Abstract
People differ in their sleep-wake behavior. This individual difference is conceptualized in different aspects, such as wake up times, bed times, times of peak performance, as well as in morning affect. A total of 14,987 visitors of an exhibition in the LWL State Museum of Natural History, Münster (Germany), did the survey on chronotype and gave their consent that these data can be used for a scientific study. Age groups were coded into 5-year bins. Mean age (mean ± SD) was 28.2 ± 17.5 years. There were 8075 females (54%) and 6912 males in the sample. The German version of the rMEQ (reduced Morningness-Eveningness-Questionnaire) was used for data collection. The data showed clear age effects. Younger children are more morning oriented and become rapidly evening oriented during puberty, while the more attenuated turn towards morningness occurs from the age of 20 years. Then between the ages 25 to 30 morningness-eveningness remained rather stable. Significant gender differences existed in the reproductive age, i.e., the age groups 20 to 50 (corresponding to the age 16–50 years). In other age groups, no gender differences could be detected. Seasonal effects were also found. Chronotype score was lowest during the summer months (and more evening oriented). Based on the single item analysis of the five questions of the rMEQ, we found age group differences in all items. Gender differences occurred in all items except item 1, which deals with the preferred wake-up time. Men always scored significantly lower (i.e. more evening oriented) than women except in item 2 (tiredness after awakening). Seasonal effects were only significant in item 3, which is related to preferred bed times. People showed a later bed time preference during summer. The classification of chronotypes according to the cut-off scores provided by Adan and Almirall (1991) and by using the 20/80 percentile provided identical cut-off scores (values of 11 and below for evening types and 18 and above for morning types).
Keywords: Developmental Biology, Psychology
1. Introduction
People differ in their sleep-wake behavior. This individual difference has received increasing attention during the last decade (Adan et al., 2012). It is conceptualized in different aspects, such as wake up times, bed times, times of peak performance, as well as in morning affect. Morning affect relates to the feeling after awakening, e.g., how tired a person feels after awakening. Different terms have been used to describe this phenomenon, such as morningness-eveningness, circadian typology, diurnal preference or chronotype. Some people are morning oriented, getting up early, reaching their peak of cognitive and physical performance early throughout the morning, and are sometimes colloquially called larks. Others reach their peak performance later in the afternoon, the evening or at night and prefer later bed and wake times. These are often called owls. Although the distinction is quite easy, many people are in-between the both ends of this continuum (Natale and Cicogna, 2002). Concerning personality, (Antúnez et al., 2014), morning types had higher scores than the evening types in persistence, while evening types scored higher in novelty seeking and sensation seeking.
The individual difference of morningness-eveningness is based on the intrinsic biological rhythm of a person. Evening and morning people differ in their genetics and about 37% of variance in morningness-eveningness can be explained by genetic influence (Watson et al., 2013). Recent studies based on a genome wide association studies revealed 12 new different loci that are associated with the morningness-eveningness trait (Lane et al., 2016). In addition, differences between morning and evening people are found in their daily fluctuation of core body temperature (Baehr et al., 2000), their peak melatonin secretion (Burgess and Fogg, 2008), as well as in their cortisol awakening levels (Randler and Schaal, 2010). Also, evening types in male University students showed a higher testosterone level (Randler et al., 2012). Thus, morningness-eveningness can be seen and understood as an individual trait which is backed up by biological processes.
Apart from the individual difference, some general facts have been recognized. For example, morningness-eveningness changes during the lifespan (Randler, 2016). Children are usually more morning oriented (Zimmermann, 2016; Randler and Truc, 2014), and during adolescence, individuals are rather dramatically evening orientated (Roenneberg et al., 2004). At the end of adolescence, people progressively are more morning oriented again (Roenneberg et al., 2004). Although this seems well-known, only a handful of studies analyzed these changes based on a sufficient sample using the same measurement instrument. Roenneberg et al. (2004) demonstrated these significant shifts in a cross-sectional study from the age of 10 until about 19/20 years in a sample of 25,000 Germans based on a questionnaire (MCTQ) that assessed primarily bed times and wake times on free days and on scheduled days. Tonetti et al. (2008) based the study on 8,972 Italians, mainly from the questions of their preferred bed and rise times from the MEQ. Tonetti et al. (2008) assessed people form ages 10 to 87 years. Females received their evening lateness at around 17 years, males at the age of 20. Also, Duarte et al. (2014) examined the chronotype of 14,650 Brazilians using the MEQ with its full 19 items. However, their study was based on people aged <20 years to ≥60 years. Until the age of 30 women were more morning oriented while from the age of 50 years onwards, men were more morning oriented. For Finland, Merikanto et al. (2012; N = 6,858; age range 26–72; based on six items from the MEQ) reported a decrease of evening types and an increase of morning types with increasing age. In a more macro-analytical approach, Randler (2016) found that adults showed a higher morningness from the age of 18 years onwards.
Concerning gender, the effects are less clear. However, most large scale studies showed a clear but small gender effect with women being more morning oriented than men (e.g., Adan and Natale, 2002). This has been corroborated by meta-analysis (Randler, 2007) as well as by large sample studies (Tonetti et al., 2008; Roenneberg et al., 2004). However, Brazilian people showed an interaction effect (Duarte et al., 2014) with men displaying later chronotypes during the younger ages and women showing later chronotypes during older ages. In Finland, evening types were more common in women than in men, while in morning types it was vice versa (Merikanto et al., 2012). Thus, there are conflicting results concerning gender effects. The differences between the studies cannot simply be ascribed to different questionnaires, because Tonetti et al. (2008), Merikanto et al. (2012) and Duarte et al. (2014) used either the MEQ or a derivate of it. As these samples were from different countries, possible reasons for the differences could also be found in socio-cultural effects.
