Abstract
While most studies have been interested in the distinct, predisposing roles of the common BDNF Val66Met variant and extraversion personality traits on episodic memory, very few studies have looked at the synergistic effects of genetic and personality factors to account for cognitive variance. This is surprising considering recent reports challenging the long-held belief that the BDNFMet variant negatively impacts cognitive function. A total of 75 young healthy adults (26 of them carried at least one copy of the BDNFMet allele) took part in this study consisting of genetic profiling from saliva, personality assessment using the Revised NEO Personality Inventory (NEO PI-R) and a short battery of neuropsychological tests. An ANOVA revealed that BDNFMet carriers were significantly less extraverted than BDNFVal carriers (F1,73 = 9.54; p < 0.01; ηp2 = 0.126). Moreover, extraversion was found to significantly moderate the relationship between the BDNF genotype and episodic memory performance (p = 0.03). Subsequent correlational analyses yielded a strong and significant correlation (r = 0.542; p < 0.005) between introversion and delayed episodic memory specific to BDNFMet individuals. The present study suggests that introversion and the BDNFMet variant synergistically interact to reduce episodic memory performance in healthy, young adults. These findings reaffirm that a more accurate explanation of cognitive variance can be achieved by looking at the synergistic effects of genotype and phenotype factors.
Keywords: brain-derived neurotrophic factor, personality trait, memory
1. Introduction
The brain-derived neurotrophic factor (BDNF) belongs to the neurotrophin family and regulates cell survival, proliferation, and synaptic growth in many regions of the peripheral and central nervous system [1,2,3,4]. BDNF is widely expressed in the hippocampus [5] and plays an important role in human learning and memory processes through its modulating effect on synaptic changes, such as long-term potentiation (LTP) of hippocampal neurons [1,2,6].
A single functional nucleotide polymorphism (SNP) in the BDNF gene, resulting in a valine (Val) to methionine (Met) substitution at codon 66 in the prodomain (BDNFMet or BDNF Val66Met), is significantly associated with reduced BDNF secretion in an activity-dependant manner along with impairment of intracellular trafficking [7]. Early studies on the cognitive consequences of this valine-to-methionine substitution have associated the Met allele with reduced indices of cognitive function, including general intelligence [8], working memory [9,10], speed of information processing [11], and episodic memory [12,13,14]. In support of this notion, functional neuroimaging studies highlighted that relative to BDNFVal homozygotes, BDNFMet carriers show a relative decrease in hippocampal activation during encoding and retrieval of declarative memory [13,14], which in turn alters declarative memory performance [12,15,16]. Moreover, reduced hippocampal volume is also a known consequence of carrying the BDNFMet allele and the latter was associated with altered hippocampal function including declarative memory [17,18,19]. However, more recent reports on the effects of the BDNFMet variant on cognitive function have yielded mixed results and the regulating role of this BDNF polymorphism on episodic memory has been challenged in recent years [20]. Episodic memory is a subtype of declarative memory that includes the personally-experienced and conscious recollection of life events [21]. The growing interest in studying the fate of BDNFMet polymorphism partly stems from its high frequency in the Caucasian population (about 20%–30% carry at least one Met allele) [16,22], making any functional consequence of sizeable societal significance [23].
Another major factor in studying individual differences in cognitive performance, other than genetic vulnerability, is personality types. These are likely to be manifested in relatively stable and consistent patterns of cognition, emotion, motivation, and behavior, in response to a variety of eliciting stimuli [24]. There is now substantial consensus on the structure of personality in adulthood, with the most influential model being the Five-Factor Model of Personality [25]. According to this hierarchical model of trait structure, specific traits are organized in terms of five broad factors (neuroticism, extraversion, openness to experience, agreeableness and conscientiousness) that appear valid cross-culturally [26,27] and relatively stable across the lifespan [27]. Interestingly, among personality traits that influence cognitive performance, extraversion was associated with intelligence, especially with verbal ability [28,29,30]. Furthermore, a 25-year follow-up study in 4039 aging adults (aged 65 and older) showed that moderate extraversion was linked to lower risk of cognitive impairment in both case-control and co-twin designs [31]. This positive association between intelligence and extraversion is at least partially explained by the higher processing speed and assertiveness in extraverted individuals, which is advantageous for performance in most type of psychometric tests [29,32]. Conversely, introversion (i.e., low extraversion) seem to be a critical trait to predict reduced cognitive performance, mainly because of its likelihood to elicit test anxiety and lack of confidence [32,33].
In older adults (above the age of 60), extraversion has been positively associated with episodic memory, such that extraverted older adults tend to exhibit better free recall abilities relative to age-equivalent introverted individuals [29,34,35]. However, much less research has attempted to study the relation between extraversion and episodic memory performance in young adults. The major interest in studying factors that may interfere with episodic memory function at an older age stems from the clinical demonstration that episodic memory decline represents the commonest early symptom of the installation of Alzheimer’s disease [36,37,38,39]. However, one might wonder whether extraversion effects on episodic memory is age-dependent as opposed to being observed consistently throughout the lifespan.
In parallel, a genome-wide association (GWA) study on a genetically isolated population of 3972 individuals in Sardinia showed that the extraversion/introversion trait was selectively associated with the BDNF gene [40]. Interestingly, BDNFMet carriers were found to be more introverted (and thus had a significantly lower score at the NEO-PI-R Extraversion scale) compared with BDNFVal carriers [40]. These results were replicated in two independent, large cohorts from Italy (n = 1560) and the United States (n = 1131) [41].
