Key Clinical Message
After risk assessment, veno‐venous extracorporeal membrane oxygenation (ECMO) has been achieved in a superobese adult patient as a bridge to recovery of respiratory failure, despite the weight‐related difficulties. Early v‐v ECMO implantation could be considered to support and to conduct weaning both from sedation and from invasive mechanical ventilation, with the goal to perform physiokinesitherapy during awake ECMO.
Keywords: Acute distress respiratory syndrome, acute respiratory failure, awake extracorporeal membrane oxygenation, extracorporeal membrane oxygenation, obesity
Aim
To describe the use of extracorporeal membrane oxygenation (ECMO) to support a superobese patient with severe acute distress respiratory syndrome (ARDS) and to wean the subject from invasive mechanical ventilation with the use of awake ECMO.
Introduction
Overweight conditions and obesity are defined as abnormal or excessive fat accumulation that may impair health. Body mass index (BMI) is a simple index of weight‐for‐height that is commonly used to classify overweight and obese patients. It is defined as a person's weight in kilograms divided by the square of height in meters (kg/m2). The WHO definition is as follows: A BMI ≥ 25 is considered overweight, and a BMI greater than or equal to 30 is classified as obese 1. Superobesity is defined as BMI >50 kg/m2. A body mass index higher than 40 kg/m2 is associated with an increased risk of developing acute distress respiratory syndrome (ARDS) along with greater morbidity, length of stay, and duration of mechanical ventilation in the intensive care unit (ICU) 2. In these patients with severe respiratory disease requiring mechanical ventilation, avoiding pulmonary complications and muscular deconditioning may be particularly important.
Under the guidelines for adult respiratory failure of Extracorporeal Life Support Organization (ELSO), there are no absolute contraindications to extracorporeal membrane oxygenation (ECMO), as each patient is considered on an individual basis with respect to the associated risks and benefits 3. Under current opinion, class III obesity (BMI > 40 kg/m2) is not associated with poorer outcomes in critically ill patients and ECMO should not be withheld from this patient population.
The term “awake ECMO” defines the application of ECMO in nonintubated patients, either spontaneously breathing or assisted by noninvasive ventilation (NIV). The rationale is to avoid risks associated with invasive mechanical ventilation (IMV), which in some selected patients are very high. In particular, awake ECMO was successfully applied in chronic respiratory failure as a bridge to lung transplantation.
Clinical Case
A 35‐year‐old superobese man (body weight 240 Kg, BMI 70 kg/m2), already intubated for 4 days for ARDS due to community‐acquired pneumonia, was transferred to our ICU with severe hypoxemia based on FiO2 1, peak inspiratory pressures (Ppeak) 50 cm H2O, and positive end expiratory pressure (PEEP) 20 cm H2O. Arterial blood gas (ABG) resulted in a pH 7.39, PaCO2 55 mmHg, PaO2 55.9 mmHg. Before the patient underwent ECMO, he had a P/F ratio of 55.9, oxygenation index of 54.5, and a RESP score with an estimated survival rate between 50% and 60% 3. In order to match the criteria for protective ventilation, we started a veno‐venous ECMO (v‐v ECMO) procedure with a percutaneous technique (23‐F drainage cannula through the right femoral vein and 21‐F return cannula through the right jugular vein), without complications. Protective mechanical ventilator settings were used as pressure control mode with Ppeak less than or equal to 30 cm H2O, a tidal volume (Vt) of 1.43 ± 0.29 mL/kg (mean ± SD), a fraction of inspired oxygen (FIO2) of 0.6, a PEEP of 19.9 ± 0.74 cm H2O, and an inspiratory‐to‐expiratory (I/E) ratio of 1:1. We started with 4.0 L/min of blood flow (BF) and 4.0 L/min of sweep gas flow (GF), and during the first 24 h, we obtained good blood gas exchanges with a BF of 4.0 L/min and a GF of 7.0 L/min; the daily ECMO setting during the IMV and NIV is shown in Figure 1. Circuit FiO2 remained at 0.7 for the entirety of the procedure. Heparin was used for anticoagulation, with a target activated clotting time of 160–180 sec. During v‐v ECMO, the hemoglobin level was maintained at around 12 g/dL with red blood cell transfusion. Hemodynamic support was essential with low‐dose noradrenaline (0.05 μg/kg per min, calculated with ideal body weight) during the first 3 days of v‐v ECMO. ABG parameters on v‐v ECMO were (mean value ± SD) pH 7.35 ± 0.2, PaCO2 52.1 ± 2.1 mmHg, and PaO2 85.2 ± 3.4 mmHg. Microbial investigation based on the culture of samples obtained from lower respiratory tract by invasive methods using bronchoalveolar lavage showed negative results for pathogens so we started an empirical antibiotic therapy with levofloxacina and piperacillina/tazobactam.
Figure 1.
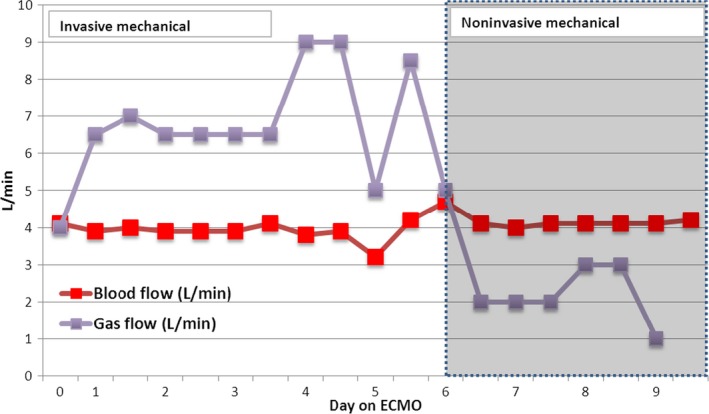
Extracorporeal membrane oxygenation (ECMO) settings of blood flow and gas flow during invasive mechanical ventilation and noninvasive ventilation periods.