Another aspect is the season of assessment. Usually, chronotype is seen and understood as a trait variable, which does not change strongly within a short time duration. However, two studies found an influence of season of assessment. Johnsen et al. (2013) reported an 8-minute difference in chronotype between summer and winter in a Norwegian population based on computing of the midpoint of sleep according to the MCTQ. Contrary to the hypothesis, chronotype was advanced when assessed during summer. Thus, people that assessed their chronotype in summer were slightly earlier chronotypes. Similar results were obtained in another study based on Estonian, Croatian and German adults, showing a more morning orientation when the assessment of chronotype was made in summer (Allebrandt et al., 2014).
Concerning measurement, there are data missing to clearly separate morning from evening types. In the first study on the German rMEQ, the cut-off scores of Adan and Almirall (1991) have been applied to the German sample, which showed a good agreement with the classification of the Composite Scale of Morningness (Randler, 2013). In this previous study, evening types scored between 4–11, and morning types from 18–25. People with a score from 12–17 were categorized as neither type. The large sample of the current study allows to re-assess the cut-off scores again.
The aim of the present study was to assess age-related differences, gender differences and their interaction in a large sample of Germans. However, above and beyond these aspects, the study wants to validate and re-assess cut-off scores for the German rMEQ, as well as assessing seasonal changes.
2. Methods
2.1. Participants and data collection
About 159.270 visitors visited the LWL museum during the exhibition consisting of 72% residents of the state of North-Rhine-Westphalia, 26% of other parts of Germany and 2.2% of neighboring countries such as the Netherlands. 14,987 visitors, nearly 10%, did the survey on chronotype and gave their consent that these data can be used for a scientific study. We consider this as an acceptable percentage because participants first established their morningness-eveningness measure for their own information, and were only subsequently asked to save their data.
The survey was performed on a 32” TFT-Public touch display. It was placed at a prominent position within the exhibition “Life in the dark”, opened from May 22nd 2015 to May 29th 2016. The touch display was at the section of the exhibition where the topics: “sleep” and the “internal clock” in human beings were presented. Visitors could work themselves through the questionnaire in a php-based touch-screen application. The application contained a general description with an explanation, followed by the questions 1–5 of the German version of rMEQ, each presented on a separate page. At the end, the users were presented with their personal score as well as the overall distribution of scores recorded so far. The results were automatically saved into a php database.
2.2. Ethical considerations
The current study is a questionnaire survey and we followed the guidelines of the Declaration of Helsinki as well as the guidelines of the ethics committee of the University of Education Heidelberg. The data were stored anonymously in the database. The questions are not of any ethical concern. Participants had the chance and possibility to drop out at any time. Further, at the end of the questionnaires, people were asked if they agree that these data can be used for scientific research. After clicking yes, data were stored and otherwise not.
2.3. Demographic data
We collected age and gender as basic demographic data. Age groups were coded into 5-year bins starting with 10 years (including 5–10 years), 15 years (including 11–15 years), 20 years (16–20 years), 25 (21–25 years), 30 (26–30 years), 35 (31–35 years), 40 (36–40 years), 45 (41–45 years), 50 (46–50 years), 55 (51–55 years), 60 (56–60 years), 65 (61–65 years), 70 (66–70 years). Table 1 gives an overview over the sample. Mean age (mean ± SD) was 28.2 ± 17.5 years. There were 8075 females (54%) and 6912 males in the sample. In addition, we collected the date of sampling. The date of sampling was later grouped into month and seasons, with march, April, May as spring, June, July, August as summer, September, October, November as autumn, and December, January and February as winter; based on the meteorological definition.
Table 1.
Overview of the sample according to age group and gender.
| Age Group | Gender |
Total | |
|---|---|---|---|
| Female | Male | ||
| 10 | 1421 | 1546 | 2967 |
| 15 | 1362 | 1329 | 2691 |
| 20 | 552 | 310 | 862 |
| 25 | 609 | 414 | 1023 |
| 30 | 563 | 408 | 971 |
| 35 | 519 | 424 | 943 |
| 40 | 675 | 474 | 1149 |
| 45 | 760 | 595 | 1355 |
| 50 | 625 | 564 | 1189 |
| 55 | 386 | 338 | 724 |
| 60 | 260 | 231 | 491 |
| 65 | 206 | 157 | 363 |
| 70 | 137 | 122 | 259 |
| Total | 8075 | 6912 | 14987 |
2.4. Measurement instruments
The German version of the rMEQ (reduced Morningness-Eveningness-Questionnaire) was used for data collection (Randler, 2013). The rMEQ is a shortened version of the Morningness-Eveningness Questionnaire (MEQ) developed by Horne and Östberg (1976). The original version was based on 19 items in a Likert type response format. Adan and Almirall (1991) developed a short form out of this original MEQ which was then applied in different countries, such as Spain (Adan and Almirall, 1991), Italy (Natale et al., 2006), Germany (Randler, 2013), Iran (Rahafar et al., 2015) and India (Biswas et al., 2014; see also Di Milia et al., 2013 for an overview). The rMEQ has good psychometric properties and validity (Di Milia et al., 2013). It has a good test-retest reliability (Carciofo et al., 2012). The scale contains five items, that are related to preferred bed time, get-up time, tiredness in the morning, peak performance and a global self-assessment item. Cronbach’s alpha was 0.62 in the present sample. The German version has been established as a reliable and valid measurement of chronotype (Randler, 2013; Di Milia et al., 2013). To group people, we classified persons with a rMEQ total score of 11 and below as evening types, between 12 and 17 as neither types, and with 18 and higher as morning types.
