Abstract
The aim of this study was to evaluate the effect of a food supplement containing α-lipoic acid and of a placebo on glyco-metabolic control and on oxidative stress markers in type 2 diabetics. We randomized 105 diabetics to either a supplementation containing 600 mg of α-lipoic acid, 165 mg of L-carnosin, 7.5 mg of zinc, and vitamins of group B, or a placebo, for three months. We evaluated body mass index, fasting plasma glucose (FPG), post-prandial-glucose (PPG), glycated hemoglobin (HbA1c), fasting plasma insulin (FPI), HOMA-index (HOMA-IR), lipid profile, high sensitivity C-reactive protein (Hs-CRP), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA). There was a reduction of FPG, PPG, and HbA1c with the food supplement containing α-lipoic acid compared with a baseline, and with the placebo. Concerning lipid profile, we observed a reduction of LDL-C, and Tg with the food supplement, compared with both the baseline, and the placebo. There was a reduction of Hs-CRP with the food supplement containing α-lipoic acid, both compared with the baseline and the placebo. An increase of SOD, and GSH-Px, and a decrease of MDA were reached by the food supplement containing α-lipoic acid, both compared with the baseline and the placebo. We can conclude that the food supplement containing α-lipoic acid, L-carnosin, zinc, and vitamins of group B improved glycemic control, lipid profile, and anti-oxidative stress markers.
Keywords: α-lipoic acid, L-carnosin, malondialdehyde, oxidative stress
1. Introduction
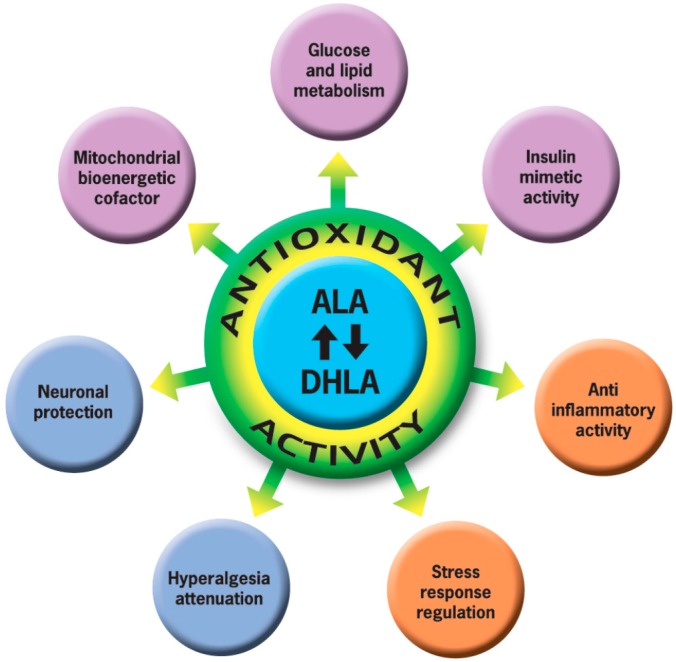
Type 2 diabetes mellitus is considered a risk factor for cardiovascular disease. Over 50% of diabetic patients have clinical manifestations of diabetic neuropathy. Diabetic neuropathy is defined by a progressive loss of nerve fibers due to a condition of chronic hyperglycemia; genetic and environmental factors also play an important role. Among all diabetic neuropathy cases, about 80% seems to be distal, symmetrical sensitive-motor neuropathy [1,2]. α-lipoic acid is the treatment of choice for treating diabetic neuropathy, in combination with symptomatic therapy and physical treatment. α-lipoic acid plays the main role as the anti-oxidant (Figure 1) and metabolic component of some enzymatic reactions involved in glucose metabolism [3]. Endogenously synthesized α-lipoic acid is covalently bound to specific proteins, cofactors for mitochondrial dehydrogenase enzyme complexes [4]. In addition, there is an increasing interest in potential therapeutic uses of pharmacological doses of free α-lipoic acid [5]. α-lipoic acid’s anti-oxidant properties are due to several factors, including (1) its capacity to scavenge reactive oxygen species (ROS) directly; (2) its ability to regenerate endogenous anti-oxidants, such as glutathione and vitamins E and C; and (3) its metal-chelating activity, resulting in reduced ROS production. Moreover, due to its anti-oxidant properties, α-lipoic acid has recently been reported to afford protection against oxidative injury in various disease processes, including neurodegenerative disorders [6]. The efficacy of α-lipoic acid was confirmed by the SIDNEY 2 study [7], where results showed that oral treatment with α-lipoic acid taken for five weeks resulted in an improvement of neuropathic symptoms in patients affected by distal symmetric polyneuropathy. Regarding the mechanism through which neuropathy develops, previously published papers showed that hyperglycemia generates free radicals, contributing to the development and progression of diabetes complications. There is large evidence that there is a relationship between the severity of diabetic neuropathy and the frequency and duration of hyperglycemic periods [8]. For this reason, improving oxidative stress may be a way to reduce diabetic complications [9,10], and this is probably the way α-lipoic acid acts. However, studies aimed to confirm the effects of α-lipoic acid on glycemic control and on anti-oxidant parameters in humans are lacking.