While most studies have been interested in the distinct, predisposing roles of the common BDNF gene polymorphism and extraversion personality traits on episodic memory decline, very few studies have looked at the synergistic effects of genetic and personality factors to account for the significant variability in cognitive function on episodic memory performance. However, in a recent study from our group, middle-aged healthy adults who carried the BDNFMet allele were found to be significantly more introverted, and among them, BDNFMet carriers with the lowest extraversion scores were also those who obtained the lowest performance score on an episodic memory task [42]. These findings suggest that at least for middle-aged healthy adults, a more accurate explanation of cognitive variance can be achieved by looking at the synergistic effects genotype and phenotype factors.
In keeping with these findings in middle-aged healthy adults, the current study aimed to examine the potential relationship between the BDNF Val66Met polymorphism, the extraversion personality trait and episodic memory performance in a population of young healthy adults. We hypothesized that BDNFMet carriers would be more introverted when compared to homozygous BDNFVal carriers. Although debated in the literature, we hypothesized that BDNFMet carriers would exhibit poorer episodic memory performance than BDNF Val homozygotes. Most importantly, it was hypothesized that episodic memory performance would be most impaired in BDNFMet carriers who exhibit a more introverted personality type.
2. Results
2.1. Univariate Analyses of Variance
Univariate analyses of variance (ANOVA) were computed to assess the effect of BDNF polymorphism on extraversion as well as on neuropsychological tests (immediate and delayed word recalls). The ANOVA revealed that BDNFMet carriers were significantly less extraverted (and therefore more introverted) than BDNFVal carriers (F1,73 = 9.54; p < 0.01; ηp2 = 0.126). The BDNFMet variant, however, was not found to significantly change performance scores on any of the neuropsychological test including both immediate and delayed free recall of the RAVLT (immediate recall: F1,73 = 0.35; p = 0.61; ηp2 = 0.006; delayed recall: F1,73 = 1.18; p = 0.29; ηp2 = 0.021). Results from ANOVAs conducted for each neuropsychological test and personality traits are presented in Table 1.
Table 1.
Participant’s demographics and genotype effects on cognition and personality.
| Variables of Interest | BDNF Genotype | F | p | |
|---|---|---|---|---|
| BDNFMet (n = 25) | BDNFVal (n = 49) | |||
| Age (year) | 20.96 (1.94) | 20.58 (1.66) | 0.79 | 0.38 |
| Education (year) | 14.81 (1.72) | 14.16 (1.53) | 2.818 | 0.09 |
| BAI | 5.03 (4.85) | 4.79 (4.25) | 0.05 | 0.83 |
| BDI | 3.07 (2.76) | 3.73 (2.97) | 0.88 | 0.35 |
| Personality NEO-PI-R | ||||
| Neuroticism | 81.76 (17.14) | 80.21 (21.18) | 0.103 | 0.75 |
| Extraversion | 120.28 (10.87) | 131.05 (12.44) | 9.54 | 0.01 * |
| Openness | 101.04 (13.79) | 105.79 (16.31) | 1.60 | 0.21 |
| Agreeableness | 120.63 (18.21) | 121.78 (12.49) | 0.10 | 0.75 |
| Conscientiousness | 119.11 (24.19) | 118.52 (16.37) | 0.02 | 0.90 |
| RAVLT | ||||
| Word Immediate recall | 12.19 (2.77) | 11.81 (2.02) | 0.46 | 0.50 |
| Word Delayed recall | 12.06 (2.30) | 11.38 (2.56) | 1.28 | 0.26 |
| RCFT | ||||
| Immediate recall | 23.06 (6.16) | 24.03 (5.21) | 0.51 | 0.47 |
| Delayed recall | 22.92 (6.05) | 24.07 (5.27) | 0.73 | 0.40 |
| D-KEFS Verbal Fluency Test | ||||
| Condition 3 Total switching accuracy | 13.47 (2.19) | 12.71 (2.69) | 1.53 | 0.22 |
| Set-Loss Errors | 0.74 (0.85) | 0.89 (1.07) | 0.38 | 0.54 |
| D-KEFS Trail Making Test | ||||
| Condition 4 Total Time | 50.62 (12.01) | 50.78 (14.23) | 0.002 | 0.96 |
| Condition 4 Total Errors | 0.61 (0.75) | 0.64 (0.81) | 0.024 | 0.87 |
| D-KEFS Color-Word Interference Test | ||||
| Condition 4 Total Time | 49.71 (8.60) | 49.97 (12.45) | 0.009 | 0.93 |
| Condition 4 Total Errors | 1.06 (0.72) | 1.31 (1.29) | 0.84 | 0.36 |
BDNF: brain-derived neurotrophic factor; Met: methionine; Val: valine; RAVLT: Rey Auditory Verbal Learning Test; RCFT: Rey Complex Figure Test; SDMT: Symbol Digit Modalities Test; D-KEFS: Delis-Kaplan Executive Function System. Numbers in parentheses represent standard deviations values. * p < 0.05.