2.5. Statistical analyses
We used T-tests to compare the scores of different age groups and general linear models to assess the effects of age, gender and season simultaneously. Partial eta-squared was used as a measure of effect size, and we considered the following cut offs: small: .0099, medium: .0588, and large: .1379. (Ferguson, 2009; Richardson, 2011). For the analyses of single items we used a non-parametric Mann-Whitney-U test for two and a Kruskal-Wallis test for more independent variables.
3. Results
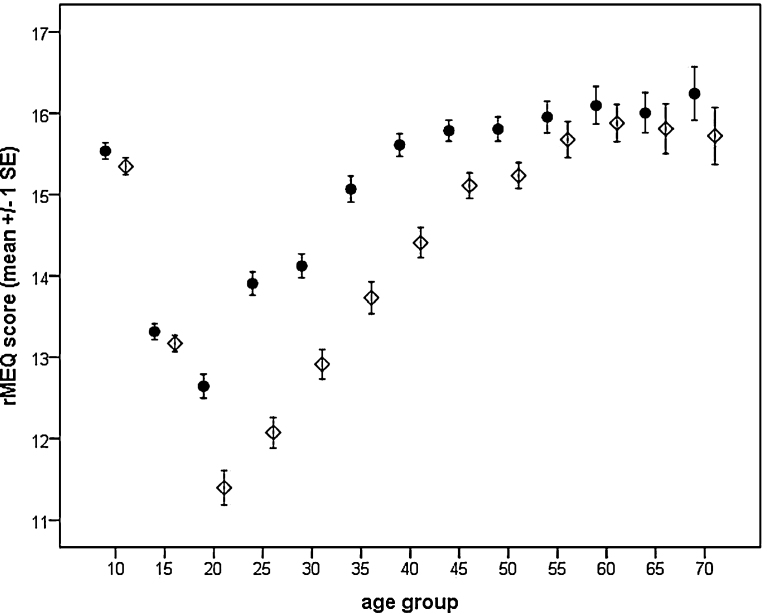
The data presented in this study show clear age effects. Younger children are more morning oriented and are strongly evening oriented during puberty, while the more attenuated turn towards morningness occurs from the age of 20 years (Fig. 1). Significant differences existed between the age groups 10 to 15 (p < .001), 15 to 20 (<.001), 20 to 25 (p < .001). Then between the ages 25 to 30 morningness-eveningness was not significantly different (p = .569). Again, from 30–35 (p < .001), and 35 to 40 (.006) a higher morningness occurred, while between the ages of 40 to 70, morningness scores remained on the same level (p = .964).
Fig. 1.
Chronotype across age groups. The rMEQ scores are depicted separately for males (open rectangles) and females (filled dots).
Significant gender differences existed in the reproductive age, i.e., the age groups 20 to 50 (corresponding to the age 16–50 years). In other age groups, no gender differences could be detected. In addition, a general linear model was applied with age group, gender and the interaction of both. The model revealed significant differences between the age groups that explained about 9% of the variance. Gender effects were smaller, which was mainly based on the fact that gender differences occurred only during the reproductive age. Also, the interaction between gender and age was significant but the effect was negligible (Table 2) because females always scored higher than males in the respective age groups.
Table 2.
General linear model using age group, gender, season and the interaction between age group and gender as independent variables and rMEQ total scores as dependent variable. * indicates a medium effect size following Richardson (2011), and the other effect sizes have no practical significance following Ferguson (2009).
| F | Sig. | Partial Eta squared | |
|---|---|---|---|
| Gender | 94.460 | <.001 | .006 |
| Season | 4.197 | .006 | .001 |
| Age Group | 127.665 | <.001 | .093* |
| Gender * Age Group | 6.546 | <.001 | .005 |
Note: R2 = .10.
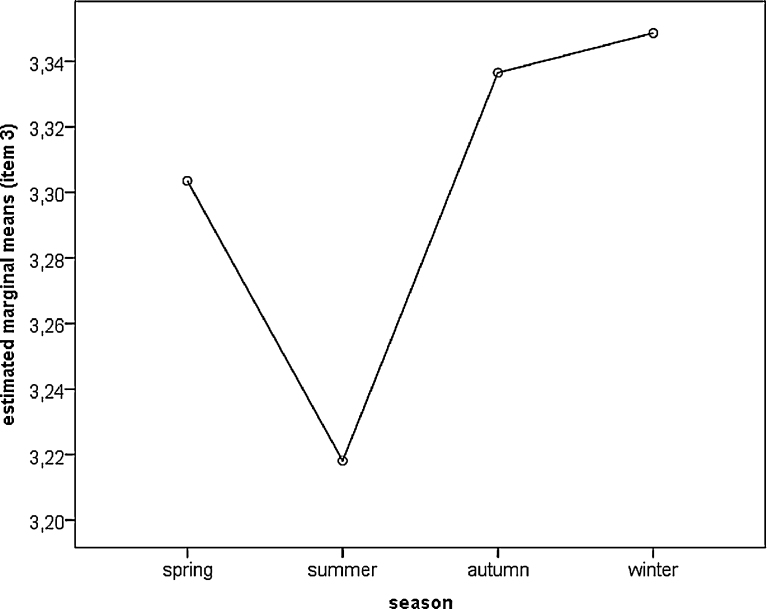
Seasonal effects were also found in a general linear model (Table 2). Chronotype score was lowest during the summer months (Table 3; Fig. 2). Significant differences existed between spring and summer (p = 0.012), summer and autumn (p = 0.001) as well as between summer and winter (p = 0.013).
