Abstract
The exposure to plant defense chemicals has negative effects on insect feeding activity and modifies insect gut microbial community composition. Dendroctonus valens is a very destructive forest pest in China, and harbors a large diversity and abundance of gut microorganisms. Host pine defensive chemicals can protect the pines from attack by the holobiont. In this study, boring length of D. valens feeding on 0 mg/g α-pinene and 9 mg/g α-pinene concentration in phloem media for 6 and 48 h were recorded, and their gut bacterial communities were analyzed in parallel. Nine milligram per gram α-pinene concentration significantly inhibited boring length of D. valens and altered its gut microbial community structure after 6 h. The inhibition of boring length from 9 mg/g α-pinene in diets ceased after 48 h. No significant differences of the bacterial communities were observed between the beetles in 0 and 9 mg/g α-pinene concentration in phloem media after 48 h. Our results showed that the inhibition of the feeding behavior of D. valens and the disturbance to its gut bacterial communities in 9 mg/g α-pinene concentration in phloem media after 6 h were eliminated after 48 h. The resilience of gut bacterial community of D. valens may help the beetle catabolize pine defense chemical.
Keywords: Dendroctonus valens, α-pinene, gut microbiota
1. Introduction
Gut microbiota are ubiquitous for insect herbivores and are often critical to insect biological performance and ecological functioning, such as augmentation of nutritional availability [1,2,3], protection of insects from pathogens and parasites [4,5], facilitation of pheromone production [6,7], and detoxification of harmful toxins produced by the host plant defense system [8,9]. The gut microbiome has also been recognized as a major force in shaping insect-plant interactions [10,11,12]. In addition to degrading plant compounds that would supplement missing nutrients [13], these microbial symbionts are capable of tolerating and detoxifying defensive chemicals of host plants [9,14,15]. On the contrary, the defensive chemicals of host plants along with decreased nutrition adversely affect insects’ growth and development [16], which can influence herbivore’s gut microbial community compositions when herbivores feed on plant tissues [17,18,19].
Bark beetles (Curculionidae: Scolytinae) are known as the most damaging pests of coniferous forests worldwide, which has caused extensive conifer tree mortality and economic damage [20,21,22,23]. Conifers are able to defend themselves against attacks with various resistance mechanisms [24], e.g., elevated levels of induced defensive chemicals, including monoterpenes, which pose significant barriers to the beetles’ utilization of plant substrates [25,26,27,28]. In addition, the defensive chemicals influence bark beetles’ associated microorganisms including growth of vectored fungi [29,30], and survival of their associated bacteria [9,31]. Some of these microbes can assist bark beetle-microorganisms holobiont to degrade or metabolize the toxic chemicals of host plants [15,32], while others help to facilitate the beetles’ pheromone production ability [7], and thus have vital ecological functions for the adaptation and reproduction of beetles. Furthermore, at the community level, a metagenome-wide study of associated microbiota of Dendroctonus ponderosae showed that many genes belonging to several genera are involved in defensive terpene degradation [33].
The red turpentine beetle (RTB), Dendroctonus valens LeConte (Scolytinae), is a very destructive pest in China, and was introduced to China in the early 1980s from North America and has caused mortality of millions of healthy pine trees [23,34]. The diversity of the gut bacteria has been well investigated using culture dependent method, and some of the gut bacteria have been proven to simultaneously help produce the pheromone verbenone of D. valens and degrade 20%–50% host defensive chemical α-pinene [7,9]. Meanwhile, the tolerance of three D. valens gut bacteria to different concentrations of α-pinene are species-dependent [9]. Although we know the toxic activities of α-pinene to both D. valens and its gut bacteria [9,35,36], the influence of α-pinene on the feeding behavior of D. valens and its gut bacterial community structure in vivo is little known. The goal of this study was to study how pine defensive chemical α-pinene influences feeding behavior of D. valens, and to further test whether the chemical influences its gut bacterial community structure.
2. Results
2.1. The Influence of α-Pinene on the Boring Length of Dendroctonus valens
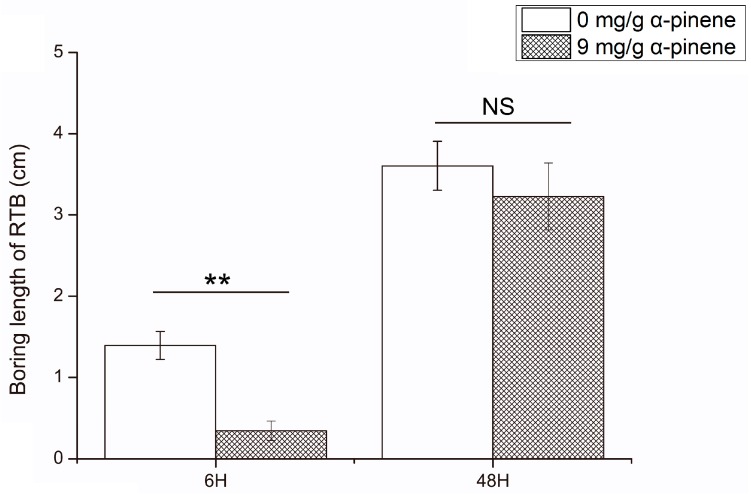
The boring length of D. valens in 9 mg/g α-pinene 6 h feeding group was significantly less than those in 0 mg/g α-pinene 6 h feeding group (Mann–Whitney U-test, p < 0.05, Figure 1). There were no significant differences of boring length of D. valens between 0 mg/g α-pinene 48 h feeding group and 9 mg/g α-pinene 48 h feeding group.
Figure 1.
The boring length (Mean ± SEM) of Dendroctonus valens feeding in 0 and 9 mg/g α-pinene phloem media after 6 h and 48 h. The data were analyzed using independent t-test or Mann–Whitney U-test depending on the results of the test of normality and homogeneity of variance. Asterisks indicate a statistically significant difference (** p < 0.01); NS, not significant; RTB, red turpentine beetle.