2.2. Regression Analyses
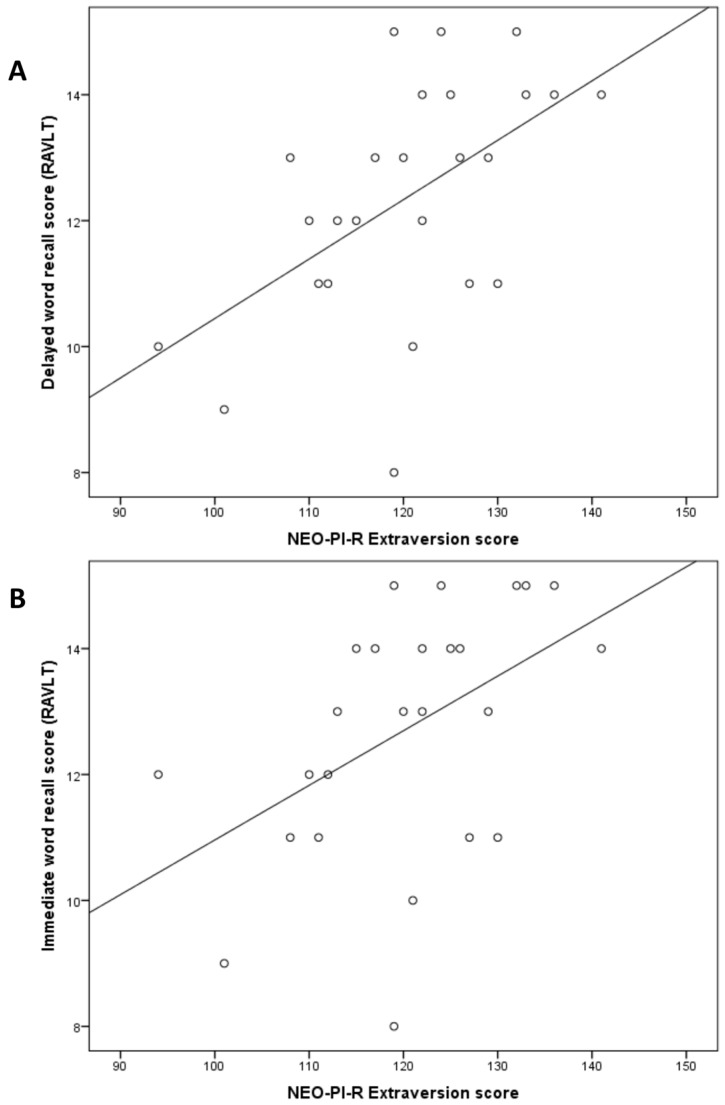
A linear regression showed that extraversion significantly moderated episodic memory performance across BDNF genotypes (β = 0.312; p = 0.03). Further correlational analyses stratified by BDNF genotype showed that for homozygous BDNFVal carriers, no significant correlation was found between scores on the NEO-PI-R Extraversion subscale and both immediate word recall score (r = −0.029; p = 0.87) and delayed word recall score (r = 0.096; p = 0.60). However, for the BDNFMet carriers, extraversion was positively correlated with both immediate word recall (r = 0.479; p = 0.015) and delayed word recall (r = 0.542; p = 0.005) (Table 2; Figure 1). These results indicate that the modulating effects of extraversion on episodic memory performance are selective to BDNFMet carriers. Other correlations stratified by BDNF genotype were also conducted to assess potential associations between extraversion and neuropsychological tests. Extraversion did not correlate with any of the other neuropsychological measures (Refer to Table 2).
Table 2.
Correlation between extraversion and cognition by genotype.
| BDNFMet | R | p | n |
| RAVLT | |||
| Word Immediate recall | 0.479 | 0.015 * | 25 |
| Word Delayed recall | 0.542 | 0.005 * | 25 |
| RCFT | |||
| Immediate recall | 0.151 | 0.46 | 25 |
| Delayed recall | 0.182 | 0.37 | 25 |
| D-KEFS Verbal Fluency Test | |||
| Condition 3 Total switching accuracy | 0.256 | 0.21 | 25 |
| Set-Loss Errors | 0.249 | 0.22 | 25 |
| D-KEFS Trail Making Test | |||
| Condition 4 Total Time | 0.321 | 0.11 | 25 |
| Condition 4 Total Errors | −0.014 | 0.94 | 25 |
| D-KEFS Color-Word Interference Test | |||
| Condition 4 Total Time | −0.131 | 0.52 | 25 |
| Condition 4 Total Errors | −0.024 | 0.91 | 25 |
| BDNFVal | R | p | n |
| RAVLT | |||
| Word Immediate recall | −0.035 | 0.81 | 49 |
| Word Delayed recall | 0.125 | 0.39 | 49 |
| RCFT | |||
| Immediate recall | 0.068 | 0.64 | 49 |
| Delayed recall | 0.050 | 0.73 | 49 |
| D-KEFS Verbal Fluency Test | |||
| Condition 3 Total switching accuracy | 0.172 | 0.24 | 49 |
| Set-Loss Errors | 0.085 | 0.56 | 49 |
| D-KEFS Trail Making Test | |||
| Condition 4 Total Time | −0.201 | 0.17 | 49 |
| Condition 4 Total Errors | −0.053 | 0.72 | 49 |
| D-KEFS Color-Word Interference Test | |||
| Condition 4 Total Time | 0.016 | 0.91 | 49 |
| Condition 4 Total Errors | −0.216 | 0.14 | 49 |
BDNF: brain-derived neurotrophic factor; Met: methionine; Val: valine; RAVLT: Rey Auditory Verbal Learning Test; RCFT: Rey Complex Figure Test; SDMT: Symbol Digit Modalities Test; D-KEFS: Delis-Kaplan Executive Function System. Numbers in parentheses represent standard deviations values. * p < 0.05.
Figure 1.
(A) Scatter plot illustrating the relation between the extraversion score at the NEO PI-R and delayed word recall performance in young healthy adults carrying at least one copy of the BDNFMet allele; (B) Scatter plot illustrating the relation between the extraversion score at the NEO PI-R and immediate word recall performance in young healthy adults carrying at least one copy of the BDNFMet allele.