Table 3.
Estimated marginal means according to seasons for the total rMEQ score and item 3, which refers to the preferred bed times. Means are estimated from the linear model, thus controlling for age and gender.
| Seasons | Sample size | Mean rMEQ score | SE | Mean item 3 | SE |
|---|---|---|---|---|---|
| Spring | 4157 | 14.75 | 0.062 | 3.30 | .015 |
| Summer | 3687 | 14.54 | 0.065 | 3.22 | .015 |
| Autumn | 3536 | 14.83 | 0.067 | 3.34 | .016 |
| Winter | 3607 | 14.75 | 0.066 | 3.35 | .016 |
Fig. 2.
Comparison of seasons for item—3 (preferred bed time). Estimated marginal means derived from the general linear model. Low values represent high evening preference.
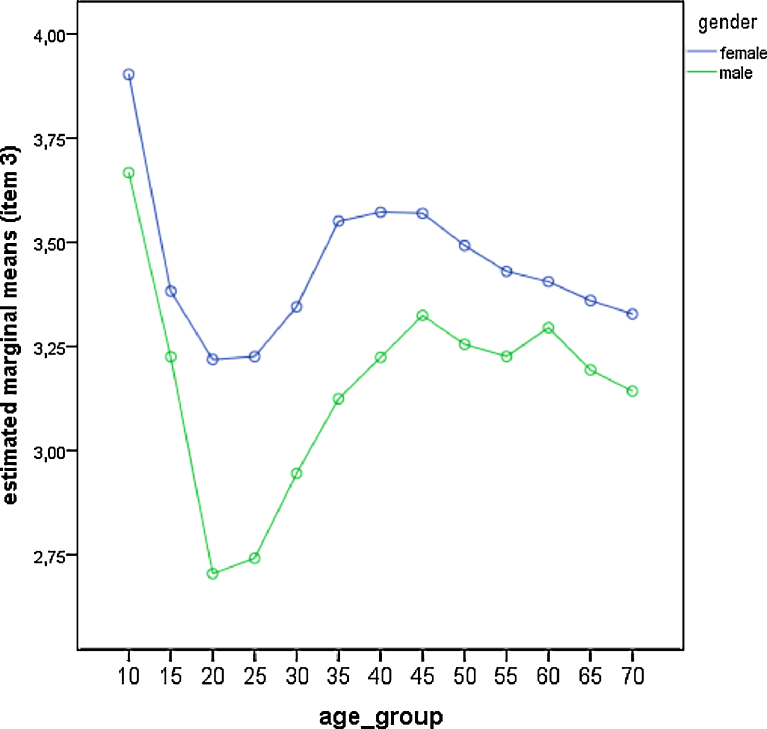
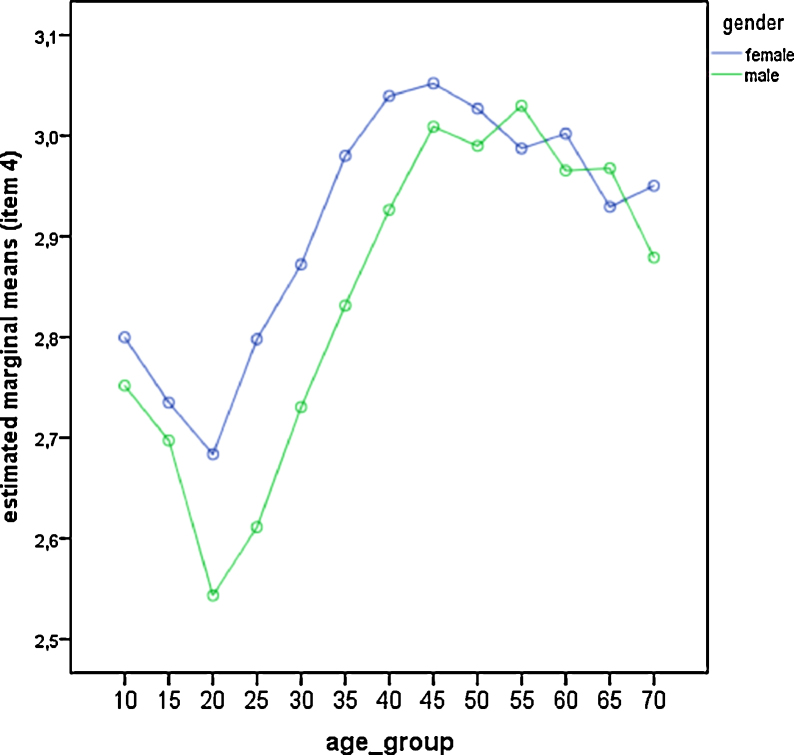
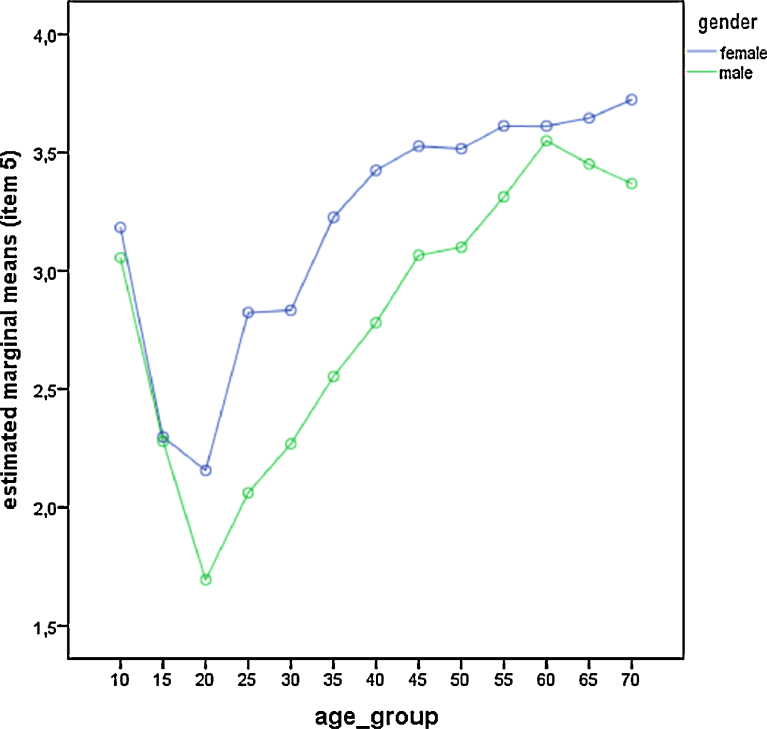
We also did a single item analysis of the five questions of the rMEQ (Table 4; Fig. 3, Fig. 4, Fig. 5, Fig. 6 and Fig. 7). Age group and gender differences emerged in all items. Men always scored significantly lower (i.e. more evening oriented) than women except in item 2. This item deals with the morning affect (tiredness after awakening). Seasonal effects were only significant in item 3 which is related to preferred bed times (Table 3 and Table 4). People showed a later bed time preference during summer. Differences were significant between