2.2. Alpha Diversity
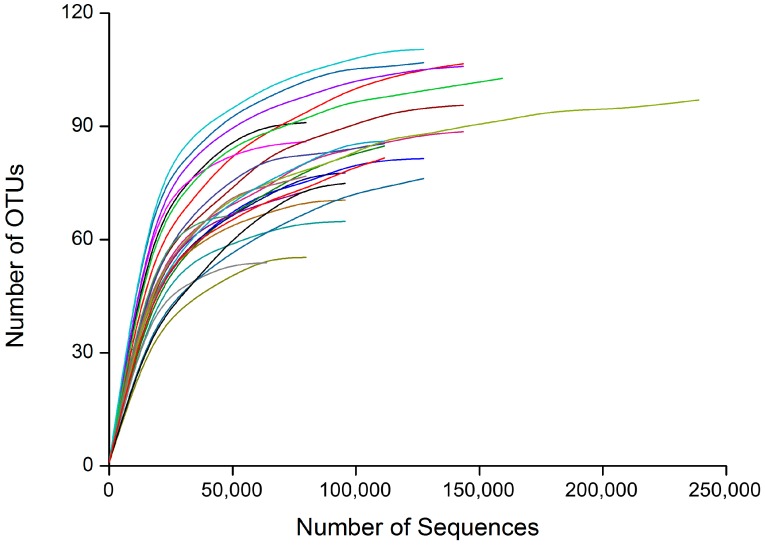
In the 25 representative gut samples of D. valens from four different treatments, we obtained a total of 2,614,017 sequences (99.8% of the total trimmed 2,618,895) and grouped into 2722 Operational Taxonomic Units (OTUs) at 97% similarity cut-off level. Rarefaction analysis showed that the OTUs of 25 samples gradually increased and then reached stable values with the increase of the number of measured sequences, which indicate that most bacterial sequences obtained by the MiSeq sequencing system reflected the abundance and diversity of the microbiota (Figure 2). Alpha diversity was estimated by five indices including number of OTUs, ACE, Chao1, Shannon, and Simpson. There were no significant differences among four groups in three diversity indices (Number of OTUs, Chao1 index, ACE index) (Table 1). Shannon diversity index of the samples in 9 mg/g α-pinene 48 h feeding group was significantly lower than the index of other three feeding groups (Table 1; F3,21 = 4.31, p < 0.05). Simpson diversity index of the samples in 9 mg/g α-pinene 6 h feeding group was significantly higher than the index in 0 mg/g α-pinene 6 h feeding group (Table 1; F3,21 = 16.07, p < 0.05).
Figure 2.
Rarefaction curves of the 20 samples (different color lines) based on MiSeq sequencing of bacterial communities. OTUs, Operational Taxonomic Units.
Table 1.
Comparison of diversity indices (Mean ± SEM) among different groups. Different superscript letters indicate significant differences across treatments (p < 0.05); OTUs, Operational Taxonomic Units.
| Index | 0 mg/g α-Pinene 6 h | 9 mg/g α-Pinene 6 h | 0 mg/g α-Pinene 48 h | 9 mg/g α-Pinene 48 h |
|---|---|---|---|---|
| Number of OTUs | 273.8 ± 28.62 | 306.6 ± 30.14 | 277.0 ± 26.36 | 268.1 ± 17.76 |
| ACE diversity | 455.4 ± 57.56 | 527.5 ± 54.89 | 441.2 ± 62.43 | 402.4 ± 33.63 |
| Chao diversity | 443.0 ± 49.51 | 532.7 ± 52.08 | 449.8 ± 64.44 | 403.1 ± 35.85 |
| Shannon diversity (H) | 1.62 ± 0.09 b | 1.86 ± 0.02 b | 1.61 ± 0.14 b | 1.52 ± 0.05 a |
| Simpson diversity | 0.49 ± 0.02 a,b | 0.60 ± 0.00 b | 0.48 ± 0.02 a,b | 0.46 ± 0.02 a |
2.3. Principal Component Analysis of the Gut Microbiota of D. valens
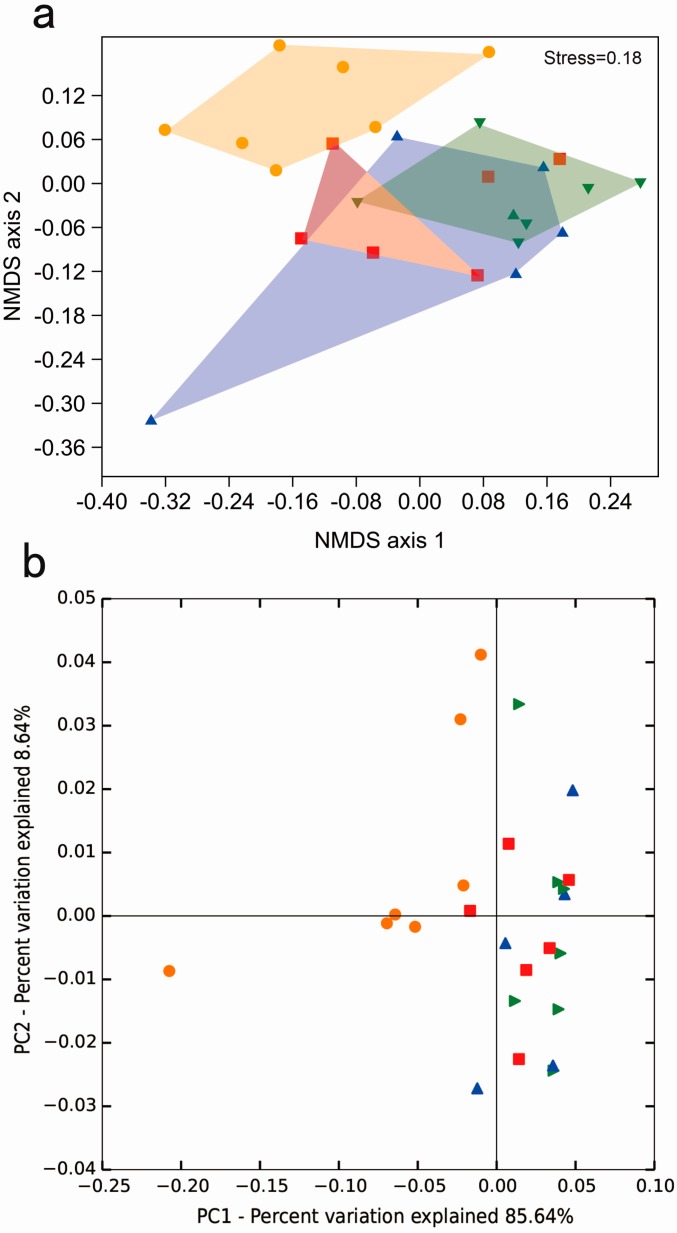
Based on the detected OTUs across the 25 samples, an non-metric multidimensional scaling (NMDS) ordination analysis result showed that D. valens gut bacterial communities in 9 mg/g α-pinene 6 h feeding group clustered independently and distinctly from other three feeding groups (0 mg/g α-pinene 6 h, 0 mg/g α-pinene 48 h, and 9 mg/g α-pinene 48 h), which were similar to each other, and the result was confirmed in the NMDS diagram using the Jaccard similarity metric (Figure 3a; analysis of similarities (ANOSIM), p < 0.001). Community compositions of gut samples in 9 mg/g α-pinene 6 h feeding group were significantly different from those gut samples in 0 mg/g α-pinene 6 h, 0 mg/g α-pinene 48 h, and 9 mg/g α-pinene 48 h feeding groups (Table S1, ANOSIM, p < 0.05), and no statistical difference exist in bacterial community composition of D. valens gut samples among 0 mg/g α-pinene 6 h, 0 mg/g α-pinene 48 h, and 9 mg/g α-pinene 48 h feeding groups (Table S1). The phylogeny-based weighted UniFrac principal coordinate analyses considering relative abundances of OTUs showed similar results, which was further corroborated by a dissimilarity test PERMANOVA (Figure 3b; PERMANOVA, p < 0.05).
