Abstract
Circulating tumor cells (CTCs) have been implicated in tumor progression and prognosis. Techniques detecting CTCs in the peripheral blood of patients with non-small cell lung carcinoma (NSCLC) may help to identify individuals likely to benefit from early systemic treatment. However, the detection of CTCs with a single marker is challenging, owing to low specificity and sensitivity and due to the heterogeneity and rareness of CTCs. Herein, the probability of cell-free RNA content in the peripheral blood as a potential biomarker for detecting CTCs in cancer patients was investigated. An immunomagnetic enrichment of real-time reverse-transcription PCR (RT-PCR) technology for analysis of CTCs in NSCLC patients was also developed. The mRNA levels of four candidate genes, cytokeratin 7 (CK7), E74-like factor 3 (ELF3), epidermal growth factor receptor (EGFR), and erythropoietin-producing hepatocellular carcinoma receptor B4 (EphB4) that were significantly elevated in tumor tissues and peripheral blood mononuclear cells (PBMCs) were determined. The expression of CK7 and ELF3 in tumor tissues and EGFR in PBMCs was associated with lymph node metastasis (all p < 0.05). The expression of CK7 in PBMCs was correlated with age and EphB4 in PBMCs correlated with histopathological type, respectively (all p < 0.05). The expression of all four genes in tumor tissues and PBMCs was significantly correlated with the clinical stage (all p < 0.01). Survival analysis showed that the patients with enhanced expression of CK7, ELF3, EGFR, and EphB4 mRNA in PBMCs had poorer disease-free survival (DFS) and overall survival (OS) than those without (all p < 0.0001). The present study showed that this alteration of cell-free RNA content in peripheral blood might have clinical ramifications in the diagnosis and treatment of NSCLC patients.
Keywords: non-small-cell lung carcinoma (NSCLC), circulating tumor cells (CTCs), peripheral blood mononuclear cells (PBMCs), cell-free RNA content, prognosis
1. Introduction
The continuous search for tumor markers has yielded several positive results [1]. Research on biomarkers playing a vital role in the early diagnosis and individualized treatments of the tumors is rapidly advancing as we begin to understand the complex mechanisms during tumor carcinogenesis and progression [1]. The identification of molecular subtypes of non-small cell lung carcinoma (NSCLC) has transformed the clinical management of this disease, which is best exemplified by the clinical success of targeting the epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) with tyrosine kinase inhibitors (TKI) as the first line of treatment [2]. However, lung cancer is still the leading cause of cancer mortality worldwide. The high mortality is due to the poor prognosis of the disease caused by late presentation of illness, tumor heterogeneities within histological subtypes, and the limited understanding of tumor biology. Importantly, difficulty in early diagnosis of lung cancer is due to the lack of a quintessential biomarker [3]. Recently, invasive biomarkers present in sputum, plasma, serum, or whole blood have increasingly been explored for the early diagnosis of NSCLC.
The practice of liquid biopsy as a diagnostic, prognostic, and theranostic tool in NSCLC patients is an appealing approach since it is noninvasive and easily reproducible. In particular, this method for the potential detection of circulating biomarkers allows patient monitoring during treatment, as well as the detection of different genomic alterations that may be accessible to targeted therapy or are associated with treatment resistance [4]. Recently, detection and molecular characterization of circulating tumor cells (CTCs) are some of the most active areas in translational cancer research; the use of CTCs as a liquid biopsy may aid in obtaining genetic follow-up data, which is an urgent prerequisite [5]. However, the challenge in the detection of CTCs is the requirement of high sensitivity combined with high specificity [5]. The high heterogeneity and low occurrence frequencies of CTCs which have been detected for a low concentration of 1–10 CTCs/mL in whole blood of patients with metastasis, hinder the development of clinical applications [4,6].
Methods for CTC detection have recently been developed, including CTC microchips, filtration devices, quantitative reverse-transcription PCR assays, and automated microscopy systems [4,7]. A successful detection of rare CTCs in peripheral blood can be achieved by coupling pre-analytical enrichment with molecular detection of enriched cells by reverse-transcription-PCR (RT-PCR) [8]. CTC detection by a panel of markers that show increased expression in tumor cells as compared to the normal epithelial cells is a critical supplement to the current tumor-node-metastasis (TNM) staging system for improved prognosis and rapid assessment of the therapeutic response. These improvements facilitate the design of enhanced therapeutic strategies for the treatment of any solid epithelial cancer [9,10]. Although many promising CTC detection techniques have been developed in recent years, the analytical specificity and specificity of these methods require substantiation in large prospective multicenter studies before clinical utility. In addition, the cost-efficiency of the detection method should also be considered.
Herein, immunobead RT-PCR was developed for the detection of CTCs originating from NSCLC. The technique combined pre-analytical enrichment by density gradient centrifugation (Ficoll-Hypaque separation), immunomagnetic, and size filtration procedures with subsequent RT-PCR detection of a panel of four markers. The markers used for RT-PCR included CK7, ELF3, EGFR, and EphB4, which were selected based on the expression in epithelial cancers including NSCLC, as described previously [11,12,13,14]. These markers can be used for the identification of circulating breast cancer cells [12], and cannot be detected in mononuclear cells from peripheral blood samples in normal individuals [12]. Therefore, these markers are specific for the detection of circulating epithelial cells.
2. Results
2.1. Expression Level of CK7, ELF3, EGFR, and EphB4 mRNA in NSCLC Tissues
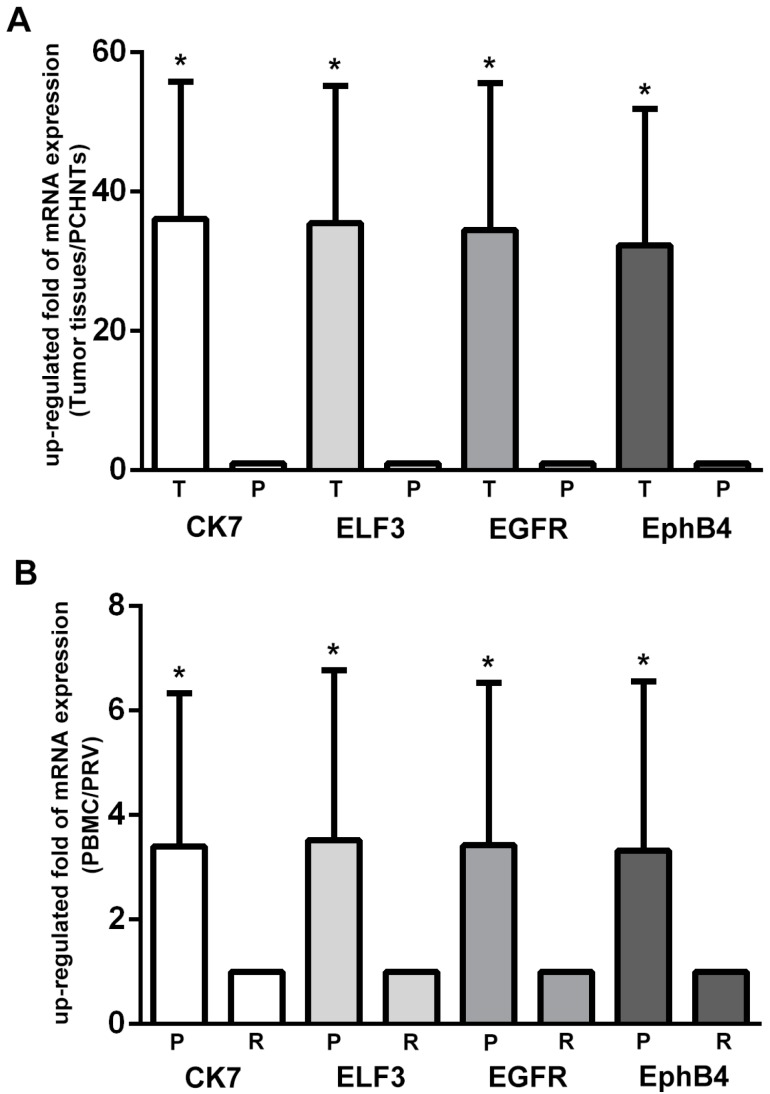
The mRNA level of CK7, ELF3, EGFR, and EphB4 in NSCLC tissues was significantly higher than that in para-cancerous histological normal tissues (PCHNTs) (all p < 0.0001, respectively, Figure 1A). The average increase of mRNA of CK7, ELF3, EGFR, and EphB4 was 36.118-, 35.476-, 34.541-, and 32.308-fold, respectively. Successively, the correlation between the four mRNA levels and the clinicopathological characteristics was analyzed (Table 1). The results indicated that the expression level of the four mRNAs was closely correlated with the clinical stage, respectively (all p < 0.0001). However, no correlation could be established between the levels of the four mRNAs and age, sex, and smoking history, respectively (all p > 0.05, Table 1).