Figure 1.
Biological functions of α-lipoic acid. α-lipoic acid (ALA) and its reduced form dihydrolipoic acid (DHLA) create a potent redox couple, often called a “universal anti-oxidant” for its capacity to regenerate several others anti-oxidants, such as vitamins C and E and glutathione. α-lipoic acid is also able to directly scavenge ROS, possesses metal chelating activity, and enhances the mitochondrial expression of key anti-oxidant enzymes. Through these properties, α-lipoic acid exerts different activities, from mitochondrial bioenergetics cofactor to stress response regulation and neuronal protection. Mitochondrial bioenergetic cofactor: α-lipoic acid is a key cofactor for mitochondrial bioenergetic enzymes, including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes, stimulating glucose and lipid metabolism. It acts as an insulin-mimetic agent, regulating the IR/PI3K/Akt pathway, so it enhances the uptake and the utilization of glucose, improving glycemic control. Stress response regulation: α-lipoic acid responds to stress factors, inhibiting stress induced transcription factor activation, such as NF-κB and AP-1, and modulating pro-inflammatory signaling, hence the anti-inflammatory activity. Neuronal protection: it is also well known that α-lipoic acid improves diabetic polyneuropathies, while attention has only recently been focused on the capacity to attenuate the hyperalgesia through the modulation of T-type calcium and transient receptor potential (TRPA1) channels.
For this reason, the aim of this study was to evaluate the effect of food supplements containing 600 mg of α-lipoic acid, 165 mg of L-carnosin, 7.5 mg of zinc, and vitamins of group B, and that of a placebo, taken once a day for three months, on glyco-metabolic control and on oxidative markers in type 2 diabetic patients.
2. Results
2.1. Study Sample
We enrolled 105 patients in this trial, 54 of which were randomized to the food supplement containing alpha-lipoic and 51 of which were randomized to the placebo. One patient in the group treated with food supplement, and two patients treated with the placebo did not complete the study; the reason for the premature withdrawal was lost to follow-up for all the three patients. Study population characteristics are described in Table 1.
Table 1.
Mean changes during the study.