spring and summer (p = 0.001), spring and autumn (p = 0.019), summer and autumn (p = 0.001), summer and winter (p < 0.001), and spring and winter (p = 0.002), but not between autumn and winter (p = 0.495).
Table 4.
Non-parametric comparisons of the gender effects (Mann-Whitney-U test), age group and seasonal effects (Kruskal-Wallis test).
| Independent Variable | Dependent Variable | P | |
|---|---|---|---|
| Season | q1—get up time | χ2 =3.55 | 0.314 |
| q2—tiredness in the morning | χ2 =1.62 | 0.654 | |
| q3—bed time | χ2 =56.32 | <0.001 | |
| q4—peak performance | χ2 =4.84 | 0.184 | |
| q5—global assessment | χ2 =5.17 | 0.159 | |
| Gender | q1—get up time | Z=2.43 | 0.015 |
| q2—tiredness in the morning | Z=3.28 | 0.001 | |
| q3—bed time | Z=−15.67 | <0.001 | |
| q4—peak performance | Z=−6.20 | <0.001 | |
| q5—global assessment | Z=−9.36 | <0.001 | |
| Age Group | q1—get up time | χ2 =1444.9 | <0.001 |
| q2—tiredness in the morning | χ2 =525.2 | <0.001 | |
| q3—bed time | χ2 =1157.1 | <0.001 | |
| q4—peak performance | χ2 =476.5 | <0.001 | |
| q5—global assessment | χ2 =790.5 | <0.001 |
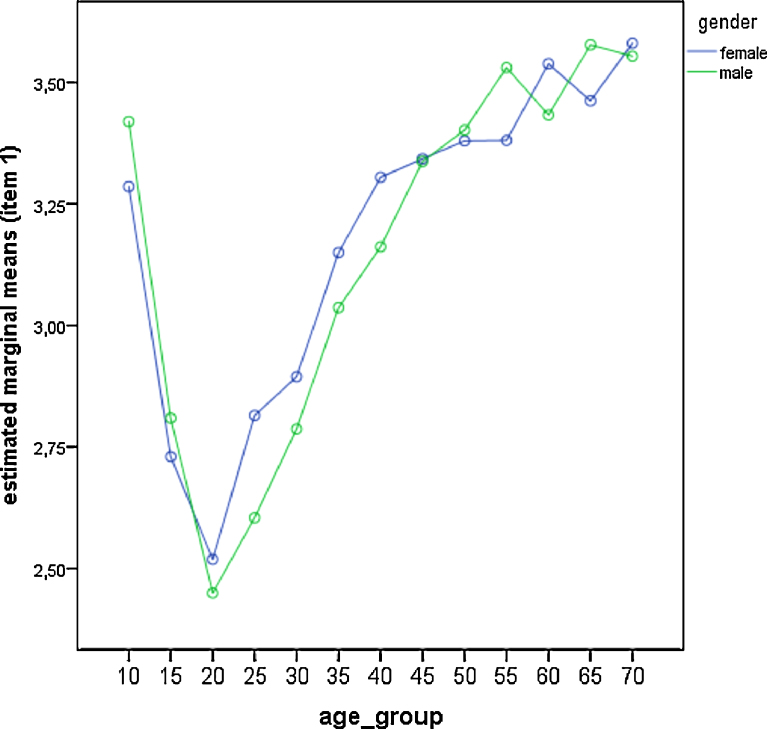
Fig. 3.
Comparison of gender differences across age groups for item—1 (preferred get-up time). Estimated marginal means derived from the general linear model. Low values represent late wake-up times.