3. Discussion
The present study found an association between polymorphism in the BDNF gene, extraversion and episodic memory performance in a population of young adults. In keeping with previous findings [40,41,42], BDNFMet carriers scored significantly lower in extraversion (i.e., higher levels of introversion) when compared to BDNFVal carriers. While no group difference by BDNF polymorphism was found for episodic memory performance, a significant and positive correlation was observed between extraversion and episodic memory performance for the young, BDNFMet carriers, which was absent in age-equivalent BDNFVal carriers. Further moderation analyses revealed that extraversion significantly moderated the relationship between BDNF polymorphism and episodic memory performance, in such a way that high levels of introversion significantly contribute the reduction in declarative memory performance in young, BDNFMet carriers, but not in age-equivalent BDNFVal carriers.
The main finding of this study is that the extraversion personality trait interacts with the BDNFMet polymorphism to alter episodic memory performance among young adults. Consistent with results obtained in a sample of 135 healthy young adults [43], the present study found an absence of BDNFMet variant effect alone on episodic memory performance. Age-associated synaptic plasticity efficiency loss may provide an explanation as to why BDNF genotype only affects episodic memory performance later in life [44]. Accordingly, more efficient synaptic plasticity in early adulthood could compensate for hippocampal vulnerabilities associated with the BDNFMet polymorphism. In addition, knowing that episodic memory performance and BDNF levels are both known to decrease steadily with the normal ageing process [45], young adults carrying the Met allele could find themselves at a lower risk of episodic memory alterations due to the abundance of BDNF [46]. Another possible explanation for this age-dependent effect of the BDNFMet variant on episodic memory performance could be that extraversion, a moderating factor of the relationship between genotype and memory performance in the present study, is considerably shaped by experiences over the lifespan, especially by social and stressful experiences, and is negatively associated with age [47,48], in part because of the lower social stimulation found in older adults [35]. In light of these results, it appears likely that among introverted BDNFMet carriers, older adults exhibit, on average, a higher level of introversion than young adults, which could exacerbate between-group differences on memory performance in older adults. Thus, the present study suggests that individual differences in memory seen in middle-aged introverted BDNFMet carriers [42] may be present from their early age. A longitudinal follow-up study would be helpful to assess whether age-associated memory performance reductions could be linked to concomitant changes in personality across the lifespan.
The combined genetic-personality trait effect on memory performance can potentially be explained by the synergistic deleterious effects of introversion and the BDNFMet variant on poor adaptation to environmental stress [49,50]. Indeed, it is well known that high stress levels indirectly lead to poorer memory processes via stress dysregulation effects on hippocampal long-term potentiation [51,52,53]. In BDNFMet knock-in mice, higher stress is found to deplete BDNF availability, which alters memory processes [54,55]. In humans, it was shown that Met allele carriers tend to exhibit higher anxiety levels, which indirectly affect memory processes through HPA stress response dysregulation [49,56,57]. Furthermore, Met allele carriers tend to benefit from less social support and they tend to be less engaged than BDNF Val homozygotes in social interactions [58], which could lead to higher stress levels and thus, to poorer memory performance. In agreement with these findings, higher levels of introversion found in Met allele carriers from this study were shown to significantly associate with lower performance scores in an episodic memory task. The current study results are in line with a previous report suggesting that extraversion is protective against stress outcomes [59] and is associated with adjustment to stress, life satisfaction and resilience [50,60]. Indeed, extraverts seek more social support/interaction than introverts [61,62,63] and engage their social networks under stressful conditions, which help them to efficiently cope with stress [63,64,65]. It is therefore possible that the quality and abundance of social interactions among extraverts provided them with plentiful opportunities to find themselves in a learning context where memory skills can be further developed.
In addition, studies show that extraverts experience more positive affects [50,66,67,68] while introverts experience more negative emotions [69]. Considering that higher positive affects experienced during encoding processes generate emotional contextual markers that are associated with event memory traces to be remembered, it is possible that a higher level of extraversion contributes to better memory performance [21,34,70]. The BDNF Met allele and introversion could be conceptualized as individual vulnerabilities that possibly predispose the individual to lower social support/interactions, poorer adaptation to stress and less positive affect, which may in turn alter memory processes. Thus, the present results extend previous findings as it shows that low extraversion interact with the BDNF Met variant to reduce episodic memory performance throughout the lifespan.
From a clinical perspective, as extraversion can be conceptualized as a buffer in the trajectory of age-related episodic memory decline [35,71] and other cognitive functions, the addition of a personality assessment in standard neuropsychological practice could further refine our clinical understanding of longitudinal changes of cognitive function. Moreover, the BDNFMet x introversion association with poorer episodic memory performance found in the current study also highlights the need for future studies to carefully take into account the modulating role of environmental factors such as stress and social interactions on memory processes.
Although this study is the first to investigate the effect of personality and BDNF on episodic memory performance in a population of young adults, it is not without limitations. A large-scale study will be helpful to replicate the current study findings due to its restricted sample size. Serum levels of BDNF could also reveal to be informative if we are to further investigate the association between memory performance and available BDNF levels.
4. Materials and Methods
4.1. Participants
All 75 participants in this study were collegial or university-level Caucasian students from French-Canadian origins, with an average education level of 14.38 years (SD = 1.61). The sample was composed of 38 women (51.0%) and 37 men (49.0%) aged between 18 and 26 years (mean age = 20.71; SD = 1.81) recruited consecutively through newspaper advertisements and posters located throughout our University institution. Participants who took part in this study were those who were not excluded after having been screened for the following exclusion criteria, which are known to influence cognitive performance: A history of alcohol and/or substance abuse, psychiatric illness (e.g., depression, anxiety), developmental disorders (e.g., ADHD, autism spectrum disorders), learning disabilities (e.g., dyslexia), neurological history (e.g., seizure, epilepsy, central nervous system neoplasm, brain tumour), a history of traumatic brain injury, and having undergone cognitive testing during the year.