Figure 1.
Real-time reverse-transcription PCR (RT-PCR) analysis of marker gene expression in non-small cell lung carcinoma (NSCLC) tissues and paired peripheral blood mononuclear cell (PBMC) specimens. Total amount of mRNA was normalized to GAPDH and the relative mRNA expressions of the samples (tumor vs. para-cancerous histological normal tissues (PCHNTs), or paired PBMCs vs. positive control) were calculated using 2−ΔΔCt formula. (A) Tumor tissues vs. PCHNTs from 111 NSCLC patients. The mRNA expression of CK7, ELF3, EGFR and EphB4 in lung cancer tissues were significantly higher than that in PCHNTs (all p < 0.0001); T: Tumor tissues; P: PCHNTs; (B) PBMCs from 111 NSCLC patients vs. positive reference value (PRV). The mRNA expression of CK7, ELF3, EGFR and EphB4 in PBMCs from NSCLC patients were significantly higher than that in healthy controls, respectively (all p < 0.0001); P: PBMC; R: PRV. *: p ˂ 0.0001.
Table 1.
Correlation of CK7, ELF3, EGFR, EphB4 expression in tissues with NSCLC’s clinicopathological characteristics.
| Clinicopathological Parameters | N | CK7 | ELF3 | EGFR | EphB4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | |||
| Gender | Male | 79 | 36.73 ± 19.44 | 0.608 | 36.64 ± 19.60 | 0.330 | 35.23 ± 20.76 | 0.590 | 32.98 ± 19.65 | 0.571 |
| Female | 32 | 34.60 ± 29.66 | 32.59 ± 20.20 | 32.84 ± 21.87 | 30.64 ± 19.76 | |||||
| Age (year) | <60 | 46 | 36.54 ± 18.05 | 0.852 | 36.24 ± 18.25 | 0.728 | 36.58 ± 20.62 | 0.393 | 34.41 ± 19.20 | 0.345 |
| ≥60 | 65 | 35.82 ± 20.96 | 34.93 ± 20.89 | 33.10 ± 21.33 | 30.82 ± 19.92 | |||||
| Smoking history | No | 46 | 36.27 ± 21.05 | 0.947 | 34.45 ± 20.20 | 0.646 | 34.08 ± 20.61 | 0.847 | 32.84 ± 19.78 | 0.812 |
| Yes | 65 | 36.01 ± 18.90 | 36.21 ± 19.57 | 34.87 ± 21.45 | 31.93 ± 19.65 | |||||
| Lesion site | Left lobe | 50 | 34.45 ± 17.73 | 0.413 | 34.92 ± 19.38 | 0.790 | 33.65 ± 20.14 | 0.686 | 31.78 ± 18.85 | 0.797 |
| Right lobe | 61 | 37.49 ± 21.27 | 35.93 ± 20.22 | 35.28 ± 21.85 | 32.74 ± 20.37 | |||||
| Histopathological type | SCC | 46 | 34.46 ± 18.00 | 0.460 | 35.14 ± 19.34 | 0.879 | 32.67 ± 19.35 | 0.423 | 31.03 ± 19.23 | 0.567 |
| ADC | 65 | 37.29 ± 20.92 | 35.72 ± 20.21 | 35.86 ± 22.17 | 33.21 ± 19.99 | |||||
| Tumor size | ≤3 cm | 24 | 37.47 ± 22.02 | 0.727 | 35.88 ± 20.95 | 0.938 | 36.82 ± 24.34 | 0.615 | 30.90 ± 18.19 | 0.653 |
| >3 cm | 86 | 35.86 ± 19.25 | 35.52 ± 19.61 | 34.07 ± 20.18 | 32.94 ± 20.08 | |||||
| Differentiation | Well/Moderate | 86 | 34.84 ± 19.72 | 0.207 | 34.09 ± 19.47 | 0.173 | 33.23 ± 21.31 | 0.224 | 30.56 ± 19.60 | 0.082 |
| Poor | 25 | 40.51 ± 19.51 | 40.23 ± 20.42 | 39.05 ± 19.73 | 38.32 ± 18.81 | |||||
| T stage | T2 | 94 | 35.55 ± 19.70 | 0.476 | 34.63 ± 19.68 | 0.289 | 33.33 ± 20.89 | 0.153 | 30.78 ± 19.18 | 0.053 |
| T3 | 17 | 39.27 ± 20.16 | 40.18 ± 20.14 | 41.27 ± 21.08 | 40.77 ± 20.45 | |||||
| Lymph node status | N0 | 18 | 31.75 ± 13.75 | 0.188 | 30.02 ± 13.90 | 0.105 | 30.47 ± 15.85 | 0.275 | 28.47 ± 15.94 | 0.296 |
| N1–3 | 93 | 36.96 ± 20.64 | 36.53 ± 20.60 | 35.33 ± 21.86 | 33.05 ± 20.24 | |||||
| Distant metastasis | M0 | 109 | 36.20 ± 19.85 | 0.735 | 35.56 ± 19.87 | 0.748 | 34.64 ± 21.12 | 0.721 | 32.39 ± 19.67 | 0.745 |
| M1 | 2 | 31.40 ± 14.85 | 31.00 ± 16.69 | 29.25 ± 18.31 | 27.80 ± 22.49 | |||||
| Clinical stage | I/II | 56 | 28.13 ± 12.80 | 1.06 × 10−5 | 27.09 ± 13.95 | 3.23 × 10−6 | 25.14 ± 15.18 | 7.62 × 10−7 | 23.87 ± 14.95 | 2.05 × 10−6 |
| III/IV | 55 | 44.25 ± 22.18 | 44.01 ± 21.22 | 44.11 ± 21.91 | 40.90 ± 20.18 | |||||
SCC: squamous cell carcinoma; ADC: adenocarcinoma; Mean: mean value of relative expression; SD: standard deviation; Numbers in bold: p < 0.05.
2.2. The mRNA Expression of Selected Markers in Paired PBMC Preparations
346 blood samples were taken from the recruited participants including 111 NSCLC patients, 115 benign pulmonary disease patients, and 120 healthy controls. No significant difference was observed in the age and gender among the different groups (p > 0.05 for both).
The expression of the four putative markers identified in paired PBMCs was validated by real-time RT-PCR. We found that these four mRNAs were detectable in the majority of PBMC samples from 111 NSCLC patients. Four markers (CK7, ELF3, EGFR, and EphB4) were significantly elevated in PBMCs (all p ˂ 0.0001) (Figure 1B). However, no significant difference was observed in the expression of the four genes in PBMCs from 115 benign pulmonary disease patients as compared to 120 healthy controls (p > 0.05). Furthermore, the results indicated that these four mRNA levels in PBMCs was closely correlated with the clinical stage (p = 0.006, 0.003, 9.62 × 10−5, and 0.006, respectively) in NSCLC patients (Table 2). The circulating CK7 mRNA was closely correlated with the patient’s age revealed during the first examination (p = 0.038). The circulating EphB4 mRNA was closely correlated with the histopathological type (p = 0.032) while the circulating EGFR mRNA was closely correlated with the lymph node metastasis (p = 0.023) (Table 2).