Figure 3.
Principal component analysis of all bacterial communities. (a) Non-metric multidimensional scaling (NMDS) diagrams of 25 samples, based on Jaccard distance matrix for bacterial communities that consisted of OTUs (97% similarity level). Bacterial communities of samples in 9 mg/g α-pinene 6 h feeding group were significantly separated from the other three feeding groups (ANOSIM, p < 0.01); (b) Principal coordinate analysis (PCoA) plots based on the weighted UniFrac metric for bacterial communities. Permutational multivariate analysis of variance indicated the bacterial community of samples in 9 mg/g α-pinene 6 h feeding group was significantly different with the other three feeding groups (PERMAOVA, p = 0.001). The red square represents samples in 0 mg/g α-pinene 6 h feeding group, orange circle represents samples in 9 mg/g α-pinene 6 h feeding group, blue triangle represents samples in 0 mg/g α-pinene 48 h feeding group, and green triangle represents samples in 9 mg/g α-pinene 48 h feeding group.
2.4. The Analysis of Community Composition at Genus Levels
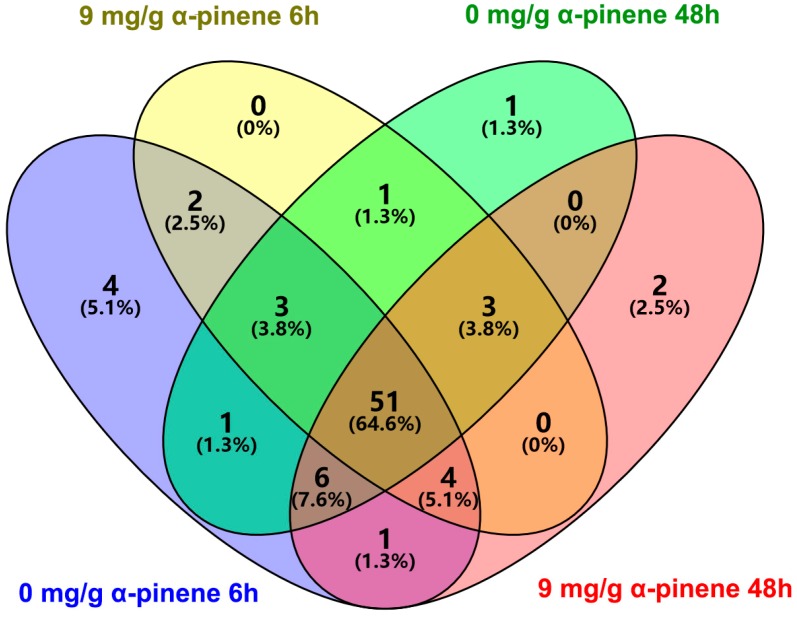
The sequences could be assigned to 79 genera and their abundance are shown in Table S2, and a total of 72 genera was shared by four different groups (Figure 4), which account for 98.66%–99.87% of the total sequences in the respective samples (Table S2). The genera with an abundance of at least 0.1% of the total number of reads in at least one sample are presented in Table 2. The gut samples in 9 mg/g α-pinene 6 h feeding group has a significantly higher proportion of Erwinia spp. and significantly lower proportion of Sphingomonas spp. than samples in 0 mg/g α-pinene 6 h, 0 mg/g α-pinene 6 h, and 9 mg/g α-pinene 48 h feeding groups (Table 2; Erwinia, One-way ANOVA, F3,21 = 9.02, p < 0.05; Sphingomonas, One-way ANOVA, F3,21 = 8.89, p < 0.05). The proportion of genera Burkholderia in gut samples of 9 mg/g α-pinene 6 h feeding group was significantly lower than those in gut samples of 9 mg/g α-pinene 48 h feeding group (Table 2; One-way ANOVA, F3,21 = 3.54, p < 0.05).
Figure 4.
Venn diagram (at distance 0.03) showing the shared and unique genera between 0 mg/g α-pinene 6 h, 9 mg/g α-pinene 6 h, 0 mg/g α-pinene 48 h, and 9 mg/g α-pinene 48 h feeding groups.
Table 2.