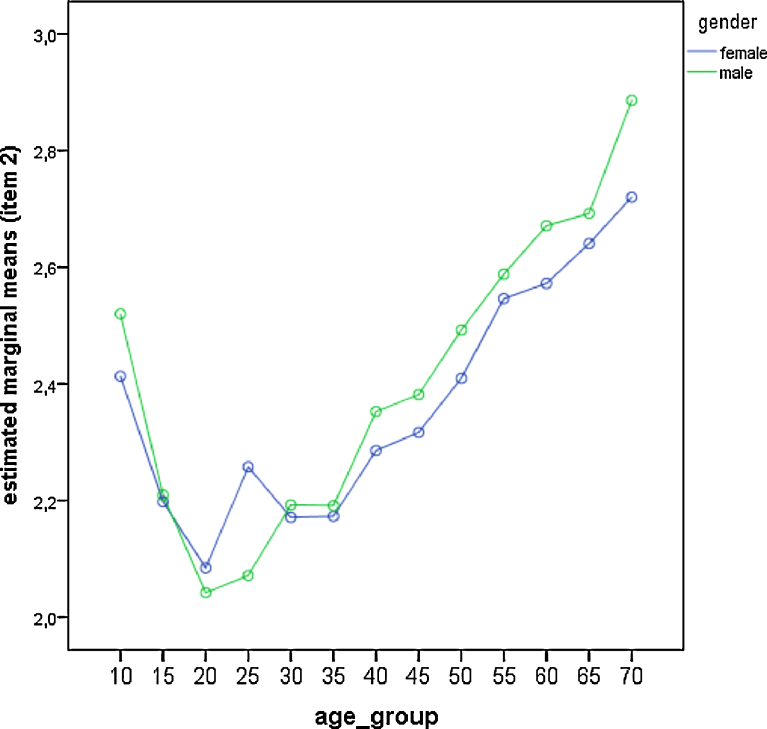
Fig. 4.
Comparison of gender differences across age groups for item—2 (feeling after awakening). Estimated marginal means derived from the general linear model. High values represent high/positive feelings after awakening.
Fig. 5.
Comparison of gender differences across age groups for item—3 (preferred bed time). Estimated marginal means derived from the general linear model. Low values represent high evening preference (late bedtimes).
Fig. 6.
Comparison of gender differences across age groups for item—4 (feeling best peak). Estimated marginal means derived from the general linear model. Low values represent late feeling best peaks.
Fig. 7.
Comparison of gender differences across age groups for item—5 (self-assessment). Estimated marginal means derived from the general linear model. Low values represent high evening preference.
The classification of chronotypes according to the cut-off scores provided by Adan and Almirall (1991) and Randler (2013) revealed 21.9% evening types and 23.8% morning types. Using the 20/80 percentile as cut-offs based on the current study suggest cut-off values of 11 and below for evening types and 18 and above for morning types. This approach is identical to the previously suggested cut-off scores.
4. Discussion
The dramatic differences between childhood and adolescence in chronotype have been described in some other research (Roenneberg et al., 2004; Tonetti et al., 2008). However, we add to previous work by showing that chronotype remains rather stable between the age of 25 to 30 years. This indicates that after the dramatic turn towards eveningness and the less pronounced turn towards morningness, a plateau during early adulthood is achieved. This stability of chronotype fits into other German data reported by Di Milia and Randler (2013), which were obtained with a different questionnaire, the Composite Scale of Morningness (CSM; Randler, 2008), however, the CSM and the rMEQ are correlated with 0.8 to 0.9 (Randler, 2009), indicating they measure a similar construct. Even when focusing on the single items, the age effects remain similar. The changes during puberty might be a result of changing gonadal hormonal levels, e.g., testosterone was associated with eveningness (Randler et al., 2012). Also, menarche seems an important biological marker. Beal et al. (2016) reported that girls stayed up later (i.e., eveningness) as they approach menarche. After menarche, no change in chronotype was observed. Frey et al. (2009) demonstrated that the changes towards morningness at the end of adolescence is related to a time span of about 5 years after menarche. Thus, in girls and women, it may be important to assess gynecological age in addition to chronological age (Beal et al., 2016).
Gender differences occurred only during the reproductive age in our sample. One reason might be the biological basis, with women having a shorter intrinsic period than men (Duffy et al., 2011) and reaching their acrophase of melatonin earlier (Gibertini et al., 1999). This is corroborated by the findings of Roenneberg et al. (2004) and Tonetti et al. (2008) who obtained similar results as in our study. In contrast, Duarte et al. (2014) found no significant gender differences in the age groups between 30 and 44 years in Brazil. However, these age groups are well within the reproductive period. This is might be owed to the interaction effect (see below). This is interesting because it questions some of the proximate and ultimate factors (see below). Therefore, the difference of the Brazilian data may suggest that future studies should include social and psychological factors as well as analyzing chronotype across the lifespan. If we view the morningness-eveningness trait as a biological individual difference, we can apply the concept of Tinbergen’s four Whys. From the ontogenetic point, the changes during the lifespan are described above and eveningness may be a step towards adulthood. From the proximate point, we assume that hormones are involved in these changes (Randler et al., 2012), and from the ultimate aspect, we hypothesize that the dimorphism between males and females is related to sexual selection (Piffer, 2010). The phylogenetic aspects cannot be applied in this setting because research on animal sleep behavior is just emerging (Randler, 2014), but the great apes should be an interesting comparison group for phylogenetic aspects. So future studies should emphasize in assessing the proximate causes, as well as social and psychological variables.
The Morning Affect was higher in men, i.e. they felt less tired after awakening than women. This is a new finding, which has not been found previously. In another study on the MEQ, the difference between men and women was not significant in this specific Morning Affect item (based on a large sample of Spaniards aged 18–30; Adan and Natale, 2002). Thus, we found here a different result. Also, women scored always higher on morningness in all items compared to men when there was a difference between the sexes (Adan and Natale, 2002).