Table 2.
Correlation of CK7, ELF3, EGFR, EphB4 expression in PBMCs with NSCLC’s clinicopathological characteristics.
| Clinicopathological Parameters | N | CK7 | ELF3 | EGFR | EphB4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Up-Regulated (%) | χ2 | Up-Regulated (%) | χ2 | Up-Regulated (%) | χ2 | Up-Regulated (%) | χ2 | |||
| p-Value | p-Value | p-Value | p-Value | |||||||
| Gender | Male | 79 | 40 (50.63) | 0.432 | 41 (51.89) | 0.605 | 41 (51.90) | 0.014 | 39 (49.37) | 0.699 |
| Female | 32 | 14 (43.75) | 0.511 | 14 (43.75) | 0.437 | 17 (55.13) | 0.907 | 13 (40.63) | 0.403 | |
| Age (year) | <60 | 46 | 17 (36.96) | 4.299 | 20 (43.47) | 1.158 | 22 (47.83) | 0.617 | 19 (41.30) | 0.969 |
| ≥60 | 65 | 37 (56.92) | 0.038 | 35 (53.84) | 0.282 | 36 (55.38) | 0.432 | 33 (50.77) | 0.325 | |
| Smoking history | No | 46 | 26 (56.52) | 1.949 | 26 (56.52) | 1.528 | 28 (60.87) | 2.338 | 24 (52.17) | 0.895 |
| Yes | 65 | 28 (43.08) | 0.163 | 29 (44.61) | 0.216 | 30 (46.15) | 0.126 | 28 (43.08) | 0.344 | |
| Lesion site | Left lobe | 50 | 25 (50.00) | 0.285 | 24 (48.00) | 0.000 | 26 (52.00) | 0.055 | 21 (42.00) | 0.437 |
| Right lobe | 61 | 28 (45.90) | 0.593 | 30 (49.18) | 0.983 | 31 (50.82) | 0.815 | 30 (49.18) | 0.509 | |
| Histopathological type | SCC | 46 | 19 (41.30) | 1.696 | 20 (43.47) | 1.158 | 22 (47.83) | 0.617 | 16 (34.78) | 4.592 |
| ADC | 65 | 35 (53.85) | 0.193 | 35 (53.84) | 0.282 | 36 (55.38) | 0.432 | 36 (55.38) | 0.032 | |
| Tumor size | ≤3 cm | 24 | 13 (54.17) | 0.316 | 14 (58.33) | 0.853 | 14 (58.33) | 0.387 | 15 (62.50) | 2.856 |
| >3 cm | 86 | 41 (47.67) | 0.574 | 41 (47.67) | 0.356 | 44 (51.16) | 0.534 | 37 (43.02) | 0.091 | |
| Differentiation | Well/Moderate | 86 | 43 (50.00) | 0.279 | 42 (48.83) | 0.078 | 44 (51.16) | 0.182 | 39 (45.35) | 0.344 |
| Poor | 25 | 11 (44.00) | 0.597 | 13 (52.00) | 0.781 | 14 (56.00) | 0.670 | 13 (52.00) | 0.557 | |
| T stage | T2 | 94 | 46 (48.94) | 0.020 | 46 (48.93) | 0.092 | 47 (50.00) | 1.248 | 43 (45.74) | 0.299 |
| T3 | 17 | 8 (47.06) | 0.887 | 9 (52.94) | 0.761 | 11 (64.71) | 0.264 | 9 (52.94) | 0.584 | |
| Lymph node metastasis | N0 | 18 | 5 (27.78) | 3.746 | 7 (38.88) | 0.977 | 5 (27.78) | 5.158 | 6 (33.33) | 1.576 |
| N1–3 | 93 | 48 (52.17) | 0.053 | 47 (51.08) | 0.323 | 52 (56.52) | 0.023 | 45 (48.95) | 0.209 | |
| Distant metastasis | M0 | 109 | 53 (48.62) | 0.000 | 54 (49.54) | 0.000 | 57 (52.29) | 0.004 | 51 (46.79) | 0.000 |
| M1 | 2 | 1 (50.00) | 1.000 | 1 (50.00) | 1.000 | 1 (50.00) | 0.949 | 1 (50.00) | 1.000 | |
| Clinical stage | I/II | 56 | 20 (35.71) | 7.569 | 20 (35.71) | 8.654 | 19 (33.93) | 15.210 | 19 (33.93) | 7.574 |
| III/IV | 55 | 34 (61.82) | 0.006 | 35 (63.63) | 0.003 | 39 (70.91) | 9.62 × 10−5 | 33 (60.00) | 0.006 | |
SCC: squamous cell carcinoma; ADC: adenocarcinoma; up-regulated: the number of patients with gene upregulation; Numbers in bold: p < 0.05.
2.3. Receiver Operating Characteristics (ROC) Curve Analysis of the Four mRNAs as a Marker of CTCs
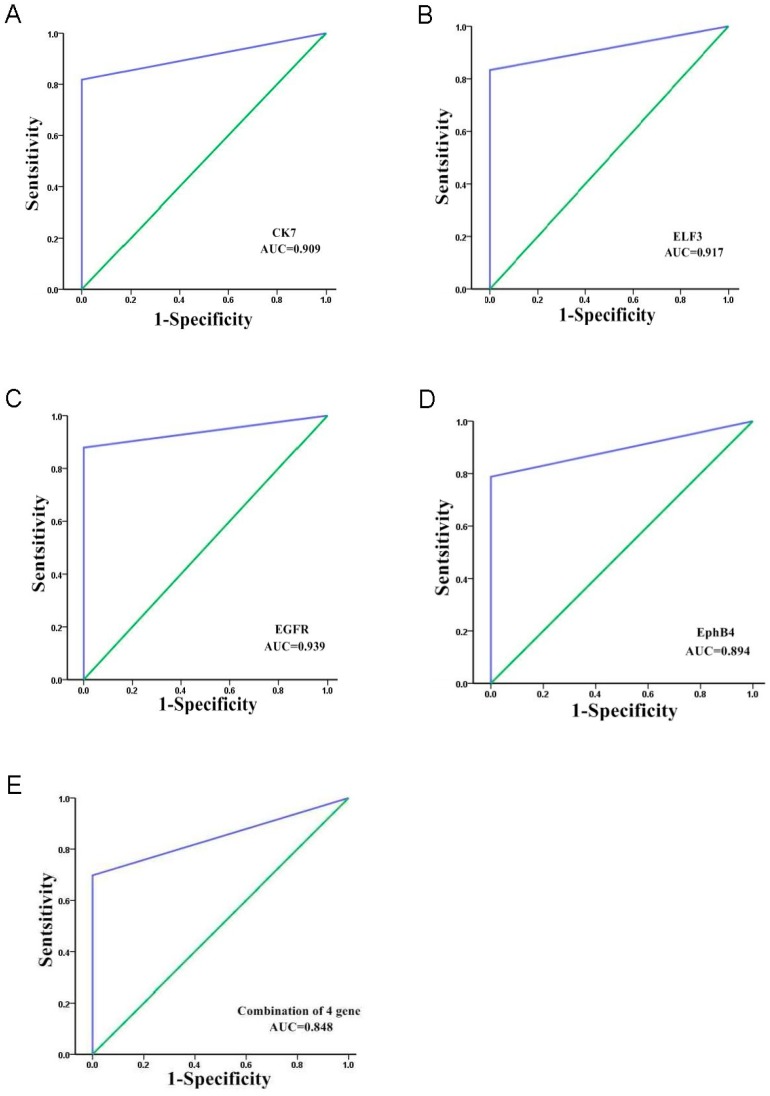
The areas under the ROC curves were 0.909 (0.853–0.965, 95.0% confidence interval (CI)) for CK7, 0.917 (0.863–0.971, 95.0% CI) for ELF3, 0.939 (0.893–0.986, 95.0% CI) for EGFR, and 0.894 (0.834–0.954, 95.0% CI) for EphB4 (Figure 2). The cut-off values of CK7, ELF3, EGFR, and EphB4 were defined as a 2-fold increase of the expression compared with the positive control. The sensitivity of each marker was: CK7 (81.8%), ELF3 (83.3%), EGFR (87.8%), EphB4 (78.8%), whereas the specificity was: CK7 (100%), ELF3 (100%), EGFR (100%), and EphB4 (100%). The expression of at least one of these four markers (a combination of the four markers) was considered as CTC positive. Thus, we determined the effect of the combination of the four markers by constructing a ROC curve and fitting a logistic model with parameters for CK7, ELF3, EGFR and, EphB4. The area under the ROC curve was 0.848 (0.779–0.917, 95.0% CI); the sensitivity and specificity were 69.7% and 100%, respectively, when the optimal threshold was defined as a 2-fold increase of expression (Figure 2). These results indicated that the detection of CTCs using the combination of four markers was not superior to the four markers individually.