Shared genera among groups. Identity of the genera shared by four different groups and their average abundance (mean ± SEM) within each group are displayed. The genera that had an abundance of at least 0.1% of the total number of reads in at least one sample were present. The sum of all taxa present in the table within each group is shown in the last row of the table. Different superscript letters indicate significant differences across treatments (p < 0.05).
| Phylum | Phylogenetic Group (Genus) | 0 mg/g α-Pinene 6 h % | 9 mg/g α-Pinene 6 h % | 0 mg/g α-Pinene 48 h % | 9 mg/g α-Pinene 48 h % |
|---|---|---|---|---|---|
| Actinobacteria | Rhodococcus | 20.42 ± 0.86 | 17.15 ± 1.99 | 20.48 ± 2.40 | 21.58 ± 0.99 |
| Salinibacterium | 0.93 ± 0.20 | 1.00 ± 0.17 | 1.17 ± 0.29 | 1.19 ± 0.20 | |
| Proteobacteria | Burkholderia | 1.05 ± 0.16 a,b | 0.69 ± 0.12 a | 1.22 ± 0.09 a,b | 1.33 ± 0.19 b |
| Erwinia | 5.80 ± 1.96 b | 24.41 ± 5.74 a | 4.05 ± 2.30 b | 2.37 ± 1.17 b | |
| Pseudomonas | 0.26 ± 0.06 | 0.10 ± 0.03 | 0.29 ± 0.09 | 0.36 ± 0.12 | |
| Sphingomonas | 70.35 ± 1.62 b | 55.85 ± 4.26 a | 71.69 ± 1.30 b | 72.24 ± 1.56 b | |
| Sum | 98.81 ± 0.00 | 99.20 ± 0.00 | 98.91 ± 0.00 | 99.06 ± 0.00 |
3. Discussion
The toxic activities and the influence of host defensive chemicals on bark beetles’ associated microbes at species level have already been investigated [9,31,37]. Host defensive chemical α-pinene has been shown to attract bark beetles [28,38], and is toxic to beetles at high concentration [25,28,36]. Some studies showed that α-pinene could be converted to verbenol which is a precursor chemical of D. valens verbenone pheromone [7,39]. The current study evaluated the influence of chemical defensive chemical α-pinene on the gut microbiota of Chinese D. valens in vivo using high throughput pyrosequencing approach, and results showed that the concentration of host defensive monoterpene α-pinene in diets altered the gut bacterial community of D. valens (Figure 3). Specifically, the gut bacterial communities of D. valens in the 9 mg/g α-pinene 6 h feeding group were significantly different in the beetles feeding on 0 mg/g α-pinene phloem media after 6 h (Figure 3). This suggests that high concentrations of α-pinene are capable of altering the community structure of D. valens gut microbiota within a short time period (6 h). Similar results have shown that plant defensive chemicals influence herbivore insect gut microbiota in other systems, e.g., aspen defense chemicals were reported to influence the midgut bacterial community composition of Lymantria dispar L. [19,40], and the gut microbial community structures of Neotoma bryanti and Neotoma lepida were altered by plant secondary metabolites [41].
The community structure change of the gut microbiota of D. valens feeding in 9 mg/g α-pinene concentration in phloem media after 6 h (Figure 3) may be linked to the toxic effects of the monoterpene to the microorganisms. Previous studies suggested that the amount of host defensive monoterpenes in beetles’ guts may accumulate to a very high level when feeding on substrates containing high concentration of the chemicals [38,42], and the tolerance of microorganisms to different concentrations of α-pinene is species dependent [9,31]. As the concentrations of α-pinene in D. valens guts in vivo were not quantified and the influence of the chemical to each gut bacteria was not assayed, this speculation need more evidence to support our claim. Meanwhile, the community structure change of gut microbiota might be due to the antifeedant effects of the defense chemical on the bark beetles (Figure 1), which would subsequently influence both nutrition and monoterpene intake of the beetle. The toxic effects of host defense chemicals like monoterpenes on bark beetles have been documented in previous studies [9,36] and other systems [43,44]. In addition to toxic substances, the available nutrition contained in food was also reported to influence the gut bacterial community structure [17,45]. We found that the relative percentage of Erwinia in 9 mg/g α-pinene 6 h feeding group increased compared to other groups (Table 2). The increase of Erwinia may attribute to the decreasing numbers of bacteria in genera Sphingomonas and Rhodococcus since the bacteria in genus Erwinia are sensitive to high concentration of α-pinene. The antifeedant effect of 9 mg/mL α-pinene at 6 h would preclude the chemical from entering the beetle’s intestinal track, which may promote its growth. Therefore, whether the differences of the community composition are caused by toxic activities of α-pinene to gut bacteria, antifeedant effects, decreased nutrition intake of D. valens, or a combination of them requires further research to verify the mechanism.
Furthermore, our evidence suggests that the influence of α-pinene on the D. valens gut bacterial community is not permanent and irreversible, and the structure change of D. valens gut microbiota of beetles feeding on 9 mg/g α-pinene in phloem media after 6 h has been recovered to a stable status after 48 h (no significance of gut community structures among 0 mg/g α-pinene 6 h, 0 mg/g α-pinene 48 h, and 9 mg/g α-pinene 48 h feeding groups) (Figure 3). A stable gut community structure is very important for insect hosts’ growth and survival, which has been proven in mammal animals and insects [46,47,48]. For example, the gut microbiota of Pyrrhocoris apterus is remarkably stable, which plays an important role for host nutrition [48,49]. The mating preference of Drosophila melanogaster could be irreversibly abolished by antibiotic treatment, which would influence its intestinal microbiota [46,50]. The functional importance of the stability of the gut bacterial community to D. valens need more research to explore and verify.
Our results support the hypothesis that the gut microbiota of D. valens is capable of helping the beetle detoxify host pine defensive chemicals. The three most abundant genera found in D. valens gut microbiota in our study are known to play a role in toxin metabolism (Table 2). The most predominant genus, Sphingomonas (Table 2), is reported to catabolize monoterpenes and other aromatic compounds [51,52,53]. The bacteria in genera Rhodococcus and Burkholderia, which also have high abundance in D. valens gut bacteria communities (Table 2), are reported to degrade monoterpenes, e.g., α-pinene, limonene [54,55,56]. It is also possible that other less abundant gut microbes (e.g., bacteria in genus Pseudomonas) help the beetle catabolize and further detoxify plant defense chemicals or perhaps other functions related to detoxification processes, such as free-radical scavenging [33,41]. In the future, we plan to conduct community metagenomic sequencing to learn about gene-centred details relevant to the detoxification of pine terpene defenses by the gut microbiota of D. valens.