In this sample, there were 4 (5.3%) homozygous Met allele carriers, 22 (29.3%) heterozygous Met allele carriers and 49 (65.3%) homozygous Val allele carriers. Due to the rare occurrence of the Met allele in a homozygous state, carriers of this genotype were added to the group of heterozygous Met allele carriers. Participants were subdivided into two groups. The first group consisted of homozygous Val allele carriers (referred to as BDNFVal; n = 49) and the second group consisted of both heterozygous and homozygous carriers of the Met allele (referred to as BDNFMet; n = 26). Groups were equivalent according to age, level of education and sex (refer to Table 1). One participant had to be excluded from further analyses after rejection for outliers (Grubb’s test) at the NEO-PI-R extraversion subscale.
4.2. Procedure
Selected participants were tested at the Université du Québec à Trois-Rivières. All participants provided written informed consent before undergoing the study procedure. Saliva samples were taken from which the DNA could be extracted and genotyped. Participants were administered standard, neuropsychological tests and questionnaires and the study was approved by the local ethics committee of the Université du Québec à Trois-Rivières (Approved date: 1 May 2014; project ID code: CER-14-176-07.11).
4.2.1. Neuropsychological Testing
The neuropsychological test battery included the Color-Word Interference test, the Trail Making Test and the Verbal Fluency Test of the D-KEFS, assessing general frontal lobe function/executive functions [72]. The Rey Complex Figure Test (RCFT), assessing visual-constructional ability, visual-spatial organization and visual memory [73] was also administered. Both immediate and delayed recall conditions of the Rey Auditory Verbal Learning Test (RAVLT) were administered to evaluate verbal episodic memory. Briefly, the RAVLT is divided into two lists (A and B), each made up of 15 unrelated nouns. List A is first read out loud five times to the participant, each time followed by free recall. Then, List B (interference) is read, followed by free recall (B). Participants are then asked to recall List A immediately (immediate recall) and 20 minutes later (delayed recall) [74]. The RAVLT has been shown to be reliable, with test-retest reliabilities between 0.63 and 0.84 for the immediate recall trials and between 0.57 and 0.78 for the delayed recall trials [75].
4.2.2. Questionnaires
First, a self-reported questionnaire (General health questionnaire) was administered to screen for medical exclusion criteria known to influence brain function. In addition, the Beck Anxiety Inventory questionnaire and the Beck Depression Inventory were administered to exclude participants who scored above the pre-established clinical threshold for anxiety and depression. Furthermore, the Revised NEO Personality Inventory (NEO-PI-R) [76] was used to assess the five personality domains (the Big Five): neuroticism, extraversion, openness to experience, agreeableness and conscientiousness. The NEO PI-R has a robust factor structure replicated in more than 50 cultures [77]. Moreover, all five subscales provide a reliable measure of its corresponding construct, as evidenced by high reliability coefficients (from 0.75 to 0.89) and test-retest reliabilities coefficients (from 0.63 to 0.91) [76].
4.2.3. Genotyping
We performed DNA extraction from the buffy coat using Qiagen EZ1 DNA kit (Hilden, Germany). A PCR method was followed by pyrosequencing to obtain genotype profiling of BDNF rs6265 (Val66Met) polymorphism. The following primer pairs were used for PCR-based amplification: forward biotin 5′-GGACTCTGGAGAGCGTGAAT-3′ and reverse 5′-CCGAACTTTCTGGTCCTCATC-3′. Genomic DNA (250–500 ng) was amplified with 10 pM/of each primer, 1× PCR buffer (Quiagen kit), 0.4 mM dNTP, 1.0 mM MgCl2, and 0.01 U of Quiagen Taq polymerase. We then used a Biometra Tprofessional Basic thermocycler (Biometra, Göttingen, Germany) to conduct a 35-cycle amplification stage preceded by a 2-min hot start at 95 °C and followed by a final 4-min extension to the last cycle at 72 °C. Each cycle consisted of the following steps: 30 s at 95 °C, 30 s at 61.2 °C and 1 min at 72 °C. A 1.2% agarose gel was used to visualize PCR products. The Val66Met polymorphism was sequenced with a pyrosequencing protocol [78] routinely used in our laboratory with a slight modification using the sequencing oligomer 5′-GCTGACATTTCGAACA-3′. The sequence to analyze was: CA/GTGATAGAAGAG.
4.3. Statistical Analyses
All values are expressed as means (SDs). Data were analyzed with SPSS 21 (SPSS, Chicago, IL, USA). The significance level was set at α = 0.05, bilaterally. Groups were subjected to standard descriptive statistics. Effect sizes for mean differences were estimated with partial eta squared (ηp2). Between-group differences in neurocognitive test results and NEO-PI-R subscales scores were assessed using univariate analyses of variance (ANOVA). For each group separately, two-tailed Pearson correlations were computed between extraversion score and neuropsychological test scores. A linear regression analysis was performed to test the moderating effect of introversion on episodic memory performance in BDNFMet carriers.
5. Conclusions
Despite its limitations, this study is the first to investigate the effect of personality and BDNF genotype on episodic memory performance in a population of young adults. Our findings show that introversion and BDNFMet polymorphism interact to significantly affect episodic memory performance in young adults. Results from this study also suggest that reduced episodic memory found in middle-aged introverted BDNFMet carriers are present throughout the lifespan. Furthermore, we observed that in young adults, BDNFMet polymorphism alone was not associated with episodic memory performance, therefore reaffirming the pertinence to consider personality, especially the extraversion trait, along with genetic factors to account for cognitive variance at all ages.
Acknowledgments
This study was supported by research grants from Fonds de Recherche du Quebec en Sante awarded to Andreanne Bombardier and Louis De Beaumont.
