Abstract
Objectives:
The plantaris tendon is increasingly recognised as an important factor in midportion Achilles tendinopathy. Its innervation pattern is completely unknown.
Methods:
Plantaris tendons (n=56) and associated peritendinous tissue from 46 patients with midportion Achilles tendinopathy and where the plantaris tendon was closely related to the Achilles tendon were evaluated. Morphological evaluations and stainings for nerve markers [general (PGP9.5), sensory (CGRP), sympathetic (TH)], glutamate NMDA receptor and Schwann cells (S-100β) were made.
Results:
A marked innervation, as evidenced by evaluation for PGP9.5 reactions, occurred in the peritendinous tissue located between the plantaris and Achilles tendons. It contained sensory and to some extent sympathetic and NMDAR1-positive axons. There was also an innervation in the zones of connective tissue within the plantaris tendons. Interestingly, some of the nerve fascicles showed a partial lack of axonal reactions.
Conclusion:
New information on the innervation patterns for the plantaris tendon in situations with midportion Achilles tendinopathy has here been obtained. The peritendinous tissue was found to be markedly innervated and there was also innervation within the plantaris tendon. Furthermore, axonal degeneration is likely to occur. Both features should be further taken into account when considering the relationship between the nervous system and tendinopathy.
Keywords: Plantaris Tendon, Achilles Tendinopathy, Pain, Innervation, Peritendinous Connective Tissue
Introduction
Midportion Achilles tendinopathy is known as a troublesome painful condition in the Achilles tendon midportion, characterised by local swelling, tenderness and disability[1]. It accounts for the majority of Achilles tendon disorders[2]. There are now promising treatments for this tendinopathy[3] but all underlaying pain mechanisms have not been clarified[4].
Immunohistochemical studies on the innervation patterns in chronic painful midportion Achilles tendinopathy tendons have shown that the tendon proper is poorly innervated showing no, or only minor, differences in the innervation compared with a healthy Achilles tendon[5,6]. The vast majority of the nerve structures are located outside the tendon, especially in the ventral loose connective tissue, here often being accompanied by blood vessels[6]. Treatment approaches targeting this ventral peritendinous tissue have shown good outcome indicating the importance of this tissue for tendon pain[7,8].
Recently, there has been an increased interest around a possible involvement of the plantaris tendon in midportion Achilles tendinopathy, and a thick plantaris tendon seemingly interfering with the medial side of the thickened Achilles midportion has frequently been found during surgical treatment[9]. The plantaris tendon has also been found to be affixed to the medial side in Achilles tendinopathy, as seen in an anatomical study[10]. The results of release and excision of the plantaris tendon for patients with midportion Achilles tendinopathy have been found to be good[8,9,11,12]. Treatment with plantaris tendon excision, together with the traditional scraping of the ventral Achilles, has also shown very good clinical results[8,9]. Morphological studies have observed tendinosis-like tissue changes in the plantaris tendons for patients with midportion Achilles tendinopathy where the plantaris tendons were excised (n=17)[13]. Thus, there is evidence of a possible involvement of the plantaris tendon in a subgroup of patients with this condition. The operations including the plantaris tendon are made in the chronic phase of tendinopathy. The time duration for symptoms up to the time of operation related to scraping of the ventral tendon combined with plantaris tendon excision is traditionally at least 3 months[13]. However on the whole, the pain time duration for Achilles operations varies considerably, in some occasions being up to 120 months[14].
It is reasonable to suggest that there is an interaction in the form of compression between the Achilles and plantaris tendons, possibly leading to tissue degeneration[9,15]. This hypothesis is supported by the fact that the plantaris tendon is stronger and stiffer than the Achilles tendon[16], and that plantaris tendons lying close by to the Achilles tendons often co-occur with medial Achilles tendon tissue degeneration[17]. It cannot be excluded that the innervation of the plantaris tendon, including that of the peritendinous connective tissue attaching to the Achilles tendon, can be responsible for pain in patients with midportion Achilles tendinopathy. There is, however, no knowledge about the innervation pattern in the plantaris tendon itself and in the peritendinous connective tissue between the Achilles and plantaris tendons.
The aim with this study was to evaluate the innervation patterns of the plantaris tendon and the peritendinous connective tissue located in between the plantaris and Achilles tendons. Based on previous knowledge on innervation patterns for the Achilles tendon[5,6,18], stainings using antibodies against general nerve marker, and markers for sensory and sympathetic nerve fibres and fibres expressing glutamate NMDA receptor (NMDAR1) were performed. Information on these patterns is necessary in order to further understand the pain mechanisms in midportion Achilles tendinopathy.
Material and methods
Patients
Plantaris tendons from 46 patients (32 men - mean age 46 years; 14 women - mean age 53) suffering from chronic (duration of symptoms >3 months) painful midportion Achilles tendinopathy, were included. Clinical examination showed a tender thickening of the Achilles tendon midportion, and Ultrasound and Colour Doppler (US/CD) examination verified midportion Achilles tendinopathy. Before surgery the mean VAS (evaluating pain during activity related Achilles tendon loading, 0 referring to no pain and 100 to worst pain) was 67.5 (SD 18.6), and the mean VISA-A score (0 referring to optimal function and 100 to worst function) was 42.2 (SD 16.1). All patients had failed conservative treatment (eccentric training).
All patients underwent a surgical procedure including plantaris tendon removal and scraping of the ventral Achilles tendon (see below). For 10 patients that had tendinopathy bilaterally, the plantaris tendons from both sides were examined. Thus, in total, 56 tendons were evaluated and examined (see below). Patients were recruited within a time frame of three years.