4. Materials and Methods
4.1. Insects
Adult beetles were captured during the dispersal phase using Lindgren funnel traps baited with kairomone lure ((+)-α-pinene: (−)-β-pinene: (+)-3-carene = 1:1:1) (Aldrich, Shanghai, China) from May to June 2014. Field trapping was conducted in the Tunlanchuan Forestry Station (N 37°48′, E 111°44′, average elevation 1400 m), west of Gujiao, China. Sexes of bark beetles were distinguished by listening for stridulation produced by males [57,58]. Log bolts (≥30 cm) were cut into 0.5 m lengths and two pairs of adult beetles were introduced to each bolt after the cut ends of bolts were waxed, then bolts were placed in plastic boxes (40 cm diameter, 50 cm height) at room temperature [9,36]. Beetles from the next generation were collected as they emerged from infested bolts.
4.2. The Boring Lengths of D. valens at 0 and 9 mg/g Concentration of Host Defensive Compound α-Pinene
To make phloem medium, Pinus tabuliformis phloem was freeze-dried, ground, and autoclaved to sterilize and remove volatile monoterpenes, as described previously [7]. Six grams of agar (NewProbe, Beijing, China) was mixed with 180 mL boiling distilled water and twelve grams of ground phloem, then the corresponding amount of α-pinene was dissolved in pentane (HPLC purity; J&K Scientific) [36], and it was added into medium after cooling to about 50 °C (final concentration of α-pinene: 0, 9 mg/g, respectively) [7]. Nine milligram per gram was set as a concentration of α-pinene of host pines after beetles’ attack because the mean quantities of α-pinene range from 0.1 mg/g to 1.6 mg/g in phloem tissue of healthy tree [36], and the concentration of the chemical around beetle’s galleries would increase 5–10-folds after beetles’ attack [59]. About 3.5 mL of phloem medium was then poured into each glass tube (1 cm diameter, 5 cm height), and the glass tubes were stoppered at both ends by plastic caps and sealed with parafilm, and then phloem medium was dried for 12 h.
Adult beetles (n = 200) were randomly chosen and separated into two groups and weighed, and then introduced into the media individually after they had been starved for 12 h. It has been reported that the long chain of behavioral steps of bark beetles from landing to continued oviposition was divided into several phases, and the beetles walk on the logs under attack within the first day, and then they would complete nuptial chamber construction in the next 1–2 days [60]. Therefore, 6 and 48 h was selected as two time-points. After 6 h feeding at room temperature, the boring lengths of half of the beetles in each group were measured (40 beetles in 0 mg/g α-pinene concentration in phloem media, 47 beetles in 9 mg/g α-pinene concentration in phloem media) (AL 204, Mettler Toledo, Inc., Shanghai, China), and 13 beetles (6 beetles in 0 mg/g α-pinene concentration in phloem media, 7 beetles in 9 mg/g α-pinene concentration in phloem media) were randomly chosen for gut microbiota analysis. After 48 h feeding, the boring lengths of the remaining beetles were measured individually (39 beetles in 0 mg/g α-pinene concentration in phloem media, 39 beetles in 9 mg/g α-pinene concentration in phloem media), and 12 beetles (5 beetles in 0 mg/g α-pinene concentration in phloem media, 7 beetles in 9 mg/g α-pinene concentration in phloem media) were randomly chosen for gut microbiota analysis. Boring lengths for those beetles that failed to enter the media were scored as zero and dead beetles were discarded.
4.3. DNA Extraction, PCR, Pyrosequencing, and Sequence Processing
The beetles from 0 mg/g α-pinene phloem media and 9 mg/g α-pinene phloem media after 6 h (0 mg/g α-pinene 6 h feeding group and 9 mg/g α-pinene 6 h feeding group) and 48 h (0 mg/g α-pinene 48 h feeding group and 9 mg/g α-pinene 48 h feeding group) were dissected, and then the bacteria genomic DNA of each insect gut sample was extracted by using a TIANamp Bacteria DNA kit (TianGen, Beijing, China) according to the manufacturer’s instructions, respectively. The PCR reactions were carried out in a 20 μL of solution containing 10 ng of DNA, 1 μL of 10 μM of each primer, 2 μL of 2.5 mM dNTPs, 0.3 μL Fastpfu polymerase (Transgene, Beijing, China), and 4 μL 5× Fastpfu buffer. The amplifications were performed in an ABI GeneAmp® 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) with an initial denaturation step at 95 °C for 10 min followed by 30 cycles of annealing and extending (each cycle occurred at 95 °C for 30 s followed by 55 °C for 30 s and an extension step at 72 °C for 45 s) and the final extension at 72 °C for 10 min using 16S rRNA primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [61]. The final PCR products were analyzed by electrophoresis in 1.5% agarose gel followed by staining with ethidium bromide and visualization under ultraviolet light. The purified amplicons were pyrosequenced on an Illumina platform (Illumina MiSeq PE250, Illumina, CA, USA).
Paired-end reads were assembled with FLASH (V1.2.7, Available online: www.ccb.jhu.edu) and low-quality reads were filtered using the QIIME (Quantitative Insights Into Microbial Ecology) software packages (V1.9.0, Available online: www.qiime.org) with default parameters [62]. Chimeras were checked and removed with UCHIME [63] and qualified sequences were clustered into Operational Taxonomic Units (OTUs) at 97% sequence similarity with a UPARSE algorithm [64]. The representative OTU was selected based on the most abundant sequence in each OTU, and then taxonomic identification was performed using the RDP classifier [65] algorithm implemented in QIIME and using the Greengene database under a confidence threshold of 80% (Available online: http://greengenes.secondgenome.com) [66].
4.4. Statistical Analysis
In comparisons of the boring length of beetles between 0 and 9 mg/g α-pinene feeding groups, means of cases were tested using independent t-test or Mann–Whitney U-test, depending on the results of the test of normality and homogeneity of variance. Data were analyzed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA) for Windows, and figures were drawn using Origin 8.5 (Origin Lab Corporation, Northampton, MA, USA).