Author Contributions
Louis De Beaumont and Judes Poirier conceived and designed the experiments; Andreanne Bombardier and Maude Beauchemin performed the experiment; Andreanne Bombardier, Louis De Beaumont and Nadia Gosselin analyzed the data; Andreanne Bombardier, Maude Beauchemin, and Louis De Beaumont wrote the paper; and Judes Poirier and Nadia Gosselin significantly contributed to editing the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Poo M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 2.Tyler W.J., Alonso M., Bramham C.R., Pozzo-Miller L.D. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramham C.R., Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Angelucci F., Brene S., Mathe A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 5.Conner J.M., Lauterborn J.C., Yan Q., Gall C.M., Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figurov A., Pozzo-Miller L.D., Olafsson P., Wang T., Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z.Y., Patel P.D., Sant G., Meng C.X., Teng K.K., Hempstead B.L., Lee F.S. Variant brain-derived neurotrophic factor (BDNF)(Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J. Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai S.J., Hong C.J., Yu Y.Y., Chen T.J. Association study of a brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and personality trait and intelligence in healthy young females. Neuropsychobiology. 2004;49:13–16. doi: 10.1159/000075333. [DOI] [PubMed] [Google Scholar]
- 9.Rybakowski J.K., Borkowska A., Czerski P.M., Skibińska M., Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disord. 2003;5:468–472. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 10.Rybakowski J.K., Borkowska A., Skibinska M., Szczepankiewicz A., Kapelski P., Leszczynska-Rodziewicz A., Czerski P.M., Hauser J. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin. Neurosci. 2006;60:70–76. doi: 10.1111/j.1440-1819.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- 11.Raz N., Rodrigue K.M., Kennedy K.M., Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempster E., Toulopoulou T., McDonald C., Bramon E., Walshe M., Filbey F., Wickham H., Sham P.C., Murray R.M., Collier D.A. Association between BDNF Val66Met genotype and episodic memory. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;134:73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- 13.Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 14.Hariri A.R., Goldberg T.E., Mattay V.S., Kolachana B.S., Callicott J.H., Egan M.F., Weinberger D.R. Brain-derived neurotrophic factor Val66Met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan H.Y., Chen Q., Sust S., Buckholtz J.W., Meyers J.D., Egan M.F., Mattay V.S., Meyer-Lindenberg A., Weinberger D.R., Callicott J.H. Epistasis between catechol-o-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc. Natl. Acad. Sci. USA. 2007;104:12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pezawas L., Verchinski B.A., Mattay V.S., Callicott J.H., Kolachana B.S., Straub R.E., Egan M.F., Meyer-Lindenberg A., Weinberger D.R. The brain-derived neurotrophic factor Val66Met polymorphism and variation in human cortical morphology. J. Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montag C., Weber B., Fliessbach K., Elger C., Reuter M. The BDNF Val66Met polymorphism impacts parahippocampal and amygdala volume in healthy humans: Incremental support for a genetic risk factor for depression. Psychol. Med. 2009;39:1831–1839. doi: 10.1017/S0033291709005509. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo K., Walss-Bass C., Nery F.G., Nicoletti M.A., Hatch J.P., Frey B.N., Monkul E.S., Zunta-Soares G.B., Bowden C.L., Escamilla M.A. Neuronal correlates of brain-derived neurotrophic factor Val66Met polymorphism and morphometric abnormalities in bipolar disorder. Neuropsychopharmacology. 2009;34:1904–1913. doi: 10.1038/npp.2009.23. [DOI] [PubMed] [Google Scholar]
- 19.Kambeitz J.P., Bhattacharyya S., Kambeitz-Ilankovic L.M., Valli I., Collier D.A., McGuire P. Effect of BDNF Val66Met polymorphism on declarative memory and its neural substrate: A meta-analysis. Neurosci. Biobehav. Rev. 2012;36:2165–2177. doi: 10.1016/j.neubiorev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Kim A., Fagan A.M., Goate A.M., Benzinger T.L., Morris J.C., Head D. Lack of an association of BDNF Val66Met polymorphism and plasma BDNF with hippocampal volume and memory. Cogn. Affect. Behav. Neurosci. 2015;15:625–643. doi: 10.3758/s13415-015-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tulving E. Episodic and semantic memory 1. Organ. Memory Lond. Acad. 1972;381:382–404. [Google Scholar]
- 22.Shimizu E., Hashimoto K., Iyo M. Ethnic difference of the BDNF 196G/A (Val66Met) polymorphism frequencies: The possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;126:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- 23.Cheeran B., Talelli P., Mori F., Koch G., Suppa A., Edwards M., Houlden H., Bhatia K., Greenwood R., Rothwell J.C. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrae R.R., Allik I.U. In: The Five-Factor Model of Personality Across Cultures. Marsella A.J., editor. Volume 1. Springer Science & Business Media; New York, NY, USA: 2002. pp. 1–4. [Google Scholar]
- 25.McCrae R.R., Costa P.T. Validation of the five-factor model of personality across instruments and observers. J. Personal. Soc. Psychol. 1987;52:81–90. doi: 10.1037/0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- 26.McCrae R.R., Costa P.T., Jr. Personality in Adulthood: A Five-Factor Theory Perspective. Volume 1. Guilford Press; New York, NY, USA: 2003. pp. 1–268. [Google Scholar]