Exclusion criteria were acute and chronic inflammatory disease, previous intratendinous treatments and partial tears in the Achilles tendon.
Surgical procedure: Plantaris excision and ventral scraping
Surgery was performed by one of the authors (HA) according to a previously described procedure[7,9]. Via a short longitudinal skin incision on the medial side of the Achilles tendon midportion, the medial side of Achilles and the plantaris tendons were visualised. The plantaris tendon was removed in all patients because it was found to be located close to, and seemingly compressing, the medial side of the Achilles tendon. In some cases, the plantaris tendon was “invaginated” into the medial side of the Achilles tendon. There was always fatty richly vascularised peritendinous connective tissue in between the plantaris and Achilles tendons. The plantaris tendon was released in proximal and distal directions, and 5-6 cm of its length was excised together with attached fatty peritendinous connective tissue. For four patients, a “tissue block” being 1-2x3-4 mm in size, containing a small part of the medial side of the Achilles tendon, the plantaris tendon and the entire fatty peritendinous connective tissue in between the two tendons, was taken. In this way, the entire peritendinous tissue between the Achilles and plantaris tendon could be evaluated.
The study was approved by the Regional Ethical Board in Umeå (dnr 04-157M; 2011-83-32M). The experiments were conducted according to the principles expressed in the Declaration of Helsinki. All patients signed an informed consent.
Sampling, fixation and sectioning
Directly after the surgical procedure, the excised samples were put in fixative solution containing 4% formaldehyde in 0.1 M phosphate buffer (pH 7.0) and were then incubated overnight at 4°C. Thereafter the samples were washed for three times (including one wash overnight) at 4°C in Tyrode’s solution containing 10% sucrose (pH 7.2).
With a knife, each plantaris tendon was divided into at least three parts (middle and the two end parts) before being mounted on a thin cardboard in OCT embedding medium (TissueTek, Miles Laboratories, Naperville, IL, USA). Then, the specimens were frozen in propane chilled liquid nitrogen and stored at -80°C until use.
The middle part and at least one of the end parts (2-5 specimens per tendon sample) were finally cryosectioned with a thickness of 7 μm (Leica Microsystem CM 300, Heidelberg, Germany) and mounted on superfrost plus slides (Thermo Scientific, Braunschweig, Germany). In total, 137 tendon specimens from the 56 tendons evaluated in the study were further used for morphological and immunohistochemical analyses.
Morphological analysis (H&E staining)
In order to visualise the morphology, the sections were stained with haematoxylin and eosin (H&E) according to previously described routine process[19].
Immunohistochemistry
Antibodies
An antibody against protein gene product 9.5 (PGP 9.5), a well-studied marker for all types of nerve fibres in peripheral tissues, was used. In order to further characterize the nerve axons, further stainings against tyrosine hydroxylase (TH) showing sympathetic nerve fibres and, against calcitonin related gene peptide (CGRP) indicating sensory nerve fibres, were performed in a subset of samples. Schwann cells were detected in a subset of slides using an antibody against S-100β. The glutamate system was analysed via stainings against the Vesicular Glutamate Transporter 2 (VGluT2) and the ionotrophic N-Methyl-D-Aspartate receptor (NMDAR1). Mast cells were detected via an antibody against mast cell tryptase. More information about the antibodies is stated in Table 1.
Table 1.
Antibodies used in the present study.
| Antigen | Host | Code | Source | Dilution |
|---|---|---|---|---|
| CGRP | Goat pAb | sc-8856 | Santa Cruz (Dallas, TX, USA) | 1:100 |
| CGRP | Rabbit pAb | ab47027 | abcam (Cambridge, UK) | 1:1000 |
| Mast cell tryptase | Mouse mAb | ab2378 | abcam (Cambridge, UK) | 1:500 |
| NMDAR1 | Goat pAb | sc-1467 | Santa Cruz (Dallas, TX, USA) | 1:500 |
| PGP9.5 | Rabbit pAb | 7863-0504 | Serotec (Kidlington, UK) | 1:500 |
| S-100β | Mouse mAb | S2657 | Sigma Aldrich (St.Louis, MS, USA) | 1:1000 |
| TH | Rabbit pAb | P40101 | Pel-Freez Arkansas (Rogers, AR, USA) | 1:1000 |
| VGluT2 | Goat pAb | sc-26026 | Santa Cruz (Dallas, TX, USA) | 1:500 |
pAb= polycloncal, mAb = monoclonal.
Staining procedure
Initially the sections were pre-incubated in potassium permanganate for 2 min to enhance visualisation of specific immunofluorescence reaction sites. When staining for S-100β this step was not performed. After three washes for 5min each in 0.01 M phosphate-buffered saline (PBS, pH7.4), containing 0.1% sodium azide as preservative, the slides were kept in 1% Triton X-100 in 0.01 M PBS (pH7.4) for 20 min followed by another washing step. After that, sections were treated with 5% normal serum for 15 min and then incubated with the primary antibody (4°C, overnight). On the next day, the slides were washed and again treated with 5% normal serum followed by incubation with the secondary antibody (37°C, 30 min). Finally, the samples were washed again and mounted with Vectashield mounting medium (H-1000). Microscopical evaluation was performed using a Zeiss Axioscope 2 plus microscope equipped with epifluorescent technique and an Olympus DP70 digital camera.
Normal serum and primary and secondary antibodies were diluted in 0.1% bovine serum albumin (BSA) in 0.01 M PBS (pH 7.4). However, when using goat antibodies all dilutions were made without BSA.