| Parameters | Food Supplement Containing Alpha-Lipoic Acid | Placebo | ||
|---|---|---|---|---|
| – | Baseline | 3 Months | Baseline | 3 Months |
| Number | 54 | 53 | 51 | 49 |
| Sex (Male/Female) | 26/28 | 25/28 | 25/26 | 24/25 |
| Age (years) | 52.5 ± 7.9 | – | 53.1 ± 8.2 | – |
| Smoking status (M/F) | 12/10 | 11/10 | 13/12 | 13/12 |
| Height (m) | 1.68 ± 0.05 | – | 1.69 ± 0.06 | – |
| Weight (kg) | 80.1 ± 7.1 | 79.8 ± 6.9 | 80.4 ± 7.3 | 80.9 ± 7.6 |
| BMI (kg/m2) | 28.4 ± 2.5 | 28.3 ± 2.4 | 28.1 ± 2.2 | 28.3 ± 2.4 |
| FPG (mg/dL) | 119.5 ± 16.3 | 102.2 ± 9.6 *,° | 122.3 ± 17.1 | 120.4 ± 16.6 |
| PPG (mg/dL) | 164.2 ± 22.1 | 141.3 ± 18.4 *,° | 162.3 ± 21.2 | 159.1 ± 19.8 |
| HbA1c (%) | 7.8 ± 0.4 | 7.2 ± 0.3 *,° | 7.9 ± 0.5 | 7.7 ± 0.3 |
| FPI (µU/mL) | 11.1 ± 3.6 | 10.6 ± 3.3 | 10.2 ± 2.6 | 10.4 ± 2.8 |
| HOMA-IR | 3.26 ± 1.86 | 2.67 ± 1.23 *,° | 3.08 ± 1.64 | 3.09 ± 1.71 |
| TC (mg/dL) | 192.5 ± 17.3 | 171.3 ± 14.6 *,° | 191.7 ± 16.8 | 193.4 ± 17.7 |
| LDL-C (mg/dL) | 123.2 ± 11.5 | 105.7 ± 9.8 *,° | 122.5 ± 11.0 | 124.9 ± 11.8 |
| HDL-C (mg/dL) | 45.7 ± 5.5 | 46.1 ± 5.7 | 45.9 ± 5.6 | 45.6 ± 5.4 |
| Tg (mg/dL) | 118.2 ± 25.8 | 97.4 ± 19.5 *,° | 116.5 ± 24.2 | 114.7 ± 22.2 |
| Hs-CRP (mg/L) | 2.3 ± 0.6 | 1.8 ± 0.3 *,° | 2.5 ± 0.8 | 2.2 ± 0.7 |
| SOD (U/mL) | 94.7 ± 19.3 | 111.4 ± 24.9 *,° | 96.2 ± 21.4 | 98.1 ± 22.3 |
| GSH-Px (EE/U) | 97.2 ± 39.8 | 119.6 ± 43.3 *,° | 93.7 ± 36.5 | 94.4 ± 35.1 |
| MDA (nmol/mL) | 42.8 ± 18.5 | 33.9 ± 14.8 *,° | 44.6 ± 22.7 | 41.5 ± 20.8 |
Data are expressed as mean ± standard deviation; * p < 0.05 vs. baseline; ° p < 0.05 vs. placebo. BMI: body mass index; FPG: fasting plasma glucose; PPG: post-prandial-glucose; HbA1c: glycated hemoglobin; FPI: fasting plasma insulin; HOMA-IR: HOMA-index; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; Tg: triglycerides; Hs-CRP: high sensitivity C-reactive protein; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; MDA: malondialdehyde.
2.1.1. Body Weight
We did not record any variations in body weight or BMI.
2.1.2. Glycemic Control
There was a reduction of FPG, PPG, and HbA1c with the food supplement containing α-lipoic acid compared with the baseline (p < 0.05 for all) and with the placebo (p < 0.05 for all). There was a reduction in HOMA-IR, compared with both the baseline and the placebo (p < 0.05 for both).
2.2. Lipid Profile
We recorded a decrease in LDL-C and TG with the food supplement containing α-lipoic acid, compared with both the baseline (p < 0.05) and the placebo (p < 0.05 for both).
2.3. Inflammatory and Oxidative Parameters
A reduction of Hs-CRP was reduced by the food supplement containing α-lipoic acid, both compared with the baseline and with the placebo (p < 0.05 for both). An increase of SOD, and GSH-Px, and a decrease of MDA were reached with the food supplement containing α-lipoic acid, compared with both the baseline and the placebo.
2.4. Side Effects
No side effects were reported. No patients interrupted the food supplement containing α-lipoic acid due to side effects.
