Abstract
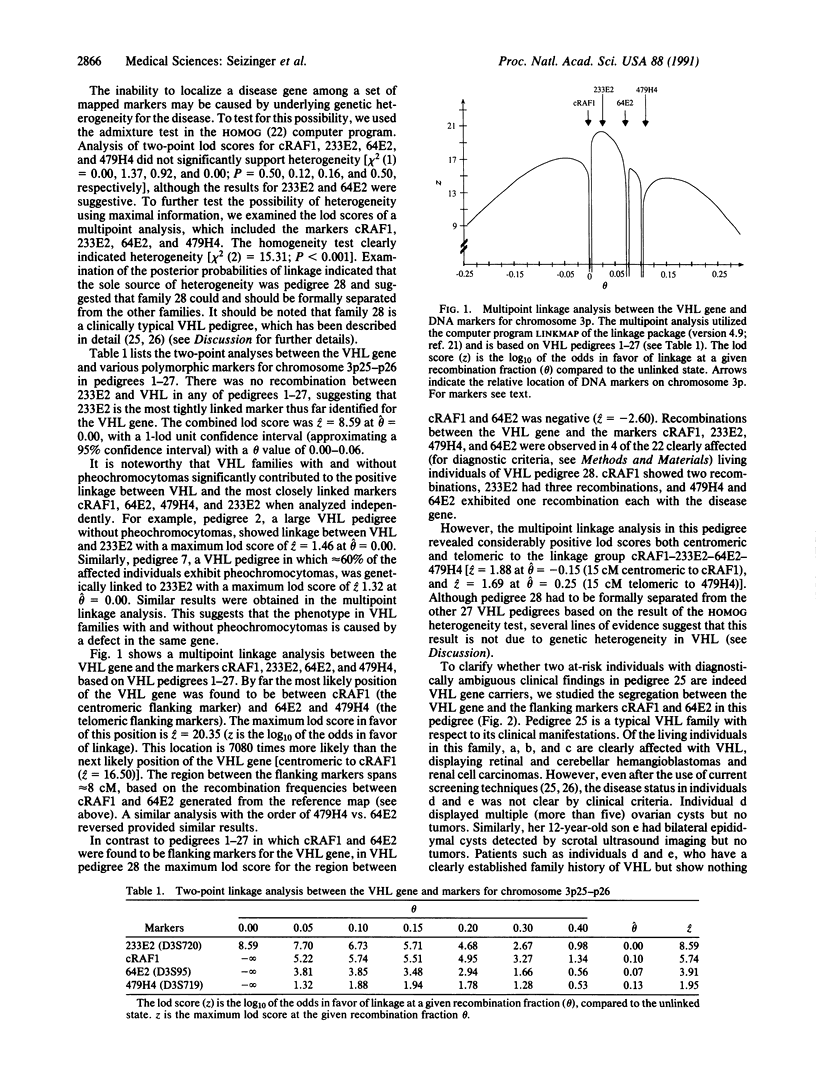
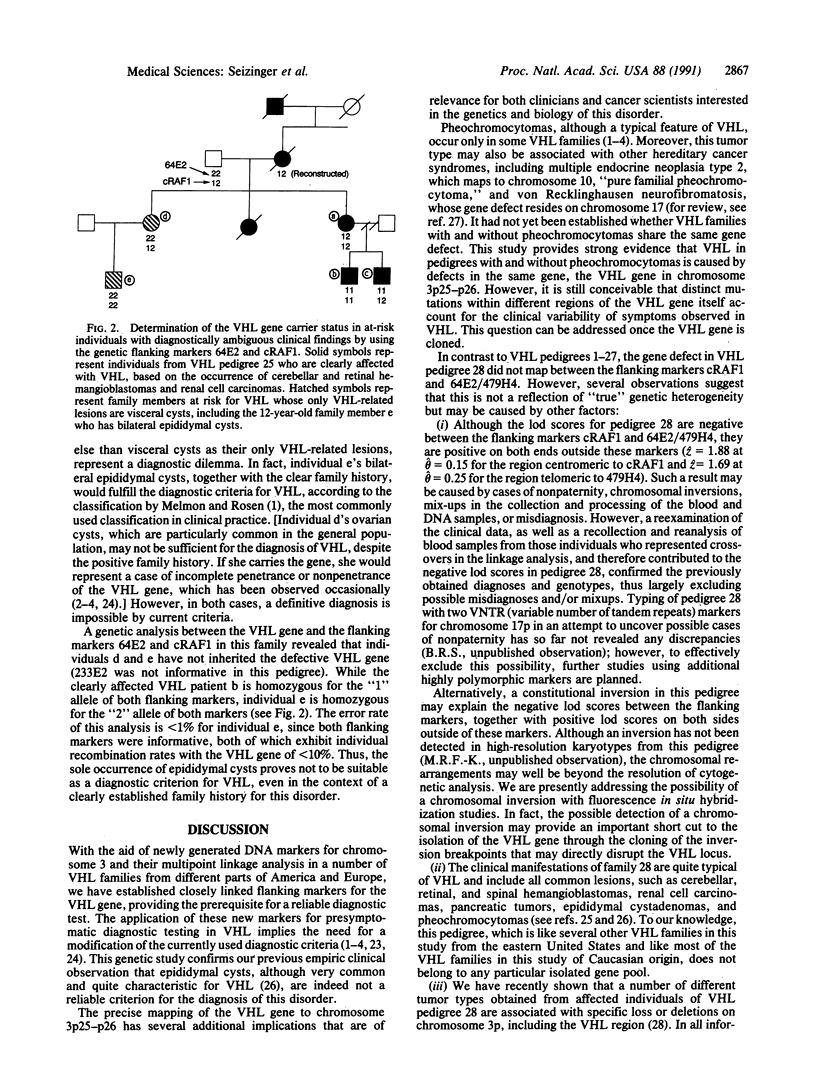
Von Hippel Lindau disease (VHL) is a hereditary syndrome, associated with tumors and cysts in multiple organ systems, whose expression and age of onset are highly variable. The availability of a genetic test for the early and reliable detection of individuals carrying the defective gene would be beneficial for VHL patients and their relatives, since many of the manifestations of VHL can be successfully treated if detected in their early stages, while the complications of undetected disease can be devastating. We have previously shown that the VHL gene maps to chromosome 3p. To provide genetic markers for the development of a reliable diagnostic test, and to further narrow and eventually clone the VHL defect, we have generated DNA markers for chromosome 3p. With these markers, we have performed a multipoint genetic linkage analysis in 28 VHL pedigrees, comprising 470 individuals, 164 of whom were affected with VHL. Here we report the identification of tightly linked markers, including flanking markers that bracket the VHL gene to a small region on chromosome 3p25-p26. This finding has several major implications. While visceral cysts of the kidney, pancreas, and epididymis are commonly found in VHL and are considered diagnostic criteria for this disorder, they also occur in the general population. The presence of cysts, unaccompanied by other more typical lesions such as retinal and cerebellar hemangioblastoma, may therefore represent a major diagnostic problem, leading to errors in the assessment of disease status. The application of flanking markers for the VHL gene for presymptomatic diagnostic testing confirms that epididymal cysts are indeed not suitable as a diagnostic criterion in this disorder. Pheochromocytomas occur nonuniformly in VHL families and may also be associated with other hereditary tumor syndromes; our genetic studies imply that the phenotype in VHL families with and without pheochromocytomas is caused by defects within the same gene. The absence or presence of this tumor type is therefore due to the pleiotropic expression of a single gene rather than to the existence of several different genes for VHL. The region on chromosome 3p13-p14 known to contain several chromosomal translocation breakpoints in families with "pure familial renal cell carcinoma" is quite proximal to the VHL locus in 3p25-p26 we have identified. Chromosome 3p may therefore contain two loci for renal cell carcinoma: one gene (or genes) in 3p13-p14 and the VHL gene in 3p25-p26, whose aberration is also associated with other typical manifestations of VHL. Since renal cell carcinoma, pheochromocytoma, and visceral cysts can occur sporadically even in young people and may also be associated with other tumor syndromes, the availability of flanking markers for the VHL gene will be useful in identifying VHL gene carriers, particularly among those individuals at risk in whom these are the only manifestations of disease. The isolation and characterization of the VHL gene, based on the identification of flanking markers, will have important implications for diagnosis and treatment of patients with VHL, as well as for a much larger number of individuals having the sporadic counterparts of VHL-associated tumor types.