Donkey normal serum (code no. 017-000-121, Jackson Immune Research Laboratories Inc., West Grove, PA, USA) and FITC-conjugated donkey anti-goat secondary antibody (code no. 705-095-147, Jackson Immune Research Inc.) were used for the staining procedures for all goat primary antibodies. Rabbit normal serum (code no. X0902, DAKO Cytomation, Glostrup, Denmark) and TRITC-conjugated rabbit anti-mouse (R0276, DAKO Cytomation) was applied for the stainings using mouse primary antibodies. When using rabbit primary antibodies, swine normal serum (code no. 014-000-121, Jackson Immune Research Inc.) and TRITC-conjugated swine anti-rabbit (code no. R0156, DAKO) was used.
Control stainings
For all antibodies control stainings using PBS instead of primary antibody were performed. Additionally, preabsorption of the antibody with the corresponding antigen (20 μg/ml) at 4°C overnight was performed for NMDAR1 (antigen: sc-1467-P, Santa Cruz Biotechnology), VGluT2 (antigen: sc-26026-P, Santa Cruz Biotechnology) and CGRP (antigen: sc-8856-P, Santa Cruz Biotechnology) respectively.
The specificity of the TH antiserum has been previously evaluated[6,20]. The PGP9.5 antibody is well established as general nerve marker and has been frequently used for tendon tissue[5,6,21]. The mast cell tryptase antibody has been frequently used for identification of mast cells[22].
Double staining
Double stainings were performed for PGP9.5 together with NMDAR1, TH, CGRP and S-100β on a subgroup of samples that showed high level of innervation based on the PGP9.5 immunoreaction pattern.
Results
Morphology
Peritendinous connective tissue
The samples contained different amounts of peritendinous connective tissue. The peritendinous connective tissue contained a large number of vessels, including large and small arterioles/venoles and fine blood vessels (Figures 1 A,B,E). In addition, the peritendinous connective tissue always contained fat tissue as evaluated from samples containing a large amount of peritendinous connective tissue (Figures 1 C-E). Furthermore, mast cells were seen in this tissue (Figure 1D). The samples (six) showing an almost normal morphology in the tendon proper (see further below) were noted to contain small parts of peritendinous connective tissue. Nevertheless, blood vessels occurred also in the peritendinous tissue in these samples.
Figure 1.
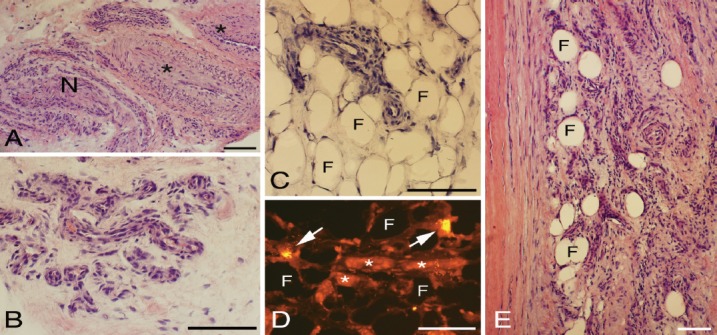
Peritendinous tissue located close to the plantaris tendon and where this tendon is facing the Achilles tendon. Staining for H&E in (A-C and E). Staining with antibody against mast cell tryptase in (D). There is a presence of large arterioles (asterisks, A) and numerous small vessels (B,E). The small vessels are partially embedded within the here occurring fat tissue (asterisks, C-E). Also in (D), there is a presence of fine vessels. Tendon tissue proper to the left in (E). Arrows in (D) point at mast cells. F= fat tissue (C-E); N= nerve fascicle (A). Bars=100 μm.
Tendon tissue proper
Morphological examinations revealed clear tendinosis-like changes in tendon samples in 50/56 tendons (40/46 patients), i.e. changes that are typical for tendinopathy samples in Achilles and patellar tendinopathy (tendinosis)[23-25]. That included hypercellularity in the tendon proper and abnormal tenocyte appearances (rounded/wavy shapes, lined up in long rows). There was a high frequency of blood vessels in the connective tissue zones of the tendon tissue proper. These zones of connective tissue which are typical constituents of tendon tissue proper, and conform to zones of endotendon were clearly observed in the H&E stainings. Specimens from different parts of the tendons exhibited the same tissue pattern. However, there was a heterogeneity in the degree of the pathology between the tendons from the various patients. The tendons from six patients (four men, two women; mean age: 45 years) showed an almost normal morphology, exhibiting few to moderate numbers of tenocytes, these in principle being slender-shaped.
General nerve pattern (PGP9.5 innervation)
Peritendinous connective tissue
In clearly evaluated peritendinous connective tissue (in samples that had a large amount of this tissue; n=70/137), PGP9.5 immunoreactions conforming to reactions in perivascular nerve fibres (Figure 2A), in blood vessel walls (Figure 2B), in isolated fine nerve fibres (Figure 2C) and in nerve fascicles (Figure 2D) were always seen. Some of the PGP9.5 immunoreactive nerves of the peritendinous tissue were located very close to the tendon tissue proper. PGP9.5 immunoreactions were also always observable in the peritendinous connective tissue of the tendons showing an almost normal morphology of tendon tissue.
Figure 2.