3. Discussion
Diabetes and insulin resistance are involved in the onset of cardiovascular and nervous diseases. The mechanism responsible for these disorders involves, mainly, oxidative stress generated by reactive oxygen species (ROS) and reactive nitrogen species (RNS). It has been largely reported that diabetes impairs endothelial nitric oxide synthase (eNOS) activity, thus increasing ROS production, with a consequent reduction of NO bioavailability. α-lipoic acid has shown beneficial effects both in the prevention and in the treatment of diabetes because of its insulin-mimetic and anti-inflammatory action; it is also involved in mitochondrial bioenergetic reactions [11]. The positive effects of α-lipoic acid on oxidative stress parameters have been shown by our study: α-lipoic acid increased SOD, an enzyme with the role of commuting highly reactive O2− into H2O2, which is subsequently reduced in H2O by mitochondrial glutathione peroxidase and catalase [12]. Throughout this mechanism, SOD neutralizes superoxide radicals, as reported in diabetic peripheral nerve tissue [13,14,15]. Furthermore, SOD inhibits inflammatory response, with a consequent reduction of hyperalgesia, typical of diabetic neuropathy [16]. In our study, α-lipoic acid also reduced GSH-Px, whose main biological role is to protect the organism from oxidative damage.
In our study, we recorded an improvement of glycemic control and insulin resistance with α-lipoic acid supplementation. This effect of α-lipoic acid on glycemic control is in line with reported by Ansar et al. [17], which similarly reported an improvement of FPG, PPG, and HOMA-index. These effects may be due to the insulin signaling pathway, with an increase in PI 3-kinase and protein kinase B (Akt) [18,19,20]. The α-lipoic acid increased intrinsic activity of GLUT in an insulin-like manner, even if p38 mitogen-activated protein kinase may also mediate the activation of GLUT. Chronic α-lipoic acid treatment improved both insulin stimulated glucose oxidation and glycogen synthesis; consequently, it is related to decreased levels of insulin and free fatty acids.
Concerning the effects of α-lipoic acid on LDL-C and Tg levels, they have been previously reported as acting on rats by Thirunavukkarasu et al. [21]. These results might be due to the Alfa lipoic acid effect on glucose utilization reported above and to the consequent reduced levels of free fatty acids.
4. Material and Methods
4.1. Study Design
This randomized, double-blind, placebo-controlled trial took place at the Centre of Diabetes and Metabolic Diseases, Department of Internal Medicine and Therapeutics, University of Pavia and Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy.
The study protocol was conducted in accordance with the Declaration of Helsinki and its amendments, and was approved by the local review board. Suitable patients were identified from case notes, computerized clinic registers, or both and were contacted by the clinicians personally or by telephone. All eligible candidates had to provide signed informed consent before enrollment in the study.
4.2. Patients
We enrolled 105 Caucasian adults, of both sexes, 18–75 years of age, with type 2 diabetes according to the ESC (European Society of Cardiology) and EASD (European Association for the Study of Diabetes) Guidelines criteria [22], and with a glycated hemoglobin (HbA1c) level >7.0%. All participants were overweight (BMI ≥25, and <30 kg/m2).
We excluded patients with previous ketoacidosis or unstable or rapidly progressive diabetic retinopathy, nephropathy, neuropathy, impaired hepatic or renal function, or severe anemia. Patients with a myocardial infarction or stroke within 6 months before study enrollment were not enrolled. Women with childbearing potential and not taking adequate contraceptive precautions were not considered for inclusion in this trial.
4.3. Treatments
Subjects fulfilling all inclusion criteria reported above were randomized to receive either, in a 1:1 ratio, a supplementation containing LICA®, a patented association of components (600 mg of α-lipoic acid, 165 mg of L-carnosin, and 7.5 mg of zinc) and vitamins of group B (Table 2) produced by DIFASS Italia (Prato, Italy), or a placebo, once a day for three months, in a single oral dose, with a glass of water.
Table 2.
Food supplement composition.
| Components | Dose |
|---|---|
| α-lipoic acid | 600 mg |
| L-carnosine | 165 mg |
| Zinc | 7.5 mg |
| Vitamin PP | 9.0 mg |
| Vitamin B5 | 3.0 mg |
| Vitamin B6 | 1.0 mg |
| Vitamin B1 | 0.7 mg |
| Vitamin B2 | 0.8 mg |
| Vitamin B12 | 0.5 mcg |
| Folic acid | 100 mcg |
The food supplement containing α-lipoic acid was added to the previously taken anti-diabetic therapy, including insulin. The methods used to ensure the blind status of the trial, to perform randomization and to assess medical compliance, have been previously described [23]. Patients did not sustain any costs for the medications provided.