Figure 2.
Receiver operating characteristics (ROC) curve analysis of the four mRNAs as a marker of circulating tumor cells (CTCs). The areas under the ROC curves are as follows: (A) CK7, 0.909 (0.853–0.965, 95.0% CI) with a sensitivity of 81.8% and a specificity of 100.0% for the discrimination between CTCs and the normal subjects; (B) ELF3, 0.917 (0.863–0.971, 95.0% CI) with a sensitivity of 83.3% and a specificity of 100.0% for the discrimination between CTCs and the normal subjects; (C) EGFR, 0.939 (0.893–0.986, 95.0% CI) with a sensitivity of 87.8% and a specificity of 100.0% for the discrimination between CTCs and the normal subjects; (D) EphB4, 0.894 (0.834–0.954, 95.0% CI) with a sensitivity of 78.8% and a specificity of 100.0% for the discrimination between CTCs and the normal subjects; (E) The area under the ROC curve was 0.848 (0.779–0.917, 95.0% CI), the sensitivity and specificity were 69.7% and 100%, respectively, when CTCs was detected using the combination of four markers; AUC: Area under curve.
2.4. Cell-Free RNA Content in Peripheral Blood Is Closely Associated with the Prognosis of NSCLC Patients
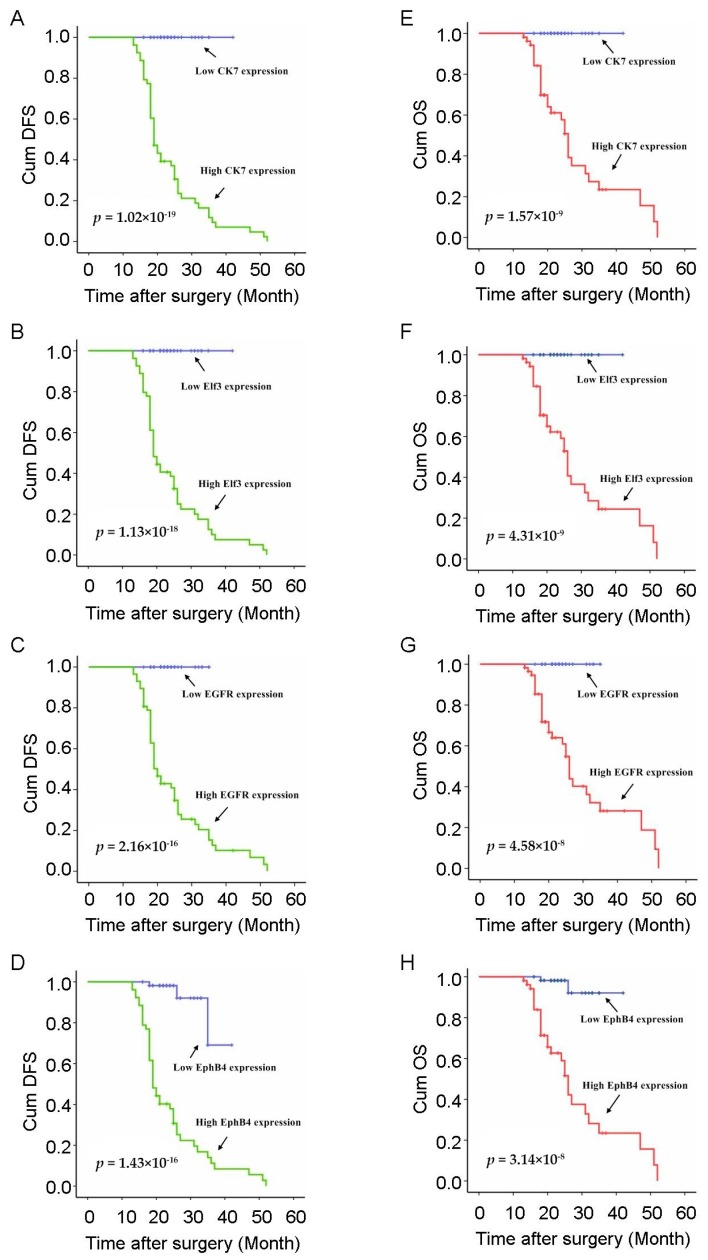
Next, we investigated the association of circulating CK7, ELF3, EGFR, and EphB4 mRNA with patients’ survival. In both disease-free survival (DFS) and overall survival (OS) situations, the four circulating mRNAs were significantly associated with poor prognosis, short DFS, and low OS, respectively. The average durations of DFS and OS in patients with high level circulating CK7, ELF3, EGFR, and EphB4 mRNAs (tumor PBMCs vs. positive control: >2-fold) were significantly shorter than those of patients with normal or downregulation of the four mRNAs in the PBMCs (Figure 3). The average DFS in patients with high level circulating CK7, ELF3, EGFR, and EphB4 mRNAs was 17.6, 17.9, 18.4, and 17.7 months vs. 24.3, 24.2, 24.0, and 24.1 months in patients with a normal or low level of the four mRNAs, respectively. The average OS in patients with high level circulating CK7, ELF3, EGFR, and EphB4 mRNA was 22.7, 22.9, 23.1, 22.6 months vs. 24.3, 24.2, 24.0, and 24.4 months in patients with a normal or low level of the four mRNAs, respectively (Table 3). All of the four circulating mRNAs, including CK7, ELF3, EGFR, and EphB4 mRNA, were strongly associated with NSCLC patients’ prognosis; however, the association of the cell-free RNA content with patient survival was independent of the tumor stage (Figure 3). In addition to the four circulating mRNAs, we observed that a number of other previously characterized clinical parameters were associated with patient survival (Table 3), including gender, smoking, and TNM stage. However, no significant correlation was found between a majority of the clinical characteristics such as age at diagnosis, lesion site, histopathological type, tumor size, differentiation, invasive depth, nodal metastasis, and distant metastasis with the patients’ prognosis. In summary, these data indicate that in addition to a minority of clinical characteristics that have been shown to affect the prognosis of NSCLC patients, these four circulating mRNAs might serve as a biomarker to predict the DFS and OS of the patients.
Figure 3.
Kaplan-Meier survival analysis of DFS and OS based on the selected gene expression. Comparison of survival data from NSCLC patients positive or negative for epithelial cells in blood. Cum: Cumulative; (A) Kaplan-Meier curves of DFS in patients with NSCLC treated with curative surgery according to the expression of circulating CK7; (B) Kaplan-Meier curves of DFS in patients with NSCLC treated with curative surgery according to the expression of circulating ELF3; (C) Kaplan-Meier curves of DFS in patients with NSCLC treated with curative surgery according to the expression of circulating EGFR; (D) Kaplan-Meier curves of DFS in patients with NSCLC treated with curative surgery according to the expression of circulating EphB4; (E) Kaplan-Meier curves of OS in patients with NSCLC treated with curative surgery according to the expression of circulating CK7; (F) Kaplan-Meier curves of OS in patients with NSCLC treated with curative surgery according to the expression of circulating ELF3; (G) Kaplan-Meier curves of OS in patients with NSCLC treated with curative surgery according to the expression of circulating EGFR; (H) Kaplan-Meier curves of OS in patients with NSCLC treated with curative surgery according to the expression of circulating EphB4.