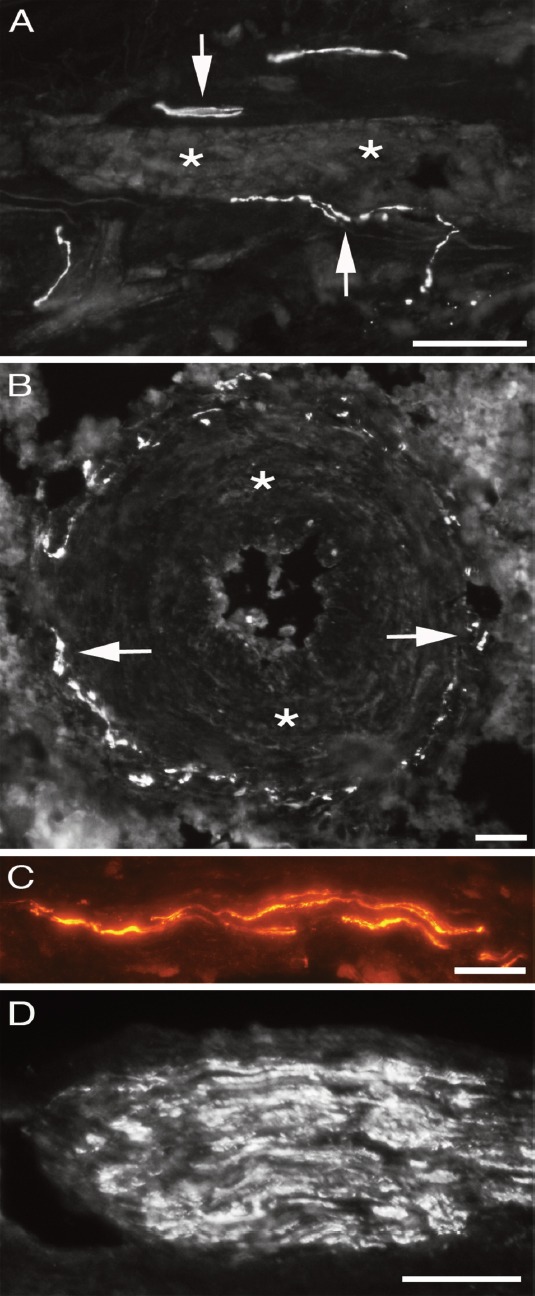
Peritendinous connective tissue in between Achilles and plantaris tendons showing PGP9.5 positive immunoreactions (arrows) in close vicinity of a longitudinally/obliqually cut blood vessel (A), in the media-adventitia junction of the wall of a large arteriole (B), in the form of fine isolated nerve fibres (C) and in a nerve fascicle (D). Asterisks in the media of the blood vessel walls. Bars=40 μm.
Tendon tissue proper
PGP9.5 positive reactions, in the form of single nerve fibres or very thin nerve fascicles, were detected in the zones of connective tissue inside the tendon tissue proper (Figures 3 A,B). This was the case in 48 out of the 137 tendon specimens examined (35%) conforming to 37/56 examined plantaris tendons (66.1%) and 32/46 patients (69.6%) (Table 2). Concerning the specimens with tendinosis-like morphology the values were 47 out of 125 (37%) tendon specimens, 36/50 (72%) tendons and 31/40 (77.5%) patients. As revealed via examination of more than one level along the tendon samples it was thus noted that the magnitude of PGP9.5-innervation was not the same in all sections examined. Accordingly, immunoreactive nerve fibres were present in one specimen level whilst that was sometimes not the case for another/others. This shows the importance of evaluating more than one sample level.
Figure 3.
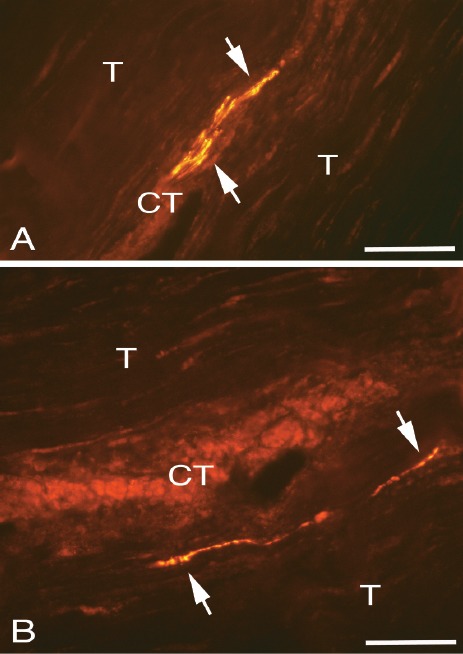
Tendon tissue proper of plantaris tendons (A,B) showing PGP9.5 positive immunoreactive fine nerve fibres (arrows) located in zones of loose connective tissue spaces (CT). Tendon tissue (T) containing tenocytes to the left above and to the right below. Bars=40 μm.
Table 2.
Ratios of occurrence of PGP9.5 positive reactions in the zones of connective tissue of the tendon tissue proper related to numbers of patients, legs and specimens.
| Nerves in internal sheet | Patients | Legs | Specimens |
|---|---|---|---|
| All patients | 32/46 (69.6%) | 37/56 (66.1%) | 48/137 (35%) |
| Tendinosis | 31/40 (77.5%) | 36/50 (72%) | 47/125 (37%) |
| group | |||
| Group with in | |||
| principle normal | 1/6 (16.7%) | 1/6 (16.7%) | 1/12 (8.3%) |
| morphology |
PGP9.5 positive immunoreactions were less frequent in the zones of connective tissue in the tendon tissue proper in the samples of the six patients showing an almost normal morphology. Thus, in only 1 out of 12 tendon specimens (from 6 tendon samples) these reactions were detected (Table 2).