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Choyke P. L., Filling-Katz M. R., Shawker T. H., Gorin M. B., Travis W. D., Chang R., Seizinger B. R., Dwyer A. J., Linehan W. M. von Hippel-Lindau disease: radiologic screening for visceral manifestations. Radiology. 1990 Mar;174(3 Pt 1):815–820. doi: 10.1148/radiology.174.3.2305064. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., Li F. P., Berg S., Marchetto D. J., Tsai S., Jacobs S. C., Brown R. S. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979 Sep 13;301(11):592–595. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- Drabkin H., Sage M., Helms C., Green P., Gemmill R., Smith D., Erickson P., Hart I., Ferguson-Smith A., Ruddle F. Regional and physical mapping studies characterizing the Greig polysyndactyly 3;7 chromosome translocation, t(3;7)(p21.1;p13). Genomics. 1989 May;4(4):518–529. doi: 10.1016/0888-7543(89)90275-9. [DOI] [PubMed] [Google Scholar]
- Filling-Katz M. R., Choyke P. L., Patronas N. J., Gorin M. B., Barba D., Chang R., Doppman J. L., Seizinger B., Oldfield E. H. Radiologic screening for von Hippel-Lindau disease: the role of Gd-DTPA enhanced MR imaging of the CNS. J Comput Assist Tomogr. 1989 Sep-Oct;13(5):743–755. doi: 10.1097/00004728-198909000-00001. [DOI] [PubMed] [Google Scholar]
- Gerber M. J., Drabkin H. A., Firnhaber C., Miller Y. E., Scoggin C. H., Smith D. I. Regional localization of chromosome 3-specific DNA fragments by using a hybrid cell deletion mapping panel. Am J Hum Genet. 1988 Oct;43(4):442–451. [PMC free article] [PubMed] [Google Scholar]
- Gilliam T. C., Tanzi R. E., Haines J. L., Bonner T. I., Faryniarz A. G., Hobbs W. J., MacDonald M. E., Cheng S. V., Folstein S. E., Conneally P. M. Localization of the Huntington's disease gene to a small segment of chromosome 4 flanked by D4S10 and the telomere. Cell. 1987 Aug 14;50(4):565–571. doi: 10.1016/0092-8674(87)90029-8. [DOI] [PubMed] [Google Scholar]
- Go R. C., Lamiell J. M., Hsia Y. E., Yuen J. W., Paik Y. Segregation and linkage analyses of von Hippel Lindau disease among 220 descendants from one kindred. Am J Hum Genet. 1984 Jan;36(1):131–142. [PMC free article] [PubMed] [Google Scholar]
- Green J. S., Bowmer M. I., Johnson G. J. Von Hippel-Lindau disease in a Newfoundland kindred. CMAJ. 1986 Jan 15;134(2):133-8, 146. [PMC free article] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J. L., Ozelius L. J., McFarlane H., Menon A., Tzall S., Martiniuk F., Hirschhorn R., Gusella J. F. A genetic linkage map of chromosome 17. Genomics. 1990 Sep;8(1):1–6. doi: 10.1016/0888-7543(90)90218-j. [DOI] [PubMed] [Google Scholar]