For MiSeq data analysis, rarefaction curves were estimated using the “alpha_rarefaction.py” script in QIIME to test whether the sequencing efforts adequately represented the bacterial diversity within each sample. Two richness estimators (the abundance-based coverage estimator (ACE) and a nonparametric richness estimator based on distribution of singletons and doubletons (Chao1)) and two diversity indices (Shannon and Simpson index) were calculated for the samples using the “alpha_diversity.py” script in QIIME. The diversity indices of four groups and the relative abundances of different genera were compared using One-way ANOVA test followed by Bonferroni test (equal variances) or One-way Brown-Forsythe’s ANOVA test followed by Dunnett’s T3 test (unequal variances). Non-metric multidimensional scaling (NMDS) was used to visualize the phylogenetic distance (Jaccard similarity) between the bacterial communities from different samples. Composition differences were tested using ANOSIM with 10,000 permutations using PAST software [67,68]. The representative sequences of all OTUs were used to construct neighbor-joining trees. The phylogenetic tree together with sample sequence abundance data were used for weighted Unifrac PCoA (principal coordinate analysis) which considers both relative abundance and different branch lengths in a tree, through the online Fast Unifrac program [69]. A Permutational Multivariate Analysis of Variance based on the weighted UniFrac distance (PERMANOVA, “PermanovaG” function in the “GUniFrac” package of R) was used to test for differences in community composition between four sample groups [70].
5. Conclusions
In summary, our results suggested that 9 mg/g α-pinene concentration significantly inhibited feeding behavior of D. valens and altered its gut microbial community structure after 6 h. The inhibition of feeding behavior from 9 mg/g α-pinene in diets ceased after 48 h. No significant differences of the bacterial communities were observed between the beetles in 0 and 9 mg/g α-pinene concentration in phloem media after 48 h. We found that both the inhibition of the feeding behavior of D. valens and the disturbance to its gut bacterial communities by high concentration of host defensive α-pinene in phloem media after 6 h were eliminated soon after 48 h, which suggests the quick adaptation of both the beetle and its gut microflora to high concentration of host defensive chemical.
Acknowledgments
We appreciate Jacob Wickham (Institute of Zoology, Chinese Academy of Science) for reviews of earlier versions of the manuscript. We thank Zhudong Liu (Institute of Zoology, Chinese Academy of Sciences) and Jianqiang Li for field assistance. This work was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB11050000) and the National Natural Science Foundation of China (31222013 and 31110103903), and the State Key Laboratory of Integrated Management of Pest Insects and Rodents (Grant No. ChineseIPM1411).
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/17/10/1734/s1.
Author Contributions
Letian Xu, Min Lu and Jianghua Sun conceived and designed the experiments; Letian Xu performed the experiments and analyzed the data; Letian Xu prepared the manuscript; and Zhanghong Shi, Bo Wang, Min Lu, Jianghua Sun edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Geib S.M., Filley T.R., Hatcher P.G., Hoover K., Carlson J.E., del Mar Jimenez-Gasco M., Nakagawa-Izumi A., Sleighter R.L., Tien M. Lignin degradation in wood-feeding insects. Proc. Natl. Acad. Sci. USA. 2008;105:12932–12937. doi: 10.1073/pnas.0805257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morales-Jiménez J., de León A.V.-P., García-Domínguez A., Martínez-Romero E., Zúñiga G., Hernández-Rodríguez C. Nitrogen-fixing and uricolytic bacteria associated with the gut of Dendroctonus rhizophagus and Dendroctonus valens (Curculionidae: Scolytinae) Microb. Ecol. 2013;66:200–210. doi: 10.1007/s00248-013-0206-3. [DOI] [PubMed] [Google Scholar]
- 3.Ayayee P., Rosa C., Ferry J.G., Felton G., Saunders M., Hoover K. Gut microbes contribute to nitrogen provisioning in a wood-feeding cerambycid. Environ. Entomol. 2014;43:903–912. doi: 10.1603/EN14045. [DOI] [PubMed] [Google Scholar]
- 4.Dillon R., Vennard C., Buckling A., Charnley A. Diversity of locust gut bacteria protects against pathogen invasion. Ecol. Lett. 2005;8:1291–1298. doi: 10.1111/j.1461-0248.2005.00828.x. [DOI] [Google Scholar]
- 5.Koch H., Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl. Acad. Sci. USA. 2011;108:19288–19292. doi: 10.1073/pnas.1110474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillon R., Vennard C., Charnley A. A note: Gut bacteria produce components of a locust cohesion pheromone. J. Appl. Microbiol. 2002;92:759–763. doi: 10.1046/j.1365-2672.2002.01581.x. [DOI] [PubMed] [Google Scholar]
- 7.Xu L., Lou Q., Cheng C., Lu M., Sun J. Gut-associated bacteria of Dendroctonus valens and their involvement in verbenone production. Microb. Ecol. 2015;70:1012–1023. doi: 10.1007/s00248-015-0625-4. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi Y., Hayatsu M., Hosokawa T., Nagayama A., Tago K., Fukatsu T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA. 2012;109:8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L., Lu M., Sun J. Invasive bark beetle-associated microbes degrade a host defensive monoterpene. Insect Sci. 2016;23:183–190. doi: 10.1111/1744-7917.12255. [DOI] [PubMed] [Google Scholar]
- 10.Feldhaar H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011;36:533–543. doi: 10.1111/j.1365-2311.2011.01318.x. [DOI] [Google Scholar]
- 11.Douglas A.E. Microbial brokers of insect-plant interactions revisited. J. Chem. Ecol. 2013;39:952–961. doi: 10.1007/s10886-013-0308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer T.J., Bowers M.D. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia. 2015;179:1–14. doi: 10.1007/s00442-015-3327-1. [DOI] [PubMed] [Google Scholar]