Patterns of PGP9.5 immunoreactions in nerve fascicles
The majority of the nerve fascicles present in the peritendinous connective tissue showed a fairly homogenous pattern of PGP9.5 immunoreaction (Figure 2D). However, some of the nerve fascicles were found to exhibit a partial lack of immunoreactions (Figures 4 A,B). Thus, only parts of these fascicles were positively stained for PGP9.5. This observation was based on a thorough evaluation of the nerve fascicles at the various focus planes (the sections were 7μm thick). Double staining with S-100β showed on the other hand a homogenous S-100β immunoreaction, including S-100β immunoreaction for the PGP9.5 negative spaces (Figure 4C).
Figure 4.
Nerve fascicles in peritendinous connective tissue in between Achilles and plantaris tendons, stained for PGP9.5. Nerve fascicle exhibiting inhomogenous immunostaining are shown in (A) and (B) (asterisks in non-reactive parts). Double staining PGP9.5 (green)/S-100β (red) reveals that, whilst only parts of axons are PGP9.5 immunoreactive, there is an overall reaction for S-100β (C). Bars=20 μm.
Nerve characterisation
The features for tendon samples showing a tendinosis-like appearance (n=50):
Sensory innervation
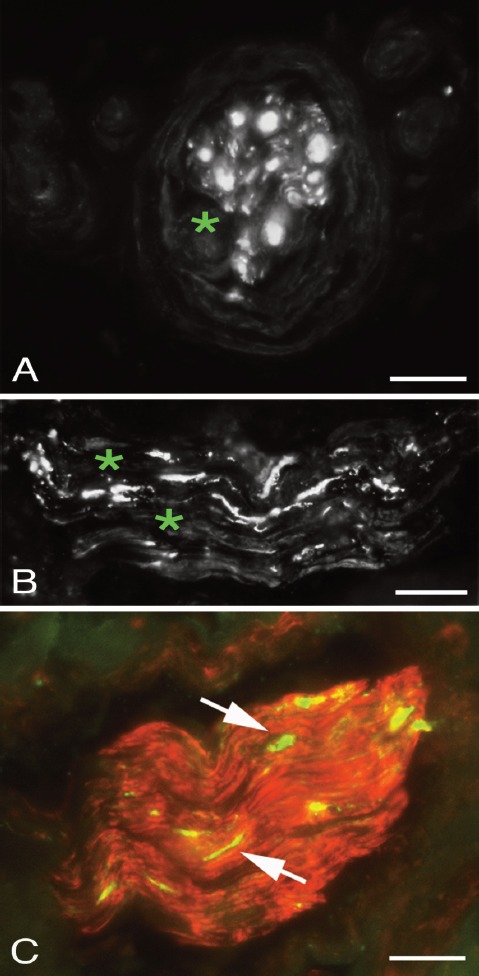
Positive stainings for CGRP were frequently seen in freely coursing nerve fibres (Figure 5A) and in nerve fascicles (Figure 5B). The reactions showed punctuate and partially varicose appearance. CGRP immunoreaction was present both in the peritendinous connective tissue and in the zones of connective tissue inside the tendon tissue proper.
Figure 5.
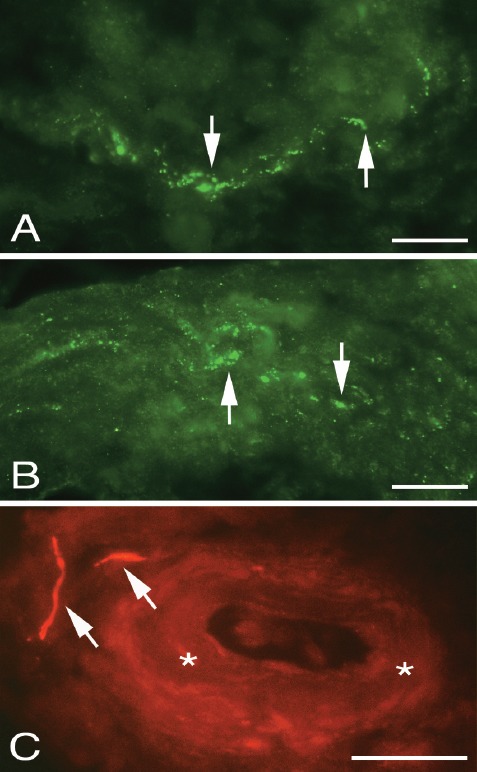
Peritendinous connective tissue in between the Achilles and plantaris tendons stained for CGRP (A,B) and TH (C). Immunoreactions for CGRP (arrows) can be seen in an isolated fine nerve fibre (A) and a large nerve fascicle (B). TH immunoreactive reactions (arrows) are seen in vicinity of a blood vessel (asterisks, C). Bars=20 μm.
Sympathetic innervation
Immunoreactions for TH could be detected in the peritendinous connective tissue in most of the samples that showed a tendinosis-like appearance and contained a large amount of peritendinous tissue. Reactions were mainly seen in perivascular location (Figure 5C). No TH was detected in the zones of connective tissue in the tendon tissue proper as well as in samples containing only very little peritendinous tissue.
Excitatory innervation (Glutamate innervation)
Immunoreactions for NMDAR1, but not VGluT2, could be detected in nerve fibres in a subgroup of nerve fascicles in the peritendinous connective tissue but were not detected in the zones of connective tissue in the tendon tissue proper (Figure 6).
Figure 6.