Table 3.
Univariate and multivariate analysis of survival in 110 patients with NSCLC according to clinicopathologic factors and circulating mRNA levels of CK7, ELF3, EGFR and EphB4.
| Clinicopathologic Factor | DFS | OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Survival (mo.) | Univariate Analysis | Multivariate Analysis | Survival (mo.) | Univariate Analysis | Multivariate Analysis | ||||||||
| N | χ2 | p-Values | HR (95% CI) | p-Values | χ2 | p-Values | HR (95% CI) | p-Values | ||||||
| Age | <60 | 45 | 21.69 ± 6.90 | 2.577 | 0.108 | 1.604 | 0.842–3.057 | 0.151 | 22.67 ± 6.43 | 2.608 | 0.106 | 2.184 | 0.894–5.337 | 0.086 |
| ≥60 | 65 | 20.66 ± 7.75 | 24.15 ± 7.76 | |||||||||||
| Gender | Male | 78 | 19.92 ± 6.85 | 4.447 | 0.035 | 0.237 | 0.098–0.574 | 0.001 | 22.65 ± 5.95 | 8.597 | 0.003 | 0.120 | 0.026–0.553 | 0.006 |
| Female | 32 | 23.91 ± 8.02 | 25.72 ± 9.50 | |||||||||||
| Smoking | No | 45 | 21.33 ± 7.95 | 0.580 | 0.446 | 0.456 | 0.215–0.969 | 0.041 | 23.96 ± 8.00 | 0.707 | 0.401 | 0.578 | 0.220–1.519 | 0.266 |
| Yes | 65 | 20.91 ± 7.05 | 23.26 ± 6.74 | |||||||||||
| Lesion site | Left lobe | 50 | 20.94 ± 7.79 | 0.003 | 0.957 | 0.873 | 0.476–1.601 | 0.660 | 24.26 ± 7.74 | 0.254 | 0.615 | 0.933 | 0.409–2.130 | 0.869 |
| Right lobe | 60 | 21.20 ± 7.12 | 22.95 ± 6.83 | |||||||||||
| Histopathological type | SCC | 45 | 22.60 ± 7.85 | 2.286 | 0.131 | 1.374 | 0.654–2.888 | 0.401 | 24.76 ± 7.76 | 0.068 | 0.794 | 1.338 | 0.518–3.452 | 0.548 |
| ADC | 65 | 20.03 ± 6.94 | 22.71 ± 6.82 | |||||||||||
| Tumor size | ≤3 cm | 24 | 20.38 ± 6.28 | 0.833 | 0.361 | 0.894 | 0.421–1.897 | 0.769 | 24.12 ± 8.22 | 0.342 | 0.559 | 1.528 | 0.476–4.903 | 0.476 |
| >3 cm | 85 | 21.34 ± 7.73 | 23.47 ± 7.00 | |||||||||||
| Differentiation | Well/Moderate | 40 | 21.55 ± 6.92 | 0.016 | 0.898 | 0.685 | 0.326–1.439 | 0.318 | 23.80 ± 6.43 | 0.000 | 1.000 | 0.653 | 0.237–1.796 | 0.409 |
| Poor | 70 | 20.81 ± 7.70 | 23.40 ± 7.73 | |||||||||||
| T stage | T2 | 93 | 20.92 ± 7.23 | 0.000 | 0.993 | 0.776 | 0.313–1.925 | 0.584 | 23.30 ± 6.92 | 0.269 | 0.604 | 0.410 | 0.120–1.407 | 0.156 |
| T3 | 17 | 21.94 ± 8.45 | 24.88 ± 8.99 | |||||||||||
| Lymph node status | N0 | 18 | 19.28 ± 4.99 | 0.585 | 0.444 | 0.757 | 0.256–2.243 | 0.616 | 20.39 ± 3.27 | 0.455 | 0.500 | 0.497 | 0.133–1.859 | 0.299 |
| N1–3 | 92 | 21.43 ± 7.76 | 24.16 ± 7.66 | |||||||||||
| Distant metastasis | M0 | 108 | 20.82 ± 7.09 | 2.229 | 0.135 | 0.000 | 0.000–9.74 × 10233 | 0.968 | 23.28 ± 6.82 | 0.417 | 0.519 | 1.451 | 0.139–15.122 | 0.756 |
| M1 | 2 | 35.00 ± 14.14 | 38.00 ± 18.38 | |||||||||||
| Clinical stage | I/II | 56 | 21.96 ± 6.89 | 5.723 | 0.017 | 1.878 | 0.903–3.903 | 0.091 | 23.29 ± 6.58 | 1.075 | 0.300 | 1.418 | 0.532–3.781 | 0.486 |
| III/IV | 54 | 20.17 ± 7.85 | 23.81 ± 7.95 | |||||||||||
| Circulating CK7 mRNA | >2-fold | 53 | 17.62 ± 7.95 | 82.565 | 1.02 × 10−9 | 132.315 | 10.691-1637.622 | 1.41 × 10−4 | 22.74 ± 9.00 | 36.447 | 1.57 × 10−9 | 91.148 | 3.863–2150.588 | 0.005 |
| Normal/low | 57 | 24.30 ± 5.11 | 24.30 ± 5.11 | |||||||||||
| Circulating ELF3 mRNA | >2-fold | 54 | 17.87 ± 8.00 | 77.808 | 1.13 × 10−18 | 119.681 | 10.024–1428.965 | 1.56 × 10−4 | 22.89 ± 8.91 | 34.479 | 4.31 × 10−9 | 84.458 | 3.655–1951.764 | 0.006 |
| Normal/low | 56 | 24.18 ± 5.19 | 24.18 ± 5.19 | |||||||||||
| Circulating EGFR mRNA | >2-fold | 57 | 18.39 ± 8.51 | 67.452 | 2.16 × 10−16 | 101.954 | 8.565–1213.683 | 2.53 × 10−4 | 23.14 ± 9.13 | 29.888 | 4.58 × 10−8 | 72.525 | 3.178–1655.090 | 0.007 |
| Normal/low | 53 | 23.98 ± 4.49 | 23.98 ± 4.49 | |||||||||||
| Circulating EphB4 mRNA | >2-fold | 52 | 17.69 ± 7.69 | 68.262 | 1.43 × 10−16 | 26.490 | 8.111–86.516 | 5.76 × 10−8 | 22.62 ± 8.88 | 30.619 | 3.14 × 10−8 | 19.010 | 4.487–80.547 | 6.40 × 10−5 |
| Normal/low | 58 | 24.12 ± 5.64 | 24.38 ± 5.34 | |||||||||||
SCC: squamous cell carcinoma; ADC: adenocarcinoma; DFS: disease free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; mo.: month; Normal: 0.5 ≤ 2−ΔΔCt ≤ 2; low: 2−ΔΔCt < 0.5.