4.4. Diet and Exercise
Subjects followed a controlled-energy diet according to the American Heart Association (AHA) recommendations [24].
Standard diet advice was given by a dietician, a specialist doctor, or both as previously described [23].
4.5. Assessments
At the start of the study, patients were interviewed about their medical history, underwent physical examination, examined for vital signs, and underwent a 12-lead electrocardiogram. We evaluated body mass index (BMI), fasting plasma glucose (FPG), post-prandial-glucose (PPG), glycated hemoglobin (HbA1c), fasting plasma insulin (FPI), HOMA-index (HOMA-IR), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), high sensitivity C-reactive protein (Hs-CRP), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA).
We measured these parameters both at the baseline and after 3 months of the food supplement containing α-lipoic acid or the placebo. All adverse events were recorded to assess tolerability. For a complete description of how various parameters were assessed, see our previous papers [23,25]. Superoxide dismutase (SOD) was evaluated according to the method described by Marklund et al. [26]. We assessed GSH-Px activity according to Paglia et al. [27]. Malondialdehyde was obtained via acid hydrolysis of 1,1,3,3-tetraethoxy-propane according to Requena et al. [28].
4.6. Statistical Analysis
We conducted an intention-to-treat (ITT) analysis in patients receiving ≥1 dose of medication. The statistical significance of the independent effects of treatments on each variable was determined using ANCOVA. We used a 1-sample t-test to compare values obtained before and after treatment, and 2-sample t-tests for between-group comparisons. Statistical analysis of data was performed using the Statistical Package for Social Sciences software version 11.0 (SPSS Inc., Chicago, IL, USA). Data in the text were presented as mean (±standard deviation). A p-value of <0.05 was considered statistically significant [29].
5. Conclusions
The use of a food supplement containing α-lipoic acid seems to bring an improvement in glycemic control and in lipid profile, even if only slightly. Moreover, α-lipoic acid seems to improve markers of oxidative stress, such as GSH-Px, SOD, and MDA, and of inflammation (Hs-CRP).
Acknowledgments
Difass International s.r.l. covered the costs for publishing in open access. The funding source did not have any role in the study design, or in data collection or interpretation or in the writing of the manuscript.
Author Contributions
Design and conduction of the study: Giuseppe Derosa, Pamela Maffioli; data collection: all authors; data interpretation and manuscript writing: Giuseppe Derosa, Pamela Maffioli. All authors read and approved the final manuscript.
Conflicts of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
- 1.Aring A.M., Jones D.E., Falko J.M. Evaluation and prevention of diabetic neuropathy. Am. Fam. Physician. 2005;71:2123–2128. [PubMed] [Google Scholar]
- 2.Bansal V., Kalita J., Misra U.K. Diabetic neuropathy. Postgrad. Med. J. 2006;82:95–100. doi: 10.1136/pgmj.2005.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasso S., Bramanti V., Tomassoni D., Bronzi D., Malfa G., Traini E., Napoli M., Renis M., Amenta F., Avola R. Effect of lipoic acid and α-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J. Neurosci. Res. 2014;92:86–94. doi: 10.1002/jnr.23289. [DOI] [PubMed] [Google Scholar]
- 4.Bramanti V., Tomassoni D., Bronzi D., Grasso S., Currò M., Avitabile M., Li Volsi G., Renis M., Ientile R., Amenta F., et al. α-lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase expression in astroglial cell cultures. Neurochem. Res. 2010;35:2070–2077. doi: 10.1007/s11064-010-0256-6. [DOI] [PubMed] [Google Scholar]
- 5.Kramer K., Packer L., Hoppe P. R-α-Lipoic Acid. Nutraceuticals in Health and Disease Prevention. Marcel Dekker, Inc.; New York, NY, USA: 2001. pp. 129–164. [Google Scholar]
- 6.Evans J.L., Goldfine I.D. α-lipoic acid: A multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol. Ther. 2000;2:401–413. doi: 10.1089/15209150050194279. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler D., Ametov A., Barinov A., Dyck P.J., Gurieva I., Low P.A., Munzel U., Yakhno N., Raz I., Novosadova M., et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy: The SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 8.Babizhayev M.A., Strokov I.A., Nosikov V.V., Savel’yeva E.L., Sitnikov V.F., Yegorov Y.E., Lankin V.Z. The Role of oxidative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem. Biophys. 2015;71:1425–1443. doi: 10.1007/s12013-014-0365-y. [DOI] [PubMed] [Google Scholar]