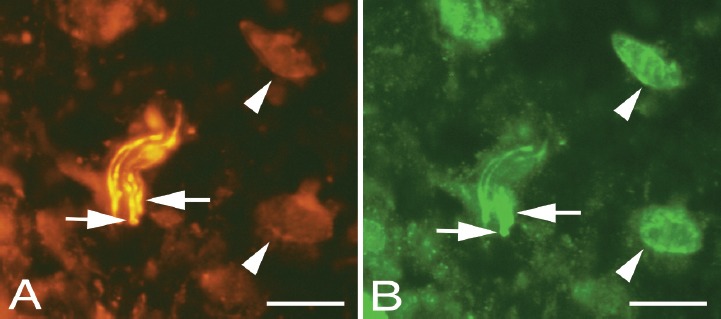
Peritendinous connective tissue in between Achilles and plantaris tendons double stained for PGP9.5 (yellow, A) and NMDAR1 (green/white, B). Overlapping positive stainings are seen in some nerve fibres (arrows). Cells in the peritendinous tissue (arrowheads) are seen to exhibit NMDAR1 immunoreaction, but only unspecific staining in the PGP9.5 stained section. Bars=20 μm.
Features for tendons showing a morphology that in principle is normal (n=6):
CGRP-immunoreactions could be seen in the peritendinous connective tissue and occasionally in the zones of connective tissue within the tendon tissue proper. TH- and NMDAR1 immunoreactions were occasionally detected for nerve fascicles of the peritendinous connective tissue, whilst VGluT2 reactions were never seen. Concerning the peritendinous tissue the immunoreaction patterns seen for CGRP, TH and NMDAR1 were thus similar to those seen for tendons showing a tendinosis-like appearance. As the sizes of this tissue in the specimens of tendons showing an almost normal appearance was comparably small (c.f. above), a truthful comparison concerning the magnitudes of reactions could not be made.
Discussion
We have here shown that the fatty peritendinous connective tissue in between the Achilles and plantaris tendons is richly innervated in patients with midportion Achilles tendinopathy. The innervation was found to contain sensory, sympathetic and NMDAR1 positive nerve fibres. Furthermore, there was an innervation in the zones of connective tissue within the plantaris tendon tissue proper. There were thus fine PGP9.5-immunoreactive nerve fibres in this location in 48/137 examined tendon specimens from 32/46 patients. Another observation was the unexpected relative absence of axons in the peritendinous tissue, as evidenced by the occurrence of partial lack of immunoreaction for the general nerve marker PGP9.5 in parts of some of the large nerve fascicles. These observations represent completely new information concerning tendon innervation.
A strength of this study is the fact that the plantaris samples were sectioned and stained at more than one location along their course close to the Achilles tendon. Altogether we found PGP9.5 positive nerve fibres in the zones of connective tissues in the tendon proper in 69.6% of patients but in only 35% of examined tendon specimens. These findings show the importance of evaluations of more than one part of the plantaris tendon samples in order to conclude how the inner part of the tendon is innervated. Such a thorough evaluation of tendon-associated innervation cannot be done for larger tendons like the Achilles and patellar tendons.
A drawback with the present study is that tendons of completely healthy individuals were not evaluated. This is totally related to ethical aspects. Nevertheless, we carefully evaluated the morphology of the tendon tissue proper of the plantaris tendons, and in six out of the 46 examined patients we noted a morphology that to a large extent resembled that of a normal tendon structure. This shows great variability in morphology for tendons of tendinopathic patients, some tendons showing a rather normal morphology. These tendons had a tendency of having less PGP9.5 immunoreactive nerve fibres in the zones of connective tissue of the tendon tissue proper (1 out of 12 examined tendon specimens) than the other 40 patients. The findings can lead support for a theory of nerve fibre ingrowth in tendinopathy, a theory previously put forward for the Achilles[26,27] and patellar[28] tendons. However, due to the small number of specimens exhibiting an almost normal structure, this information does not have a statistical impact. The peritendinous connective tissue of the samples showing a rather normal morphology of the tendon tissue proper, as well as those showing a tendinosis-like feature, contained PGP9.5, CGRP and glutamatergic innervation. As the sizes of the tissue part conforming to peritendinous tissue largely varied, it was not possible to clarify if the magnitude in innervation differed between the two types of tendons. On the other hand, as is commented on above, this was possible concerning the tendon tissue proper.
A conspicuous and interesting finding was the observation that nerve fascicles in some specimens were found to exhibit a relative lack of PGP9.5 immunoreactions. Based on this lack but the marked occurrence of S-100β staining in these fascicles it cannot be excluded that a partial degeneration of axons has occurred. In our previous studies on Achilles[5,6] and patellar[21] tendinopathy tendons we did not observe these features. The findings raise a possibility for the occurrence of not only nerve proliferation and ingrowth in chronic tendinopathy[26-28] but also nerve degeneration in chronic tendinopathy. This is a new aspect concerning the features of nerve structures in relation to tendinopathy.
It cannot be excluded that the CGRP-positive (sensory) nerve fibres in the peritendinous tissue, and the tissue in the zones of connective tissue in the tendon proper, are a main source of pain in midportion Achilles tendinopathy. It nevertheless remains unclear to what extent the innervation of the zones inside the tendon proper vs. the innervation located in the peritendinous tissue, contributes to tendon pain.
Overall, the study shows that there is a high frequency of nerves and blood vessels in the peritendinous connective tissue in between the plantaris and Achilles tendon. In previous studies, the importance of the peritendinous connective tissue in relation to tendon loading was highlighted[29]. It might be that this tissue is also of importance in tendinopathy. The peritendinous connective tissue located ventrally to the Achilles tendon has also been shown to contain a large number of vessels and nerves[6]. Tendinosis treatments such as polidocanol injections and mini-invasive scraping for this tissue has been found to have a favourable outcome[7,30].