3. Discussion
Metastasis is a multistep event. Some tumor cells acquire metastasis ability independent of the in situ tumor and then migrate or invade into the blood and lymph vessels to act as circulating tumor cells [15]. More than 90% of the tumor-related deaths arise out of the progression of hematogenously disseminated metastasis [16]. CTCs play a vital role in metastasis, and detection of CTCs can contribute towards the evaluation of progression, prognosis, and personal treatment [4,5]. However, because of low quantity and heterogeneity of CTCS, a unified detection method to count and classify CTCs has not yet been described [5,6]. Herein, we collected PBMCs from 111 NSCLC patients to detect the target genes’ expression originating from epithelium instead of acquiring and examining the CTCs. The elevated levels of gene expression from epithelial origin indicate the existence of CTCs or circulating tumor nuclei in peripheral blood; these cannot be detected in the normal peripheral blood [17,18]. Lung cancer is a heterogeneous disease with respect to histological and biological characteristics [19]. Owing to the heterogeneity of the tumor cells and the limited reliability of single marker, we selected four genes of epithelial origin, described previously, for the analysis of gene expression [17,18]. These four markers cannot be detected in normal mononuclear cells by optimized experiments and are specific for the detection of circulating epithelial cells [12]. The analysis of CK7, ELF3, EGFR, and EphB4 mRNAs in PBMCs for the detection of CTCs have been reported in various epithelial cancers including NSCLC [11,12,13,14], respectively; however, a combined of these four markers in NSCLC has not yet been reported.
CK7, a star member in lung cancer, has been reported in primary or metastatic lung cancer, cavity liquid, and pleural mesothelioma. The frequency of CK7 upregulation in adenocarcinoma (ADC) was higher than that in squamous cell carcinoma (SCC); it can be used as a potential marker to distinguish the two histological types [19,20]. However, our results were inconsistent with the previous reports. We found that neither CK7 expression in tissues nor peripheral mononuclear cells was associated with histological types (all p > 0.05). CK7 mRNA was up-regulated in all 111 NSCLC patients, including 65 ADC and 46 SCC patients. The CK7 level in both tissues (p ˂ 0.0001) and PBMCs (p = 0.006) was significantly correlated with the clinical stage. Moreover, circulating CK7 mRNA was significantly correlated with age during diagnosis (p = 0.038). NSCLC patients with low CK7 expression (fold < 2) have a long DFS while 92.5% (49/53) patients with high CK7 expression (fold ≥ 2) suffer progression, metastasis, or death. Therefore, CK7 overexpression in peripheral cells may be a good biomarker for predicting poor prognosis in NSCLC. CK7 is almost exclusively expressed in epithelial tissues, especially lung tissues. The expression of CK7 in lung cancer tissues is elevated and can be used to distinguish between primary lung cancer and lung metastasis of other cancers [21,22,23].
ELF3 is limited to cells of epithelial origin and is firstly found in lung tissues. Moreover, ELF3 is a transcriptional factor in the ETS-domain family and is known to regulate the expression of several growth-related genes, including angiopoietin 1, collagenase, as well as other transformed growth and invasion-related genes [24,25]. ELF3 may play a crucial role in lung tumorigenesis and progression. However, the additional functions of ELF3 in various kinds of tumors have frequently been studied for many years since its expression in lung cancer tissues and lung cancer cell lines was first discovered in 1997 [25]. ELF3 was demonstrated to possess dual functions in the transcriptional regulation of genes involved in squamous epithelial differentiation. ELF3 suppresses the basal keratin4 promoter activity while simultaneously activating the late differentiation linked small proline-rich protein 2A (SPRR2A) promoter in both esophageal and cervical epithelial cancer cell lines [26]. Nakarai et al. evaluated the mRNA expression of ELF3 and carcinoembryonic antigen (CEA) in the lymph node and the tissue from patients with colorectal cancer (CRC) and controls. The results showed that ELF3 may sufficiently assess the lymph node metastases of CRC [27]. In addition, ELF3 expression plays a critical role in lung tumorigenesis and is regulated by oncogenic protein kinase C (PKC) [28]. Our data indicate that ELF3 expression in NSCLC tissues is associated with lymph node metastasis and clinical stage; however, its expression in PBMCs is not correlated with lymph node metastasis. Moreover, the increased ELF3 expression correlated with poor prognosis. Hitherto, there is no report describing the association of ELF3 expression and metastasis of lung cancer. Hence, these results should be verified by a large sample analysis in the future studies.
EGFR is a biomarker used for the prediction of chemotherapy and targeted treatment. It is a representative marker and abnormal expression of EGFR influences the treatment and prognosis of lung cancer. When patients with lung cancer carry activating EGFR mutations, their first-line of treatment can be selected with EGFR-TKI, like gefitinib [29]. The prognosis of NSCLC patients with mutated EGFR was superior to that of the wild-type EGFR in NSCLC [30]. We found that high EGFR expression level was associated with short DFS and is an independent influencing factor for the prognosis in NSCLC (p ˂ 0.0001). Also, it has been demonstrated that patients showed higher EGFR expression in SCC than non-SCC (p < 0.05) [31], and co-expression with insulin-like growth factor 1 receptor (IGF1R) was associated with poor survival. We found the EGFR expression did not correlate with the histological type (p > 0.05).
EphB4 plays a key role in numerous kinds of tumor, including lung cancer [32], esophageal cancer [33], pancreatic cancer [34], and gliomas [35]. As a member of receptor tyrosine kinases, EphB4 is frequently implicated in tumor pathogenesis. Moreover, the inhibition of some tyrosine kinases targets EphB4, and is deemed efficient treatment Ferguson et al. [36] found that EphB4 overexpression promotes cellular proliferation, colony formation, and motility while EphB4 inhibition reduces cell viability in vitro. The growth of the established tumors in mouse xenograft models can be halted by single-target strategy, and EphB4 may be a potential novel therapeutic target in lung cancer. EphB4 expression in tumors was increased significantly compared to the control (adjacent normal tissues), which was correlated with differentiation, lymph node metastasis, and TNM stage [32]. However, we found that EphB4 expression in PBMCs was not associated with the differentiation and lymph node metastasis, but correlated with the histological type and TNM stage. The survival curve of EphB4 was different from the other three genes. The patients with low EphB4 expression also underwent progression, metastasis, or death.
4. Material and Methods
4.1. Patients
A total of 111 NSCLC patients who underwent curative surgery, without prior treatments, at Zhejiang Cancer Hospital (Hangzhou, China) from January 2010 to December 2011, were enrolled in this study. All the patients showed no history of other tumors by examination of a plain chest radiograph, CT scan, fiber optic bronchoscopy, and bone scan and were diagnosed with NSCLC by histopathology. The patients’ medical records were reviewed to obtain data including age at diagnosis, sex, and smoking history (Table 1). Tumor stage was determined according to the American Joint Committee on Cancer (AJCC)/ Union for International Cancer Control (UICC) TNM tumor classification [37]. The mean age of the patients at tumor resection was 62 years (range 44–82 years); 79 (71.2%) were males and 32 (28.8%) were females. The tumor specimens and paired PCHNTs were collected at the time of surgery. The paired PCHNTs were obtained from the proximity of 5 cm from the tumor edge and was assessed microscopically for the presence of healthy cells and absence of dysplastic cells. The paired peripheral blood samples were collected from these patients during diagnosis before surgery, radiation, or chemotherapy. The tissue and blood specimens for the present study were procured and used after obtaining informed consent from all the participants. As a measure of prognosis, we analyzed the clinical data concerning DFS and OS. All recruited patients had been followed-up periodically until the due date. The mean follow-up duration was 29 months; the average survival time was 32 months (range, 13–52 months) for all patients.
In addition, peripheral blood mononuclear cells (PBMCs) from 115 benign pulmonary disease patients and 120 healthy subjects as normal controls were also included to examine the specificity of these markers. PBMCs were prepared as described below.
Informed consent was obtained from all individual participants included in the study. The research involved human participants. The study was approved by the Zhejiang Cancer Hospital Institutional Review Board (2012KYB035).