No interaction between gender and age was found in such a dimension as in the Brazilian sample (Duarte et al., 2014). This is very interesting. One could hypothesize that the Brazilian results may be influenced by the questionnaire. However, Duarte et al. (2014) used the MEQ, which is a valid and reliable measurement instrument, and the rMEQ has evolved out of this measure, and usually correlates highly with the original measure (Di Milia et al., 2013), thus, we assume that indeed there are differences in the ontogeny of chronotype between Brazilians and Central European people (Germany, Italy). At least to our present knowledge, we have no explanation for this phenomenon.
Allebrandt et al. (2014) analyzed the season of assessment and reported a stronger morning orientation during the summer months in a cross-sectional design. Interestingly, we found a contradictory effect with eveningness being highest in summer, mainly because of preferred bed times which were later during the summer months. However, this makes sense because people in the temperate zone, especially in Germany, stay out later during the summer months, which should lead to later bed times and a higher evening orientation. Although Kantermann et al. (2007) modelled that the morning sun is the more important predictor of chronotype (dawn), our results seem also plausible. Probably the studies based on the assessment of clock times (Kantermann et al., 2007; Allebrandt et al., 2014) may more easily detect such differences compared to a full assessment of chronotype based on a set of variables, such bed times, rise times, morning affect and peak performance. Further, questions about clock times may be more sensitive towards changes and thus more state-like and stronger fluctuating, while the global assessment item used in our study (item 5) is a real trait-like item that does not fluctuate and change quickly.
Concerning measurement aspects, we found that the cut-off scores for the German population that have been derived from the original publication of Adan and Almirall (1991) and used by Randler (2013) in another German sample are identical to the 20/80 percentile approach. Thus, we strongly support previous work on this cut-off and suggest classifying evening types with a score of 11 and below, and morning types with a score of 18 and higher. As the cut-off scores of the first German sample were based on N = 594, the refinement was necessary to include a larger sample of adults. Nevertheless, we find the identical cut-offs.
5. Limitations
The study has several limitations. First, the Cronbach’s alpha value of our measurement (.62) is below the suggested acceptable level of .70. Compared to the original publication of Randler (2013), also in a German sample, it is lower (.723 in Randler 2013). Second, one may argue that the visitors of the exhibition in the LWL State Museum of Natural History are not representative of the German general population, just because museum visitors may be different. However, the LWL is visited by a high number of people from all sociological stratifications, so they may be nearly representative. In addition, we did not collect many demographic variables, which would have enhanced our dataset, but we feel that this would not have been possible given the nature of the data collection method (computer terminal), so you always have the decision between collecting many data with a small set of variables or few datasets with many variables. Another aspect may be the effect sizes. The effect sizes in line of Richardson’s (2011) work can be classified as medium concerning the age differences, and as negligible concerning sex differences (because lower than the cut-off value of .009).
Declarations
Author contribution statement
Christoph Randler: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Katharina Freyth-Weber, Andrea Florez Jurado: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Arash Rahafar: Analyzed and interpreted the data; Wrote the paper.
Jan-Ole Kriegs: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
No additional information is available for this paper.
Acknowledgments
We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
References
- Adan A., Almirall H. Horne & Östberg morningness-eveningness questionnaire: a reduced scale. Pers. Individual Differences. 1991;12:241–253. [Google Scholar]
- Adan A., Natale V. Gender differences in morningness/eveningness preference. Chronobiol. Int. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Adan A., Archer S.N., Hidalgo M.P., Di Milia L., Natale V., Randler C. Circadian typology: a comprehensive review. Chronobiol. Int. 2012;29:1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- Allebrandt K.V., Teder-Laving M., Kantermann T., Peters A., Campbell H., Rudan I., Wilson J.F., Metspalu A., Roenneberg T. Chronotype and sleep duration: the influence of season of assessment. Chronobiol. Int. 2014;31(5):731–740. doi: 10.3109/07420528.2014.901347. [DOI] [PubMed] [Google Scholar]
- Antúnez J.M., Navarro J.F., Adan A. Morningness–eveningness and personality characteristics of young healthy adults. Pers. Individual Differences. 2014;68:136–142. [Google Scholar]
- Baehr E.K., Revelle W., Eastman C.I. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J. Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Beal S.J., Grimm K.J., Dorn L.D., Susman E.J. Morningness–eveningness and physical activity in adolescent girls: menarche as a transition point. Child Dev. 2016 doi: 10.1111/cdev.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A., Adan A., Haldar P., Majumder D., Natale V., Randler C., Tonetti L., Sahu S. Exploration of transcultural properties of the reduced version of the Morningness-Eveningness Questionnaire (rMEQ) using adaptive neuro-fuzzy inference system. Biol. Rhythm Res. 2014;45:955–968. [Google Scholar]