When discussing pain mechanisms it should also be recalled that glutamate has recently been highlighted for painful tendons, and that we in the present study found NMDAR1 immunoreactive axons in the peritendinous connective tissue. It has been shown that glutamate injected into human tendon tissue induces tendon pain[31] and that painful tendons are characterised by high levels of free glutamate[32]. Furthermore, elevated expression of the glutamate receptor NMDAR1 has previously been observed in nerve fibres of Achilles and patellar tendinopathy tendons suggesting a link between glutamate and chronic tendon pain[18,33,34].
The possible interference between the plantaris and Achilles tendons in midportion Achilles tendinopathy and the surgical removal/release of the plantaris tendon leading to pain relief has been described in several studies[9-12]. Knowledge on the innervation in this region is therefore of great importance. That includes information on the innervation in the fatty peritendinous connective tissue, and that in the zones of connective tissue in the tendon tissue proper. In the present study we have shown that the peritendinous connective tissue located in between the two tendons, and to some extent the zones of connective tissue within the plantaris tendon tissue proper, contains a large number of nerve structures (Figure 7). More precisely, particularly a CGRP-innervation was found. This favours that the sensory innervation located here can have an impact on pain transmission, and might explain why plantaris removal can lead to pain relief. It should, however, be stressed that clinical symptoms and possible pain relief were not evaluated in the present study.
Figure 7.
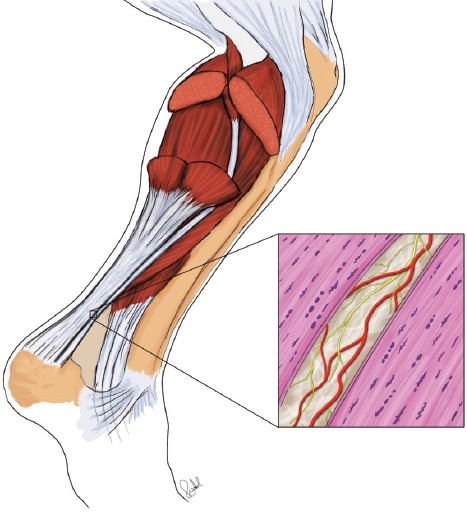
Schematic drawing showing the highly innervated and vascularised peritendinous connective tissue in between the plantaris and Achilles tendons, both showing tendinosis features such as tenocytes frequently being lined up in rows. Red marks vessels, yellow innervation.
Apart from showing innervation in the peritendinous connective tissue and in the zones within the tendon tissue proper, the present study also shows features that favour the occurrence of nerve fibre degeneration. This aspect should be further considered when discussing the relationship between the nervous system and tendinopathy. In total, it is obvious that features related to innervation of the plantaris tendon and the peritendinous connective tissue between the plantaris and Achilles tendons can play an important role for the pain in midportion Achilles tendinopathy.
Acknowledgements
The authors would like to thank MD Gustav Andersson (PhD) for providing the schematic drawing of Figure 7 and Ms Ulla Hedlund for excellent technical services. We furthermore acknowledge ass. Prof. Paul Kingham (PhD) for comments on nerve morphology.
Footnotes
Edited by: S. Warden
Financial support was obtained from the Faculty of Medicine at Umeå University, the Swedish National Centre for Research in Sports (CIF) and Idrottshögskolan, Umeå University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jarvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10(2):255–66. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Kvist M. Achilles tendon injuries in athletes. Sports Med. 1994;18(3):173–201. doi: 10.2165/00007256-199418030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Cook JL, Purdam CR. The challenge of managing tendinopathy in competing athletes. Br J Sports Med. 2014;48(7):506–9. doi: 10.1136/bjsports-2012-092078. [DOI] [PubMed] [Google Scholar]
- 4.Rio E, Moseley L, Purdam C, Samiric T, Kidgell D, Pearce AJ, et al. The pain of tendinopathy: physiological or pathophysiological? Sports Med. 2014;44(1):9–23. doi: 10.1007/s40279-013-0096-z. [DOI] [PubMed] [Google Scholar]
- 5.Bjur D, Alfredson H, Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005;320(1):201–6. doi: 10.1007/s00441-004-1014-3. [DOI] [PubMed] [Google Scholar]
- 6.Andersson G, Danielson P, Alfredson H, Forsgren S. Nerve-related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1272–9. doi: 10.1007/s00167-007-0364-2. [DOI] [PubMed] [Google Scholar]
- 7.Alfredson H. Where to now with Achilles tendon treatment? Br J Sports Med. 2011;45(5):386. doi: 10.1136/bjsm.2011.084129. [DOI] [PubMed] [Google Scholar]
- 8.Ruergard A, Alfredson H. Major physical but also psychological effects after pain relief from surgical scraping in patients with Achilles tendinopathy - A 1-year follow-up study. Pain Studies Treatment. 2014;2(1):21–25. [Google Scholar]
- 9.Alfredson H. Midportion Achilles tendinosis and the plantaris tendon. Br J Sports Med. 2011;45(13):1023–5. doi: 10.1136/bjsports-2011-090217. [DOI] [PubMed] [Google Scholar]
- 10.van Sterkenburg MN, Kerkhoffs GM, Kleipool RP, Niek van Dijk C. The plantaris tendon and a potential role in mid-portion Achilles tendinopathy: an observational anatomical study. J Anat. 2011;218(3):336–41. doi: 10.1111/j.1469-7580.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Sterkenburg MN, Kerkhoffs GM, van Dijk CN. Good outcome after stripping the plantaris tendon in patients with chronic mid-portion Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1362–6. doi: 10.1007/s00167-011-1514-0. [DOI] [PubMed] [Google Scholar]
