. 2001;56(10):819. doi: 10.1136/thorax.56.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 7.Peichl P, Marteau R, Griesmacher A, Kumpan W, Schedl R, Prosquil E, Fasol P, Bröll H. Salmon calcitonin nasal spray treatment for postmenopausal women after hip fracture with total hip arthroplasty. J Bone Miner Metab. 2005;23(3):243–252. doi: 10.1007/s00774-004-0591-2. [DOI] [PubMed] [Google Scholar]
- 8.Lussiera D, Huskeyb AG, Portenoy RK. Adjuvant Analgesics in Cancer Pain Management. The Oncologist. 2004;9(5):571–591. doi: 10.1634/theoncologist.9-5-571. [DOI] [PubMed] [Google Scholar]
- 9.Knopp-Sihota JA, Newburn-Cook CV, Homik J, Cummings GG, Voaklander D. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Osteoporos Int. 2012;23(1):17–38. doi: 10.1007/s00198-011-1676-0. [DOI] [PubMed] [Google Scholar]
- 10.Knopp JA, Diner BM, Blitz M, Lyritis GP, Rowe BH. Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int. 2005;16(10):1281–1290. doi: 10.1007/s00198-004-1798-8. [DOI] [PubMed] [Google Scholar]
- 11.Mehta NM, Malootian A, Gilligan JP. Calcitonin for osteoporosis and bone pain. Curr Pharm Des. 2003;9(32):2659–2676. doi: 10.2174/1381612033453622. [DOI] [PubMed] [Google Scholar]
- 12.Blau LA, Hoehns JD. Analgesic efficacy of calcitonin for vertebral fracture pain. Ann Pharmacother. 2003;37(4):564–570. doi: 10.1345/aph.1C350. [DOI] [PubMed] [Google Scholar]
- 13.Silverman SL, Azria M. The analgesic role of calcitonin following osteoporotic fracture. Osteoporos Int. 2002;13(11):858–867. doi: 10.1007/s001980200118. [DOI] [PubMed] [Google Scholar]
- 14.Lyritis GP, Ioannidis GV, Karachalios T, Roidis N, Kataxaki E, Papaioannou N, et al. Analgesic effect of salmon calcitonin suppositories in patients with acute pain due to recent osteoporotic vertebral crush fractures: a prospective double-blind, randomized, placebo-controlled clinical study. Clin J Pain. 1999;15(4):284–289. doi: 10.1097/00002508-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand. 1997;275:112–114. doi: 10.1080/17453674.1997.11744761. [DOI] [PubMed] [Google Scholar]
- 16.Von Feldt JM. Managing osteoporotic fractures minimizing pain and disability. J Clin Rheumatol. 1997;3(Suppl 2):65–68. doi: 10.1097/00124743-199704001-00015. [DOI] [PubMed] [Google Scholar]
- 17.Lyritis GP, Mayasis B, Tsakalakos N, Lambropoulos A, Gazi S, Karachalios T, et al. The natural history of the osteoporotic vertebral fracture. Clin Rheumatol. 1989;8(Suppl 2):66–69. doi: 10.1007/BF02207237. [DOI] [PubMed] [Google Scholar]
- 18.Sun LM, Lin MC, Muo CH, Liang JA, Kao CH. Calcitonin nasal spray and increased cancer risk: a population-based nested case-control study. J Clin Endocrinol Metab. 2014;99(11):4259–64. doi: 10.1210/jc.2014-2239. [DOI] [PubMed] [Google Scholar]
- 19.European medicines agency. European medicines agency recommends limiting long term use of calcitonin medicines. 2012 [Google Scholar]