4.2. Sample Collection and Processing
Biopsies of NSCLC and paired PCHNTs were obtained from thoracic surgery patients. The peripheral blood samples were obtained from NSCLC patients. Peripheral blood samples from 115 benign pulmonary disease patients and 120 healthy subjects were provided by our hospital. Fresh tissue samples (<300 mg) were immediately ground into powder in liquid nitrogen. Total RNA was extracted from these samples using 1 mL TRIzol (Invitrogen, Carlsbad, CA, USA) and stored at −80 °C until further use. The peripheral blood samples were collected and processed as described previously [37]. In order to avoid contamination with skin epithelial cells, the first 2 mL blood was discarded while collecting the blood samples (7.5 mL). The PBMCs containing CTCs were prepared using Red Blood Cell Lysis Buffer (Solarbio, Beijing, China) and the blood samples were processed within 30 min. After centrifugation at 2000× g for 15 min, buffy coat was collected and washed once with phosphate buffered saline (PBS; containing 0.14 g/L KH2PO4, 9 g/L NaCl, 0.8 g/L Na2HPO4, pH 7.4). The cells were pelleted by centrifugation. Subsequently, the tumor cell enrichment using anti-EpCAM antibodies, MACS HEA MicroBeads®, density gradient centrifugation, and OncoQuick® plus (Miltenyi Biotec Inc.,Bergisch Gladbach, Germany) was performed as described previously [17,38,39,40]. For each cytometric enrichment procedure, 15 mL peripheral blood was used.
4.3. RNA Extraction and cDNA Synthesis
Total RNA of fresh tissue was isolated with TRIzol (Invitrogen, Carlsbad, CA, USA). The total RNA of PBMCs was extracted using the MiRNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The concentration and purity of the total RNAs were assessed by Ultraviolet spectrophotometer, and the integrity of total RNAs was estimated by polyacrylamide electrophoresis (PAGE). cDNA synthesis was performed using PrimeScriptTM RT reagent Kit (Takara, Otsu, Japan) for tissue RNA and PrimeScriptTM miRNA cDNA Synthesis Kit (Takara, Otsu, Japan) for PBMCs RNA.
4.4. Real-Time RT-PCR
The mRNA levels of the genes of interest were estimated by real-time RT-PCR on Applied Biosystems 7500 (Foster City, CA, USA) using SYBR® Premix Ex TaqTM II (Takara, Otsu, Japan). The sequences of primers were designed as described previously [17,18]. To prepare an artificial CTC model as a positive control, a trace amount of A549 human lung adenocarcinoma cells was mixed with peripheral blood samples (five A549 cells in 7.5 mL peripheral blood). Then, the total RNA was extracted from the mixture and used as the positive control in real-time RT-PCR. The total amount of mRNA was normalized to GAPDH, and the relative mRNA expressions of the samples (tumor vs. PCHNTs, or paired PBMCs vs. positive control) were calculated by 2−ΔΔCt. The differential expression of the samples may be defined as the upregulation when the cut-off value was set at 2-fold.
4.5. Statistical Analysis
The SPSS 17.0 statistical software package was used for all the statistical analyses. The correlation between the level of the above described four mRNAs and the clinicopathological characteristics was analyzed by Person’s chi-square test or Fisher’s exact test. The survival curves and univariate analysis were generated by the Kaplan-Meier method and log-rank test. Multivariate analysis was performed by the Cox regression model and Wald test. p < 0.05 was considered statistically significant.
5. Conclusions
In present study, we selected CK7, ELF3, EGFR and EphB4, four epithelial origin markers, to evaluate their efficiency for detecting CTCs in NSCLC. The expression of CK7, ELF3, EGFR and EphB4 in NSCLC tissues and para PMBCs were both upregulated significantly. ROC curve analysis indicated that these four genes have high sensitivity and specificity for detecting CTCs. Moreover, upregulation of four genes in PMBCs are associated with tumor progression closely. The patients with high expression of these genes in PMBCs have poor survival. We speculated that CK7, ELF3, EGFR and EphB4 expression in PMBCs are appropriate markers for detecting CTCs and have potential in the evaluation of prognosis and monitoring of therapy in NSCLC.
Acknowledgments
This research was partly supported by a grant from Zhejiang Provincial Medicine and Hygiene Programs (2012KYB035), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (Zhi-Qiang Ling), the Major Training Personnel from Zhejiang Provincial Program for the Training and Development Project for 151 talents (Zhi-Qiang Ling), and the Special Fund from Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus) (Xin-Min Yu).
Author Contributions
Zhi-Qiang Ling conceived designed the project. Xin-Min Yu and Yi-Chen Wu performed most experiments and wrote the paper. Xiang Liu and Xian-Cong Huang performed the sample collection and processing experiments. Xiu-Xiu Hou and Jiu-Li Wang helped to prepare the compounds. Xiang-Liu Cheng provided technical support for RT-PCR analysis. Wei-Min Mao and Zhi-Qiang Ling read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kalia M. Biomarkers for personalized oncology: Recent advances and future challenges. Metabolism. 2015;64:S16–S21. doi: 10.1016/j.metabol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Okimoto R.A., Bivona T.G. Recent advances in personalized lung cancer medicine. Per. Med. 2014;11:309–321. doi: 10.2217/pme.14.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathuria H., Gesthalter Y., Spira A., Brody J.S., Steiling K. Updates and controversies in the rapidly evolving field of lung cancer screening, early detection, and chemoprevention. Cancers. 2014;6:1157–1179. doi: 10.3390/cancers6021157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilie M., Hofman V., Long E., Bordone O., Selva E., Washetine K., Marquette C.H., Hofman P. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann. Transl. Med. 2014;2 doi: 10.3978/j.issn.2305-5839.2014.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz L.A., Bardelli A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joosse S.A., Gorges T.M., Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2014;7:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Y., Su C., Liu Z. Methods for detection of circulating cells in non-small cell lung cancer. Front. Biosci. 2014;19:896–903. doi: 10.2741/4255. [DOI] [PubMed] [Google Scholar]
- 8.Warkiani M.E., Khoo B.L., Tan D.S., Bhagat A.A., Lim W.T., Yap Y.S., Lee S.C., Soo R.A., Han J., Lim C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst. 2014;139:3245–3255. doi: 10.1039/c4an00355a. [DOI] [PubMed] [Google Scholar]
- 9.De Albuquerque A., Kubisch I., Stölzel U., Ernst D., Boese-Landgraf J., Breier G., Stamminger G., Fersis N., Kaul S. Prognostic and predictive value of circulating tumor cell analysis in colorectal cancer patients. J. Transl. Med. 2012;10 doi: 10.1186/1479-5876-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., Qi H., Zhou H.X., Deng C.Y., Zhu H., Li J.F., Wang X.L., Li F.R. Detection of micrometastases in peripheral blood of non-small cell lung cancer with a refined immunomagnetic nanoparticle enrichment assay. Int. J. Nanomed. 2011;6:2175–2181. doi: 10.2147/IJN.S24731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felton T., Harris G.C., Pinder S.E., Snead D.R., Carter G.I., Bell J.A., Haines A., Kollias J., Robertson J.F., Elston C.W., et al. Identification of carcinoma cells in peripheral blood samples of patients with advanced breast carcinoma using RT-PCR amplification of CK7 and MUC1. Breast. 2004;13:35–41. doi: 10.1016/S0960-9776(03)00126-7. [DOI] [PubMed] [Google Scholar]