- Burgess H.J., Fogg L.F. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carciofo R., Du F., Song N., Qi Y., Zhang K. Age‐related chronotype differences in Chinese, and reliability assessment of a reduced version of the Chinese Morningness–Eveningness Questionnaire. Sleep Biol. Rhythms. 2012;10(4):310–318. [Google Scholar]
- Di Milia L., Randler C. The stability of the Morning Affect Scale across age and gender. Pers. Individ. Diff. 2013;54:298–301. [Google Scholar]
- Di Milia L., Adan A., Natale V., Randler C. Reviewing the psychometric properties of contemporary circadian typology measures. Chronobiol. Int. 2013;30:1261–1271. doi: 10.3109/07420528.2013.817415. [DOI] [PubMed] [Google Scholar]
- Duarte L.L., Menna-Barreto L., Miguel M.A.L., Louzada F., Araújo J., Alam M. Chronotype ontogeny related to gender. Braz. J. Med. Biol. Res. 2014;47(4):316–320. doi: 10.1590/1414-431X20143001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.F., Cain S.W., Chang A.-M., Phillips A.J.K., Münch M.Y., Gronfier C., Wyatt J.K., Dijk D., Wright K.-P., Jr., Czeisler C.A. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson C.J. An effect size primer: a guide for clinicians and researchers. Prof. Psychol. 2009;40(5):532. [Google Scholar]
- Frey S., Balu S., Greusing S., Rothen N., Cajochen C. Consequences of the timing of menarche on female adolescent sleep phase preference. PLoS One. 2009;4:e5217. doi: 10.1371/journal.pone.0005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertini M., Graham C., Cook M.R. Self-report of circadian type reflects the phase of melatonin rhythm. Biol. Psychol. 1999;50:19–33. doi: 10.1016/s0301-0511(98)00049-0. [DOI] [PubMed] [Google Scholar]
- Horne J.A., Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Johnsen M.T., Wynn R., Allebrandt K., Bratlid T. Lack of major seasonal variations in self-reported sleep-wake rhythms and chronotypes among middle aged and older people at 69 degrees North: The Tromsø Study. Sleep Med. 2013;14:140–148. doi: 10.1016/j.sleep.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Kantermann T., Juda M., Merrow M., Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr. Biol. 2007;17:1996–2000. doi: 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Lane J.M., Vlasac I., Anderson S.G., Kyle S.D., Dixon W.G., Bechtold D.A., Gill S., Little M.A., Luik A., Loudon A., Emsley R., Scheer F.A.J.L., Lawlor D.A., Redline S., Ray D.W., Rutter M.K., Saxena R. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nature Commun. 2016;7(10889):1–10. doi: 10.1038/ncomms10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikanto I., Kronholm E., Peltonen M., Laatikainen T., Lahti T., Partonen T. Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol. Int. 2012;29(3):311–317. doi: 10.3109/07420528.2012.655870. [DOI] [PubMed] [Google Scholar]
- Natale V., Cicogna P.C. Morningness-eveningness dimensions: is it really a continuum? Pers. Individual Differences. 2002;32:809–816. [Google Scholar]
- Natale V., Esposito M.J., Martoni M., Fabbri M. Validity of the reduced version of the Morningness-Eveningness Questionnaire. Sleep Biol. Rhythms. 2006;4:72–74. [Google Scholar]
- Piffer D. Sleep Patterns and Sexual Selection: An Evolutionary Approach. Mank. Quarter. 2010;50:361–375. [Google Scholar]
- Rahafar A., Sadeghi M., Sadeghpour A., Heidari Z., Kasaeian A. Psychometric properties of the Persian version of the reduced Morningness-Eveningness Questionnaire: further evidence. Sleep Biol. Rhythms. 2015 [Google Scholar]
- Randler C. Gender differences in morningness-eveningness assessed by self-report questionnaires: a meta-analysis. Pers. Individ. Diff. 2007;43:1667–1675. [Google Scholar]
- Randler C. Psychometric properties of the German version of the Composite Scale of Morningness. Biol. Rhythm Res. 2008;39:151–161. [Google Scholar]
- Randler C. Validation of the full and reduced Composite Scale of Morningness. Biol. Rhythm Res. 2009;40:413–423. [Google Scholar]
- Randler C. German version of the reduced Morningness–Eveningness Questionnaire (rMEQ) Biol. Rhythm Res. 2013;44:730–736. [Google Scholar]
- Randler C. Sleep, sleep timing and chronotype in animal behavior. Anim. Behav. 2014;94:161–166. [Google Scholar]
- Randler C., Schaal S. Morningness-eveningness: habitual sleep-wake variables and cortisol levels. Biol. Psychol. 2010;85:14–18. doi: 10.1016/j.biopsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Randler C., Ebenhöh N., Fischer A., Höchel S., Schroff C., Stoll J.C., Vollmer C. Chronotype but not sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. 2012;37:1740–1744. doi: 10.1016/j.psyneuen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Randler C., Truc Y. Adaptation of the Composite Scale of Morningness for parent report and results from kindergarten children. Swiss J. Psychol. 2014;73:35–39. [Google Scholar]
- Randler C. Ontogeny of morningness-eveningness across the adult human lifespan. Sci. Nat. 2016;103(3):1–4. doi: 10.1007/s00114-015-1326-z. [DOI] [PubMed] [Google Scholar]
- Richardson J.T. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011;6(2):135–147. [Google Scholar]
- Roenneberg T., Kuehnle T., Pramstaller P.P., Ricken J., Havel M., Guth A., Merrow M. A marker for the end of adolescence. Curr. Biol. 2004;14:1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Tonetti L., Fabbri M., Natale V. Sex differences in sleep-time preference, and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents and adults. Chronobiol. Int. 2008;25:745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- Watson N.F., Buchwald D., Harden K.P. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J. Clin. Sleep Med. 2013;9(12):1333. doi: 10.5664/jcsm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L.K. The influence of chronotype in the daily lives of young children. Chronobiol. Int. 2016;33(3):268–279. doi: 10.3109/07420528.2016.1138120. [DOI] [PubMed] [Google Scholar]