- 13.Douglas A. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009;23:38–47. doi: 10.1111/j.1365-2435.2008.01442.x. [DOI] [Google Scholar]
- 14.Dowd P. Symbiont-mediated detoxification in insect herbivores. In: Barbosa P., Krischik V.A., Jones C.G., editors. Microbial Mediation of Plant-Herbivore Interactions. John Wiley & Sons; New York, NY, USA: 1991. pp. 411–440. [Google Scholar]
- 15.Boone C.K., Keefover-Ring K., Mapes A.C., Adams A.S., Bohlmann J., Raffa K.F. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J. Chem. Ecol. 2013;39:1003–1006. doi: 10.1007/s10886-013-0313-0. [DOI] [PubMed] [Google Scholar]
- 16.Levin D.A. The chemical defenses of plants to pathogens and herbivores. Annu. Rev. Ecol. Syst. 1976;7:121–159. doi: 10.1146/annurev.es.07.110176.001005. [DOI] [Google Scholar]
- 17.Colman D., Toolson E., Takacs-Vesbach C. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012;21:5124–5137. doi: 10.1111/j.1365-294X.2012.05752.x. [DOI] [PubMed] [Google Scholar]
- 18.Gayatri Priya N., Ojha A., Kajla M.K., Raj A., Rajagopal R. Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS ONE. 2012;7:e30768. doi: 10.1371/journal.pone.0030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason C., Rubert-Nason K., Lindroth R., Raffa K. Aspen defense chemicals influence midgut bacterial community composition of gypsy moth. J. Chem. Ecol. 2015;41:75–84. doi: 10.1007/s10886-014-0530-1. [DOI] [PubMed] [Google Scholar]
- 20.Raffa K.F., Berryman A.A. Interacting selective pressures in conifer-bark beetle systems: A basis for reciprocal adaptations? Am. Nat. 1987;129:234–262. doi: 10.1086/284633. [DOI] [Google Scholar]
- 21.Paine T.D., Raffa K.F., Harrington T.C. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 1997;42:179–206. doi: 10.1146/annurev.ento.42.1.179. [DOI] [PubMed] [Google Scholar]
- 22.Gitau C.W., Bashford R., Carnegie A.J., Gurr G.M. A review of semiochemicals associated with bark beetle (Coleoptera: Curculionidae: Scolytinae) pests of coniferous trees: A focus on beetle interactions with other pests and their associates. For. Ecol. Manag. 2013;297:1–14. doi: 10.1016/j.foreco.2013.02.019. [DOI] [Google Scholar]
- 23.Sun J., Lu M., Gillette N.E., Wingfield M.J. Red turpentine beetle: Innocuous native becomes invasive tree killer in China. Annu. Rev. Entomol. 2013;58:293–311. doi: 10.1146/annurev-ento-120811-153624. [DOI] [PubMed] [Google Scholar]
- 24.Lieutier F. Mechanisms and Deployment of Resistance in Trees to Insects. Springer; Berlin/Heidelberg, Germany: 2002. Mechanisms of resistance in conifers and bark beetle attack strategies; pp. 31–77. [Google Scholar]
- 25.Smith R.H. Toxicity of pine resin vapors to three species of Dendroctonus bark beetles. J. Econ. Entomol. 1963;56:827–831. doi: 10.1093/jee/56.6.827. [DOI] [Google Scholar]
- 26.Byers J.A. Pheromone biosynthesis in the bark beetle, Ips paraconfusus, during feeding or exposure to vapours of host plant precursors. Insect Biochem. 1981;11:563–569. doi: 10.1016/0020-1790(81)90024-X. [DOI] [Google Scholar]
- 27.Phillips M.A., Croteau R.B. Resin-based defenses in conifers. Trends Plant Sci. 1999;4:184–190. doi: 10.1016/S1360-1385(99)01401-6. [DOI] [PubMed] [Google Scholar]
- 28.Seybold S.J., Huber D.P.W., Lee J.C., Graves A.D., Bohlmann J. Pine monoterpenes and pine bark beetles: A marriage of convenience for defense and chemical communication. Phytochem. Rev. 2006;5:143–178. doi: 10.1007/s11101-006-9002-8. [DOI] [Google Scholar]
- 29.Klepzig K., Smalley E., Raffa K. Combined chemical defenses against an insect-fungal complex. J. Chem. Ecol. 1996;22:1367–1388. doi: 10.1007/BF02027719. [DOI] [PubMed] [Google Scholar]
- 30.Hofstetter R.W., Mahfouz J.B., Klepzig K.D., Ayres M.P. Effects of tree phytochemistry on the interactions among endophloedic fungi associated with the southern pine beetle. J. Chem. Ecol. 2005;31:539–560. doi: 10.1007/s10886-005-2035-4. [DOI] [PubMed] [Google Scholar]
- 31.Adams A.S., Boone C.K., Bohlmann J., Raffa K.F. Responses of bark beetle-associated bacteria to host monoterpenes and their relationship to insect life histories. J. Chem. Ecol. 2011;37:808–817. doi: 10.1007/s10886-011-9992-6. [DOI] [PubMed] [Google Scholar]
- 32.Raffa K.F. Terpenes tell different tales at different scales: Glimpses into the chemical ecology of conifer-bark beetle-microbial interactions. J. Chem. Ecol. 2014;40:1–20. doi: 10.1007/s10886-013-0368-y. [DOI] [PubMed] [Google Scholar]
- 33.Adams A.S., Aylward F.O., Adams S.M., Erbilgin N., Aukema B.H., Currie C.R., Suen G., Raffa K.F. Mountain pine beetles colonizing historical and naïve host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl. Environ. Microbiol. 2013;79:3468–3475. doi: 10.1128/AEM.00068-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng C., Zhou F., Lu M., Sun J. Inducible pine rosin defense mediates interactions between an invasive insect–fungal complex and newly acquired sympatric fungal associates. Integr. Zool. 2015;10:453–464. doi: 10.1111/1749-4877.12138. [DOI] [PubMed] [Google Scholar]
- 35.López M.F., Cano-Ramírez C., Shibayama M., Zúñiga G. α-Pinene and myrcene induce ultrastructural changes in the midgut of Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae) Ann. Entomol. Soc. Am. 2011;104:553–561. doi: 10.1603/AN10023. [DOI] [Google Scholar]
- 36.Xu B., Liu Z., Sun J.H. The effects of α-pinene on the feeding performance and pheromone production of Dendroctonus valens. Entomol. Exp. Appl. 2014;150:269–278. doi: 10.1111/eea.12161. [DOI] [Google Scholar]
- 37.Adams A.S., Currie C.R., Cardoza Y., Klepzig K.D., Raffa K.F. Effects of symbiotic bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can. J. For. Res. 2009;39:1133–1147. doi: 10.1139/X09-034. [DOI] [Google Scholar]
- 38.Hobson K.R., Wood D.L., Cool L.G., White P.R., Ohtsuka T., Kubo I., Zavarin E. Chiral specificity in responses by the bark beetle Dendroctonus valens to host kairomones. J. Chem. Ecol. 1993;19:1837–1846. doi: 10.1007/BF00983790. [DOI] [PubMed] [Google Scholar]