- 27.McCrae R.R., Costa P.T., Jr. Personality trait structure as a human universal. Am. Psychol. 1997;52 doi: 10.1037/0003-066X.52.5.509. [DOI] [PubMed] [Google Scholar]
- 28.Rawlings D., Carnie D. The interaction of EPQ extraversion with WAIS subtest performance under timed and untimed conditions. Personal. Individ. Differ. 1989;10:453–458. doi: 10.1016/0191-8869(89)90009-3. [DOI] [Google Scholar]
- 29.Baker T.J., Bichsel J. Personality predictors of intelligence: Differences between young and cognitively healthy older adults. Personal. Individ. Differ. 2006;41:861–871. doi: 10.1016/j.paid.2006.02.017. [DOI] [Google Scholar]
- 30.Chamorro-Premuzic T., Furnham A., Petrides K. Personality and intelligence. J. Individ. Differ. 2006;27:147–150. doi: 10.1027/1614-0001.27.3.147. [DOI] [Google Scholar]
- 31.Crowe M., Andel R., Pedersen N.L., Fratiglioni L., Gatz M. Personality and risk of cognitive impairment 25 years later. Psychol. Aging. 2006;21:573–580. doi: 10.1037/0882-7974.21.3.573. [DOI] [PubMed] [Google Scholar]
- 32.Chamorro-Premuzic T., Furnham A. A possible model for understanding the personality-intelligence interface. Br. J. Psychol. 2004;95:249–264. doi: 10.1348/000712604773952458. [DOI] [PubMed] [Google Scholar]
- 33.Chamorro-Premuzic T., Furhnam A. Intellectual competence and the intelligent personality: A third way in differential psychology. Rev. Gen. Psychol. 2006;10:251–267. doi: 10.1037/1089-2680.10.3.251. [DOI] [Google Scholar]
- 34.Allen P.A., Kaut K., Baena E., Lien M.C., Ruthruff E. Individual differences in positive affect moderate age-related declines in episodic long-term memory. J. Cogn. Psychol. 2011;23:768–779. doi: 10.1080/20445911.2011.570254. [DOI] [Google Scholar]
- 35.Meier B., Perrig-Chiello P., Perrig W. Personality and memory in old age. Aging Neuropsychol. Cogn. 2002;9:135–144. doi: 10.1076/anec.9.2.135.9544. [DOI] [Google Scholar]
- 36.Mitchell D.B. How many memory systems? Evidence from aging. J. Exp. Psychol. Learn. Mem. Cogn. 1989;15:31–49. doi: 10.1037/0278-7393.15.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Light L.L. The organization of memory in old age. In: Craik F.I.M., Salthouse T.A., editors. The Handbook of Aging and Cognition. Volume 1. Psychology Press; New York, NY, USA: 1992. pp. 111–165. [Google Scholar]
- 38.Allen P.A., Sliwinski M., Bowie T., Madden D.J. Differential age effects in semantic and episodic memory. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002;57:173–186. doi: 10.1093/geronb/57.2.P173. [DOI] [PubMed] [Google Scholar]
- 39.Matthews G. Human Performance: Cognition, Stress, and Individual Differences. Volume 1. Psychology Press; New York, NY, USA: 2000. pp. 265–285. [Google Scholar]
- 40.Terracciano A., Sanna S., Uda M., Deiana B., Usala G., Busonero F., Maschio A., Scally M., Patriciu N., Chen W.M. Genome-wide association scan for five major dimensions of personality. Mol. Psychiatry. 2010;15:647–656. doi: 10.1038/mp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terracciano A., Tanaka T., Sutin A.R., Deiana B., Balaci L., Sanna S., Olla N., Maschio A., Uda M., Ferrucci L. BDNF Val66Met is associated with introversion and interacts with 5-HTTLPR to influence neuroticism. Neuropsychopharmacology. 2010;35:1083–1089. doi: 10.1038/npp.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Beaumont L., Fiocco A.J., Quesnel G., Lupien S., Poirier J. Altered declarative memory in introverted middle-aged adults carrying the BDNF Val66Met allele. Behav. Brain Res. 2013;253:152–156. doi: 10.1016/j.bbr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Richter-Schmidinger T., Alexopoulos P., Horn M., Maus S., Reichel M., Rhein C., Lewczuk P., Sidiropoulos C., Kneib T., Perneczky R. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J. Neural Transm. 2011;118:249–257. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- 44.Kolb B., Muhammad A., Gibb R. Searching for factors underlying cerebral plasticity in the normal and injured brain. J. Commun. Disord. 2011;44:503–514. doi: 10.1016/j.jcomdis.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., Virchow J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Ziegenhorn A.A., Schulte-Herbrüggen O., Danker-Hopfe H., Malbranc M., Hartung H.D., Anders D., Lang U.E., Steinhagen-Thiessen E., Schaub R.T., Hellweg R. Serum neurotrophins—A study on the time course and influencing factors in a large old age sample. Neurobiol. Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Donnellan M.B., Lucas R.E. Age differences in the Big Five across the life span: Evidence from two national samples. Psychol. Aging. 2008;23:558–566. doi: 10.1037/a0012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCrae R.R., Costa P.T., de Lima M.P., Simões A., Ostendorf F., Angleitner A., Marušić I., Bratko D., Caprara G.V., Barbaranelli C. Age differences in personality across the adult life span: parallels in five cultures. Dev. Psychol. 1999;35:466–477. doi: 10.1037/0012-1649.35.2.466. [DOI] [PubMed] [Google Scholar]