- Huson S. M., Harper P. S., Hourihan M. D., Cole G., Weeks R. D., Compston D. A. Cerebellar haemangioblastoma and von Hippel-Lindau disease. Brain. 1986 Dec;109(Pt 6):1297–1310. doi: 10.1093/brain/109.6.1297. [DOI] [PubMed] [Google Scholar]
- Kovacs G., Brusa P., De Riese W. Tissue-specific expression of a constitutional 3;6 translocation: development of multiple bilateral renal-cell carcinomas. Int J Cancer. 1989 Mar 15;43(3):422–427. doi: 10.1002/ijc.2910430313. [DOI] [PubMed] [Google Scholar]
- Lamiell J. M., Salazar F. G., Hsia Y. E. von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore) 1989 Jan;68(1):1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Laucks S. P., Jr, McLachlan M. S. Aging and simple cysts of the kidney. Br J Radiol. 1981 Jan;54(637):12–14. doi: 10.1259/0007-1285-54-637-12. [DOI] [PubMed] [Google Scholar]
- Leung M. L., Gooding G. A., Williams R. D. High-resolution sonography of scrotal contents in asymptomatic subjects. AJR Am J Roentgenol. 1984 Jul;143(1):161–164. doi: 10.2214/ajr.143.1.161. [DOI] [PubMed] [Google Scholar]
- MELMON K. L., ROSEN S. W. LINDAU'S DISEASE. REVIEW OF THE LITERATURE AND STUDY OF A LARGE KINDRED. Am J Med. 1964 Apr;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Bishop D. T. Report of the committee on the genetic constitution of chromosome 3. Cytogenet Cell Genet. 1988;49(1-3):48–51. doi: 10.1159/000132648. [DOI] [PubMed] [Google Scholar]
- Neumann H. P. Basic criteria for clinical diagnosis and genetic counselling in von Hippel-Lindau syndrome. Vasa. 1987;16(3):220–226. [PubMed] [Google Scholar]
- Pathak S., Strong L. C., Ferrell R. E., Trindade A. Familial renal cell carcinoma with a 3;11 chromosome translocation limited to tumor cells. Science. 1982 Sep 3;217(4563):939–941. doi: 10.1126/science.7112106. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Breakefield X. O. The role of 'tumor suppressor' genes in neural tumors. Trends Neurosci. 1990 Jan;13(1):3–6. doi: 10.1016/0166-2236(90)90050-k. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G., Ozelius L. J., Lane A. H., St George-Hyslop P., Huson S., Gusella J. F., Martuza R. L. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987 Apr 17;236(4799):317–319. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- Smith D. I., Mangrulker R., Geist R., Gilbert J., Kinsman K., Drabkin H., Golembieski W. Saturation of human chromosome 3 with unique sequence hybridization probes. Genomics. 1989 May;4(4):453–459. doi: 10.1016/0888-7543(89)90268-1. [DOI] [PubMed] [Google Scholar]
- Tory K., Brauch H., Linehan M., Barba D., Oldfield E., Filling-Katz M., Seizinger B., Nakamura Y., White R., Marshall F. F. Specific genetic change in tumors associated with von Hippel-Lindau disease. J Natl Cancer Inst. 1989 Jul 19;81(14):1097–1101. doi: 10.1093/jnci/81.14.1097. [DOI] [PubMed] [Google Scholar]
- Vance J. M., Small K. W., Jones M. A., Stajich J. M., Yamaoka L. H., Roses A. D., Hung W. Y., Pericak-Vance M. A. Confirmation of linkage in von Hippel-Lindau disease. Genomics. 1990 Mar;6(3):565–567. doi: 10.1016/0888-7543(90)90488-g. [DOI] [PubMed] [Google Scholar]