- 12.Raynor M., Stephenson S.A., Walsh D.C., Pittman K.B., Dobrovic A. Optimisation of the RT-PCR detection of immunomagnetically enriched carcinoma cells. BMC Cancer. 2002;2 doi: 10.1186/1471-2407-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Xie J., Yu C., Yan L., Yang Z. mRNA expression of CK19, EGFR and LUNX in patients with lung cancer micrometastasis. Exp. Ther. Med. 2014;7:360–364. doi: 10.3892/etm.2013.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang X.X., Brodeur G.M., Campling B.G., Ikegaki N. Coexpression of transcripts encoding EPHB receptor protein tyrosine kinases and their ephrin-B ligands in human small cell lung carcinoma. Clin. Cancer Res. 1999;5:455–460. [PubMed] [Google Scholar]
- 15.Chiang A.C., Massagué J. Molecular basis of metastasis. N. Engl. J. Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wicha M.S., Hayes D.F. Circulating tumor cells: Not all detected cells are bad and not all bad cells are detected. J. Clin. Oncol. 2011;29:1508–1511. doi: 10.1200/JCO.2010.34.0026. [DOI] [PubMed] [Google Scholar]
- 17.Man Y., Cao J., Jin S., Xu G., Pan B., Shang L., Che D., Yu Q., Yu Y. Newly identified biomarkers for detecting circulating tumor cells in lung adenocarcinoma. Tohoku J. Exp. Med. 2014;234:29–40. doi: 10.1620/tjem.234.29. [DOI] [PubMed] [Google Scholar]
- 18.Winter S.C., Stephenson S.A., Subramaniam S.K., Paleri V., Ha K., Marnane C., Krishnan S., Rees G. Long term survival following the detection of circulating tumor cells in head and neck squamous cell carcinoma. BMC Cancer. 2009;9 doi: 10.1186/1471-2407-9-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng X., Chen H. Tumor heterogeneity and resistance to EGFR-targeted therapy in advanced nonsmall cell lung cancer: Challenges and perspectives. Onco Targets Ther. 2014;7:1689–1704. doi: 10.2147/OTT.S66502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilo R., Capelozzi V.L., Siqueira S.A.C., Bernardi F.D.C. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum. Pathol. 2006;37:542–546. doi: 10.1016/j.humpath.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda S., Fujimori M., Shibata S., Okajima M., Ishizaki Y., Kurihara T., Miyata Y., Iseki M., Shimizu Y., Tokumoto N., et al. Combined immunohistochemistry of β-catenin, cytokeratin 7, and cytokeratin 20 is useful in discriminating primary lung adenocarcinomas from metastatic colorectal cancer. BMC Cancer. 2006;6 doi: 10.1186/1471-2407-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chhieng D.C., Cangiarella J.F., Zakowski M.F., Goswami S., Cohen J.M., Yee H.T. Use of thyroid transcription factor 1, PE-10, and cytokeratins 7 and 20 in discriminating between primary lung carcinomas and metastatic lesions in fine-needle aspiration biopsy specimens. Cancer. 2001;93:330–336. doi: 10.1002/cncr.9048. [DOI] [PubMed] [Google Scholar]
- 23.Gruver A.M., Amin M.B., Luthringer D.J., Westfall D., Arora K., Farver C.F., Osunkoya A.O., McKenney J.K., Hansel D.E. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Arch. Pathol. Lab. Med. 2012;136:1339–1346. doi: 10.5858/arpa.2011-0575-OA. [DOI] [PubMed] [Google Scholar]
- 24.Thomas R.S., Ng A.N., Zhou J., Tymms M.J., Doppler W., Kola I. The Elf group of Ets-related transcription factors. ELF3 and ELF5. Adv. Exp. Med. Biol. 2000;480:123–128. doi: 10.1007/0-306-46832-8_15. [DOI] [PubMed] [Google Scholar]
- 25.Tymms M.J., Ng A.Y., Thomas R.S., Schutte B.C., Zhou J., Eyre H.J., Sutherland G.R., Seth A., Rosenberg M., Papas T., et al. A novel epithelial-expressed ETS gene, ELF3: Human and murine cDNA sequences, murine genomic organization, human mapping to 1q32.2 and expression in tissues and cancer. Oncogene. 1997;15:2449–2462. doi: 10.1038/sj.onc.1201427. [DOI] [PubMed] [Google Scholar]
- 26.Brembeck F.H., Opitz O.G., Libermann T.A., Rustgi A.K. Dual function of the epithelial specific ets transcription factor, ELF3, in modulating differentiation. Oncogene. 2000;19:1941–1949. doi: 10.1038/sj.onc.1203441. [DOI] [PubMed] [Google Scholar]
- 27.Nakarai C., Osawa K., Matsubara N., Ikeuchi H., Yamano T., Okamura S., Kamoshida S., Tsutou A., Takahashi J., Ejiri K., et al. Significance of ELF3 mRNA expression for detection of lymph node metastases of colorectal cancer. Anticancer Res. 2012;32:3753–3758. [PubMed] [Google Scholar]
- 28.Erdogan E., Klee E.W., Thompson E.A., Fields A.P. Meta-analysis of oncogenic protein kinase Ciota signaling in lung adenocarcinoma. Clin. Cancer Res. 2009;15:1527–1533. doi: 10.1158/1078-0432.CCR-08-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 30.Liang W., Zhang Y., Kang S., Pan H., Shao W., Deng Q., Shi X., Wang W., He J. Impact of EGFR mutation status on tumor response and progression free survival after first-line chemotherapy in patients with advanced non-small-cell lung cancer: A meta-analysis. J. Thorac. Dis. 2014;6:1239–1250. doi: 10.3978/j.issn.2072-1439.2014.07.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gately K., Forde L., Cuffe S., Cummins R., Kay E.W., Feuerhake F., O’Byrne K.J. High coexpression of both EGFR and IGF1R correlates with poor patient prognosis in resected non-small-cell lung cancer. Clin. Lung Cancer. 2014;15:58–66. doi: 10.1016/j.cllc.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Zheng M.F., Ji Y., Wu X.B., Ye S.G., Chen J.Y. EphB4 gene polymorphism and protein expression in non-small-cell lung cancer. Mol. Med. Rep. 2012;6:405–408. doi: 10.3892/mmr.2012.936. [DOI] [PubMed] [Google Scholar]
- 33.Hasina R., Mollberg N., Kawada I., Mutreja K., Kanade G., Yala S., Surati M., Liu R., Li X., Zhou Y., et al. Critical role for the receptor tyrosine kinase EPHB4 in esophageal cancers. Cancer Res. 2013;73:184–194. doi: 10.1158/0008-5472.CAN-12-0915. [DOI] [PubMed] [Google Scholar]
- 34.Li M., Zhao J., Qiao J., Song C., Zhao Z. EphB4 regulates the growth and migration of pancreatic cancer cells. Tumour Biol. 2014;35:6855–6859. doi: 10.1007/s13277-014-1937-6. [DOI] [PubMed] [Google Scholar]
- 35.Chen T., Liu X., Yi S., Zhang J., Ge J., Liu Z. EphB4 is overexpressed in gliomas and promotes the growth of glioma cells. Tumor Biol. 2013;34:379–385. doi: 10.1007/s13277-012-0560-7. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson B.D., Liu R., Rolle C.E., Tan Y.H., Krasnoperov V., Kanteti R., Tretiakova M.S., Cervantes G.M., Hasina R., Hseu R.D., et al. The EphB4 receptor tyrosine kinase promotes lung cancer growth: A potential novel therapeutic target. PLoS ONE. 2013;8:e67668. doi: 10.1371/journal.pone.0067668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edge S.B., Compton C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 38.Hayes D.C., Secrist H., Bangur C.S., Wang T., Zhang X., Harlan D., Goodman G.E., Houghton R.L., Persing D.H., Zehentner B.K. Multigene real-time PCR detection of circulating tumor cells in peripheral blood of lung cancer patients. Anticancer Res. 2006;26:1567–1575. [PubMed] [Google Scholar]
- 39.Xi L., Nicastri D.G., El-Hefnawy T., Hughes S.J., Luketich J.D., Godfrey T.E. Optimal markers for real-time quantitative reverse transcription PCR detection of circulating tumor cells from melanoma, breast, colon, esophageal, head and neck, and lung cancers. Clin. Chem. 2007;53:1206–1215. doi: 10.1373/clinchem.2006.081828. [DOI] [PubMed] [Google Scholar]
- 40.Königsberg R., Gneist M., Jahn-Kuch D., Pfeiler G., Hager G., Hudec M., Dittrich C., Zeillinger R. Circulating tumor cells in metastatic colorectal cancer: Efficacy and feasibility of different enrichment methods. Cancer Lett. 2010;293:117–123. doi: 10.1016/j.canlet.2010.01.003. [DOI] [PubMed] [Google Scholar]