- 12.Pearce CJ, Carmichael J, Calder JD. Achilles tendinoscopy and plantaris tendon release and division in the treatment of non-insertional Achilles tendinopathy. Foot Ankle Surg. 2012;18(2):124–7. doi: 10.1016/j.fas.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Spang C, Alfredson H, Ferguson M, Roos B, Bagge J, Forsgren S. The plantaris tendon in association with mid-portion Achilles tendinosis: tendinosis-like morphological features and presence of a non-neuronal cholinergic system. Histol Histopathol. 2013;28(5):623–32. doi: 10.14670/HH-28.623. [DOI] [PubMed] [Google Scholar]
- 14.Alfredson H, Spang C, Forsgren S. Unilateral surgical treatment for patients with midportion Achilles tendinopathy may result in bilateral recovery. Br J Sports Med. 2014;48:1421–1424. doi: 10.1136/bjsports-2012-091399. [DOI] [PubMed] [Google Scholar]
- 15.Cook JL, Purdam C. Is compressive load a factor in the development of tendinopathy? Br J Sports Med. 2012;46(3):163–8. doi: 10.1136/bjsports-2011-090414. [DOI] [PubMed] [Google Scholar]
- 16.Lintz F, Higgs A, Millett M, Barton T, Raghuvanshi M, Adams MA, et al. The role of Plantaris Longus in Achilles tendinopathy: a biomechanical study. Foot Ankle Surg. 2011;17(4):252–5. doi: 10.1016/j.fas.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Masci L, Spang C, van Schie H, Alfredson H. Achilles tendinopathy - Do plantaris tendon removal and Achilles tendon scraping improve tendon structure? A prospective study using Ultrasound Tissue Characterisation. BMJ Open Sports Exerc Med. 2015;1:e000005. doi: 10.1136/bmjsem-2015-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfredson H, Forsgren S, Thorsen K, Fahlstrom M, Johansson H, Lorentzon R. Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc. 2001;9(2):123–6. doi: 10.1007/s001670000188. [DOI] [PubMed] [Google Scholar]
- 19.Spang C, Scott A, Danielson P, Lorentzon R, Forsgren S. VGluT2 and NMDAR1 expression in cells in the inflammatory infiltrates in experimentally induced myositis: evidence of local glutamate signaling suggests autocrine/paracrine effects in an overuse injury model. Inflammation. 2012;35(1):39–48. doi: 10.1007/s10753-010-9287-z. [DOI] [PubMed] [Google Scholar]
- 20.Bjur D, Danielson P, Alfredson H, Forsgren S. Immunohistochemical and in situ hybridization observations favor a local catecholamine production in the human Achilles tendon. Histol Histopathol. 2008;23(2):197–208. doi: 10.14670/HH-23.197. [DOI] [PubMed] [Google Scholar]
- 21.Danielson P, Alfredson H, Forsgren S. Distribution of general (PGP 9.5) and sensory (substance P/CGRP) innervations in the human patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):125–32. doi: 10.1007/s00167-005-0636-7. [DOI] [PubMed] [Google Scholar]
- 22.De Martin S, Paliuri G, Belloni A, Orso G, Zanarella E, Stellin G, et al. Expression and Distribution of the Adrenomedullin System in Newborn Human Thymus. PLoS One. 2014;9(5):e97592. doi: 10.1371/journal.pone.0097592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand. 1997;68(2):170–5. doi: 10.3109/17453679709004002. [DOI] [PubMed] [Google Scholar]
- 24.Riley G. Tendinopathy - from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4(2):82–9. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 25.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27(6):393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 26.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64(7):1083–6. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):334–8. doi: 10.1007/s00167-003-0391-6. [DOI] [PubMed] [Google Scholar]
- 28.Lian O, Dahl J, Ackermann P, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34:1801–08. doi: 10.1177/0363546506289169. [DOI] [PubMed] [Google Scholar]
- 29.Kjaer M, Bayer ML, Eliasson Heinemeier P. What is the impact of inflammation on the critical interplay between mechanical signaling and biochemical changes in tendon matrix? J Appl Physiol. 2013;115:879–883. doi: 10.1152/japplphysiol.00120.2013. [DOI] [PubMed] [Google Scholar]
- 30.Alfredson H, Ohberg L, Zeisig L, Lorentzon R. Treatment of Midportion Achilles Tendinosis: Similar Clinical Results with US and CD-Guided Surgery Outside the Tendon and Sclerosing Polidocanol Injections. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1504–9. doi: 10.1007/s00167-007-0415-8. [DOI] [PubMed] [Google Scholar]
- 31.Gibson G, Arendt-Nielsen L, Sessle BJ, Graven-Nielsen T. Glutamate and capsaicin-induced pain, hyperalgesia and modulatory interactions in human tendon tissue. Ex Brain Res. 2009;194:173–182. doi: 10.1007/s00221-008-1683-3. [DOI] [PubMed] [Google Scholar]
- 32.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):378–81. doi: 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 33.Schizas N, Lian O, Frihagen F, Engebretsen L, Bahr R, Ackermann PW. Coexistence of up-regulated NMDA receptor 1 and glutamate on nerves, vessels and transformed tenocytes in tendinopathy. Scand J Med Sci Sports. 2010;20(2):208–15. doi: 10.1111/j.1600-0838.2009.00913.x. [DOI] [PubMed] [Google Scholar]
- 34.Schizas N, Weiss R, Lian O, Frihagen F, Bahr R, Ackermann PW. Glutamate receptors in tendinopathic patients. J Orthop Res. 2012;30(9):1447–52. doi: 10.1002/jor.22094. [DOI] [PubMed] [Google Scholar]