- 49.Colzato L.S., van der Does A.W., Kouwenhoven C., Elzinga B.M., Hommel B. BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36:1562–1569. doi: 10.1016/j.psyneuen.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Costa P.T., McCrae R.R. Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. J. Personal. Soc. Psychol. 1980;38:668–678. doi: 10.1037/0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- 51.Xu L., Anwyl R., Rowan M.J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 52.Kim J.J., Foy M.R., Thompson R.F. Behavioral stress modifies hippocampal plasticity through N-methyl-d-aspartate receptor activation. Proc. Natl. Acad. Sci. USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C.C., Yang C.H., Hsu K.S. Do stress and long-term potentiation share the same molecular mechanisms? Mol. Neurobiol. 2005;32:223–235. doi: 10.1385/MN:32:3:223. [DOI] [PubMed] [Google Scholar]
- 54.Yu H., Wang D.D., Wang Y., Liu T., Lee F.S., Chen Z.Y. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J. Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ninan I., Bath K.G., Dagar K., Perez-Castro R., Plummer M.R., Lee F.S., Chao M.V. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groves J. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z.Y., Jing D., Bath K.G., Ieraci A., Khan T., Siao C.J., Herrera D.G., Toth M., Yang C., McEwen B.S. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor W.D., Züchner S., McQuoid D.R., Steffens D.C., Blazer D.G., Krishnan K.R.R. Social support in older individuals: The role of the BDNF Val66Met polymorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147:1205–1212. doi: 10.1002/ajmg.b.30754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider T.R., Rench T.A., Lyons J.B., Riffle R.R. The influence of neuroticism, extraversion and openness on stress responses. Stress Health. 2012;28:102–110. doi: 10.1002/smi.1409. [DOI] [PubMed] [Google Scholar]
- 60.Suls J. Handbook of Affect and Social Cognition. Volume 1. Lawrence Erlbaum Associates Publishers; Mahwah, NJ, USA: 2001. Affect, Stress, and Personality; pp. 392–409. [Google Scholar]
- 61.Swickert R.J., Rosentreter C.J., Hittner J.B., Mushrush J.E. Extraversion, social support processes, and stress. Personal. Individ. Differ. 2002;32:877–891. doi: 10.1016/S0191-8869(01)00093-9. [DOI] [Google Scholar]
- 62.Watson D., Clark L.A. Handbook of Personality Psychology. Volume 1. Academic Press; San Diego, CA, USA: 1997. Extraversion and Its Positive Emotional Core; pp. 767–793. [Google Scholar]
- 63.Amirkhan J.H., Risinger R.T., Swickert R.J. Extraversion: A “hidden” personality factor in coping? J. Personal. 1995;63:189–212. doi: 10.1111/j.1467-6494.1995.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 64.David J.P., Suls J. Coping efforts in daily life: Role of Big Five traits and problem appraisals. J. Personal. 1999;67:265–294. doi: 10.1111/1467-6494.00056. [DOI] [PubMed] [Google Scholar]
- 65.Watson D., Hubbard B. Adaptational style and dispositional structure: Coping in the context of the five-factor model. J. Personal. 1996;64:737–774. doi: 10.1111/j.1467-6494.1996.tb00943.x. [DOI] [Google Scholar]
- 66.Pavot W., Diener E., Fujita F. Extraversion and happiness. Personal. Individ. Differ. 1990;11:1299–1306. doi: 10.1016/0191-8869(90)90157-M. [DOI] [Google Scholar]
- 67.Watson D., Clark L.A. On traits and temperament: General and specific factors of emotional experience and their relation to the five-factor model. J. Personal. 1992;60:441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 68.Rusting C.L., Larsen R.J. Extraversion, neuroticism, and susceptibility to positive and negative affect: A test of two theoretical models. Personal. Individ. Differ. 1997;22:607–612. doi: 10.1016/S0191-8869(96)00246-2. [DOI] [Google Scholar]
- 69.Jylhä P., Melartin T., Rytsälä H., Isometsä E. Neuroticism, introversion, and major depressive disorder—Traits, states, or scars? Depress. Anxiety. 2009;26:325–334. doi: 10.1002/da.20385. [DOI] [PubMed] [Google Scholar]
- 70.Craik F.I., Lockhart R.S. Levels of processing: A framework for memory research. J. Verbal Learn. Verbal Behav. 1972;11:671–684. doi: 10.1016/S0022-5371(72)80001-X. [DOI] [Google Scholar]
- 71.Arbuckle T.Y., Gold D.P., Andres D., Schwartzman A., Chaikelson J. The role of psychosocial context, age, and intelligence in memory performance of older men. Psychol. Aging. 1992;7:25–36. doi: 10.1037/0882-7974.7.1.25. [DOI] [PubMed] [Google Scholar]
- 72.Lezak M.D. Neuropsychological Assessment. 3rd ed. Oxford University Press; New York, NY, USA: 1995. pp. 346–352. [Google Scholar]
- 73.Strauss E., Sherman E.M., Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York, NY, USA: 2006. pp. 263–266. [Google Scholar]
- 74.Fichman H.C., Teresa Dias L.B., Fernandes C.S., Lourenço R., Caramelli P., Nitrini R. Normative data and construct validity of the Rey Auditory Verbal Learning Test in a Brazilian elderly population. Psychol. Neurosci. 2010;3:79–84. doi: 10.3922/j.psns.2010.1.010. [DOI] [Google Scholar]
- 75.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Volume 1. Western Psychological Services; Los Angeles, CA, USA: 1996. pp. 6–27. [Google Scholar]
- 76.Costa P.T., McCrae R.R. Revised Neo Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Volume 1. Psychological Assesment Resources; Lutz, FL, USA: 1992. pp. 36–39. [Google Scholar]
- 77.McCrae R.R., Terracciano A. Universal features of personality traits from the observer’s perspective: Data from 50 cultures. J. Personal. Soc. Psychol. 2005;88:547–561. doi: 10.1037/0022-3514.88.3.547. [DOI] [PubMed] [Google Scholar]
- 78.Royo J.L., Hidalgo M., Ruiz A. Pyrosequencing protocol using a universal biotinylated primer for mutation detection and SNP genotyping. Nat. Protoc. 2007;2:1734–1739. doi: 10.1038/nprot.2007.244. [DOI] [PubMed] [Google Scholar]