- 39.Brand J.M., Bracke J.W., Markovetz A.J., Wood D.L., Browne L.E. Production of verbenol pheromone by a bacterium isolated from bark beetles. Nature. 1975;254:136–137. doi: 10.1038/254136a0. [DOI] [PubMed] [Google Scholar]
- 40.Mason C.J., Couture J.J., Raffa K.F. Plant-associated bacteria degrade defense chemicals and reduce their adverse effects on an insect defoliator. Oecologia. 2014;175:901–910. doi: 10.1007/s00442-014-2950-6. [DOI] [PubMed] [Google Scholar]
- 41.Kohl K.D., Dearing M.D. Experience matters: Prior exposure to plant toxins enhances diversity of gut microbes in herbivores. Ecol. Lett. 2012;15:1008–1015. doi: 10.1111/j.1461-0248.2012.01822.x. [DOI] [PubMed] [Google Scholar]
- 42.Birgersson G., Schlyter F., Lofqvist J., Bergstrom G. Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J. Chem. Ecol. 1984;10:1029–1055. doi: 10.1007/BF00987511. [DOI] [PubMed] [Google Scholar]
- 43.Wallin K.F., Raffa K.F. Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae) Environ. Entomol. 2000;29:442–453. doi: 10.1603/0046-225X-29.3.442. [DOI] [Google Scholar]
- 44.Reid M., Purcell J.R.C. Condition-dependent tolerance of monoterpenes in an insect herbivore. Arthropod Plant Interact. 2011;5:331–337. doi: 10.1007/s11829-011-9137-4. [DOI] [Google Scholar]
- 45.Santo Domingo J., Kaufman M., Klug M., Holben W., Harris D., Tiedje J. Influence of diet on the structure and function of the bacterial hindgut community of crickets. Mol. Ecol. 1998;7:761–767. doi: 10.1046/j.1365-294x.1998.00390.x. [DOI] [Google Scholar]
- 46.Sharon G., Segal D., Zilber-Rosenberg I., Rosenberg E. Symbiotic bacteria are responsible for diet-induced mating preference in Drosophila melanogaster, providing support for the hologenome concept of evolution. Gut Microbes. 2011;2:190–192. doi: 10.4161/gmic.2.3.16103. [DOI] [PubMed] [Google Scholar]
- 47.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudakaran S., Salem H., Kost C., Kaltenpoth M. Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae) Mol. Ecol. 2012;21:6134–6151. doi: 10.1111/mec.12027. [DOI] [PubMed] [Google Scholar]
- 49.Salem H., Kreutzer E., Sudakaran S., Kaltenpoth M. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae) Environ. Microbiol. 2013;15:1956–1968. doi: 10.1111/1462-2920.12001. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka S., Kobayashi T., Songjinda P., Tateyama A., Tsubouchi M., Kiyohara C., Shirakawa T., Sonomoto K., Nakayama J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009;56:80–87. doi: 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 51.White D.C., Sutton S.D., Ringelberg D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996;7:301–306. doi: 10.1016/S0958-1669(96)80034-6. [DOI] [PubMed] [Google Scholar]
- 52.Thomassin-Lacroix E.J., Yu Z., Eriksson M., Reimer K.J., Mohn W.W. DNA-based and culture-based characterization of a hydrocarbon-degrading consortium enriched from Arctic soil. Can. J. Microbiol. 2001;47:1107–1115. doi: 10.1139/w01-125. [DOI] [PubMed] [Google Scholar]
- 53.Singer A.C. Phytoremediation Rhizoremediation. Springer; Berlin/Heidelberg, Germany: 2006. The chemical ecology of pollutant biodegradation: Bioremediation and phytoremediation from mechanistic and ecological perspectives; pp. 5–21. [Google Scholar]
- 54.Van der Werf M.J., Swarts H.J., de Bont J.A. Rhodococcus erythropolis DCL14 contains a novel degradation pathway for limonene. Appl. Environ. Microbiol. 1999;65:2092–2102. doi: 10.1128/aem.65.5.2092-2102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langolf B.M., Kleinheinz G.T. A lava rock-based biofilter for the treatment of α-pinene. Bioresour. Technol. 2006;97:1951–1958. doi: 10.1016/j.biortech.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Song Y.-J. Characterization of aromatic hydrocarbon degrading bacteria isolated from pine litter. Korean J. Microbiol. Biotechnol. 2009;37:333–339. [Google Scholar]
- 57.Breznak J.A. Intestinal microbiota of termites and other xylophagous insects. Ann. Rev. Microbiol. 1982;36:323–343. doi: 10.1146/annurev.mi.36.100182.001543. [DOI] [PubMed] [Google Scholar]
- 58.Murphy K.M., Teakle D.S., MacRae I.C. Kinetics of colonization of adult Queensland fruit flies (Bactrocera tryoni) by dinitrogen-fixing alimentary tract bacteria. Appl. Environ. Microbiol. 1994;60:2508–2517. doi: 10.1128/aem.60.7.2508-2517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leufvén A., Birgersson G. Quantitative variation of different monoterpenes around galleries of Ips typographus (Colleoptera: Scolytidae) attacking Norway spruce. Can. J. Bot. 1987;65:1038–1044. doi: 10.1139/b87-144. [DOI] [Google Scholar]
- 60.Zhang Q.H., Birgersson G., Schlyter F., Chen G.F. Pheromone components in the larch bark beetle, Ips cembrae, from China: Quantitative variation among attack phases and individuals. J. Chem. Ecol. 2000;26:841–858. doi: 10.1023/A:1005447922939. [DOI] [Google Scholar]
- 61.Yu Y., Lee C., Kim J., Hwang S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005;89:670–679. doi: 10.1002/bit.20347. [DOI] [PubMed] [Google Scholar]
- 62.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edgar R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clarke K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993;18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 68.Bohlmann J. Induced Plant Resistance to Herbivory. Springer; Berlin/Heidelberg, Germany: 2008. Insect-induced terpenoid defenses in spruce; pp. 173–187. [Google Scholar]
- 69.Six D., Paine T. Effects of mycangial fungi and host tree species on progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae) Environ. Entomol. 1998;27:1393–1401. doi: 10.1093/ee/27.6.1393. [DOI] [Google Scholar]
- 70.Shrimpton D.M. Resistance of lodgepole pine to mountain pine beetle infestation. In: Berryman A.A., Amman G.D., Stark R.W., Kibbee D.L., editors. Theroy and Practice of Mountain Pine Beetle Management in Lodgepole Pine Forests. University of Idaho Press; Moscow, ID, USA: 1978. pp. 64–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.