- 9.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Zatalia S.R., Sanusi H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Medica Indones. 2013;45:141–147. [PubMed] [Google Scholar]
- 11.Rochette L., Ghibu S., Muresan A., Vergely C. α-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015;93:1021–1027. doi: 10.1139/cjpp-2014-0353. [DOI] [PubMed] [Google Scholar]
- 12.Wallace D.C. Mitochondrial DNA. Methods Mol. Biol. 2002;197:3–54. doi: 10.1385/1-59259-284-8:003. [DOI] [PubMed] [Google Scholar]
- 13.Giugliano D., Ceriello A., Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 14.Maier C.M., Chan P.H. Role of superoxide dismutase in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 15.Nourooz-Zadeh J., Tajaddini-Sarmadi J., McCarthy S., Betteridge D.J., Wolff S.P. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes. 1995;44:1054–1058. doi: 10.2337/diab.44.9.1054. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z.Q., Porreca F., Cuzzocrea S., Galen K., Lightfoot R., Masini E., Muscoli C., Mollace V., Ndengele M., Ischiropoulos H., et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 17.Ansar H., Mazloom Z., Kazemi F., Hejazi N. Effect of α-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 2011;32:584–588. [PubMed] [Google Scholar]
- 18.Moini H., Tirosh O., Park Y.C., Cho K.J., Packer L. R-α-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2002;397:384–391. doi: 10.1006/abbi.2001.2680. [DOI] [PubMed] [Google Scholar]
- 19.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Yaworsky K., Somwar R., Ramlal T., Tritschler H.J., Klip A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by α-lipoic acid in 3T3-L1 adipocytes. Diabetologia. 2000;43:294–303. doi: 10.1007/s001250050047. [DOI] [PubMed] [Google Scholar]
- 21.Thirunavukkarasu V., Anitha Nandhini A.T., Anuradha C.V. Effect of α-lipoic acid on lipid profile in rats fed a high-fructose diet. Exp. Diabesity Res. 2004;5:195–200. doi: 10.1080/15438600490486778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rydén L., Standl E., Bartnik M., van den Berghe G., Betteridge J., de Boer M.J., Cosentino F., Jönsson B., Laakso M., Malmberg K., et al. Task force on diabetes and cardiovascular diseases of the european society of cardiology (ESC); european association for the study of diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary. The task force on diabetes and cardiovascular diseases of the european society of cardiology (ESC) and of the european association for the study of diabetes (EASD) Eur. Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 23.Derosa G., Mugellini A., Pesce R.M., D’Angelo A., Maffioli P. Barnidipine compared to lercanidipine in addition to losartan on endothelial damage and oxidative stress parameters in patients with hypertension and type 2 diabetes mellitus. BMC Cardiovasc. Disord. 2016;16:66–73. doi: 10.1186/s12872-016-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein A.H., Appel L.J., Brands M., Carnethon M., Daniels S., Franch H.A., Franklin B., Kris-Etherton P., Harris W.S., Howard B., et al. Summary of american heart association diet and lifestyle recommendations revision 2006. Arterioscler. Thromb. Vasc. Biol. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 25.Derosa G., Bonaventura A., Bianchi L., Romano D., D’Angelo A., Fogari E., Maffioli P. Effects of Berberis aristata/Silybum marianum association on metabolic parameters and adipocytokines in overweight dyslipidemic patients. J. Biol. Regul. Homeost. Agents. 2013;27:717–728. [PubMed] [Google Scholar]
- 26.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 27.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 28.Requena J.R., Fu M.X., Ahmed M.U., Jenkins A.J., Lyons T.J., Baynes J.W., Thorpe S.R. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem. J. 1997;322:317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winer B.J. Statistical Principles in Experimental Design. 2nd ed. McGraw-Hill; New York, NY, USA: 1971. [Google Scholar]