After the first 5 days, during which it had been impossible to substantially decrease sedation and obtain patient cooperation, we directly extubated the patient while on ECMO. The awake ECMO strategy was combined with NIV by mask. Ventilator settings were set at pressure support 15 cm H2O, PEEP 7.5 cm H2O, and FiO2 0.5. We used NIV and an intense program of physical therapy (PT) with an overall improvement in clinical conditions. The patient was then able to move himself in bed, which was extremely helpful to ease in nursing assistance and allowing effective PT. During awake ECMO, blood flow was maintained at 4.0 L/min and sweep gas flow 2.5 L/min with FiO2 0.7 with good ABG parameters (mean value ± SD): pH 7.36 ± 0.1, PaCO2 54.7 ± 2.5 mmHg, and PaO2 70.4 ± 4.2 mmHg.
During v‐v ECMO support, we measured (by analyzing CO2 elimination from the oxygenator with a N85‐NELLCOR™ N‐85 MONITOR WITH OXIMAX™ TECHNOLOGY & MICROSTREAM™ CAPNOGRAPHY MEDTRONIC, MN, USA) the partitioning of membrane lung CO2 elimination (ML‐V'CO2) and native lung CO2 elimination (NL‐V'CO2). After extubation, carried out on day 5, we observed a major increase in NL‐V'CO2 that was interpreted as a result of the efforts of breathing. This was compensated by increasing sweep gas flow from 7.0 to 9.0 L/min in order increase CO2 removal by ML. During the following days, with improvement of lung condition witnessed by NL‐V'CO2 increase, it was possible to reduce sweep gas flow (and therefore ML‐V'CO2) and finally remove the extracorporeal support. The different partitioning of CO2 elimination during the ECMO period between native lungs and the oxygenator is shown in Figure 2.
Figure 2.
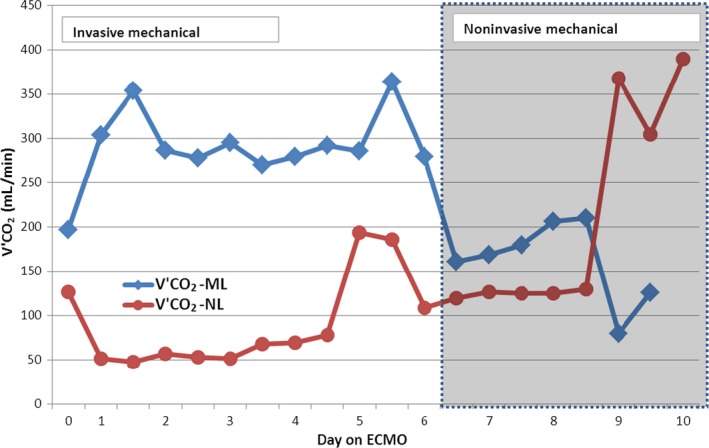
Partitioning of CO 2 elimination during extracorporeal membrane oxygenation (ECMO) run between patient native lungs (NL) and ECMO's oxygenator (ML). From day 6, the patient was extubated and he started a noninvasive ventilation (NIV) support.
Arterial blood gas values during a trial‐off made at day 9 showed pH 7.37, PaCO2 49.7 mmHg, and PaO2 83.7 mmHg, so we turned off v‐v ECMO without complications. ABG post‐v‐v ECMO showed pH 7.38, PaCO2 52.9 mmHg, and PaO2 69.4 mmHg.
veno‐venous extracorporeal membrane oxygenation was successfully stopped on day 10 after a test‐off procedure (blood flow of 2.0 L/min and zero gas flow for 2 h), and intermittent NIV was stopped on day 15 and he started high‐flow nasal cannula oxygen therapy (HFO) with a total flow of 35 L/min, FiO2 40%. Finally, the patient was discharged from ICU on day 17.
Discussion
Obesity is generally associated with an increased risk of other significant comorbid illnesses; however, there are growing data to support the protective effect of obesity compared with normal weight subjects in critical illnesses 4. This likely explains why obesity has not been associated with worse outcomes in conventionally managed patients with ARDS 5. Several postulates have been proposed to explain this paradoxical protective effect, including more adequate nutritional stores and immune modulation by adipose tissue, but it has not been demonstrated yet 6. For this reason, there are mounting data to support the use of ECMO in patients with increased body weight and particularly in critically ill patients 7.
In order to establish whether the patient was ready for v‐v ECMO weaning and removal, a thorough assessment of his respiratory function was needed. Based on the variations during the course of the disease, some main functional parameters, such as the increase in the fraction of oxygen delivery provided by the NL compared with that provided by the artificial lung, the improvement of respiratory mechanics (e.g., increase in static compliance of the respiratory system) and the improvement of gas exchange, were evaluated with PaO2 and PaCO2 values. In a large cohort of ARDS patients treated with ECMO, Mols et al. reported the successful weaning off of ECMO when at least 80% of total oxygen delivery was supplied by the patient's own lung 8.
A decrease in sweep gas flow nonlinearly reduced the artificial lung CO2 extraction, and consequently if the ventilation of the natural lung was not adjusted, PaCO2 increased. In the spontaneously breathing patient, a reduction in the sweep gas flow was associated with an increased respiratory drive, leading to an increase in minute ventilation and V'CO2NL.
However, if the gas flow was reduced too early or too quickly, the patient's respiratory efforts became excessive, leading in both cases to a dangerous elevation of plateau pressures and to an unendurable increase in breathing work and total CO2 production, with higher O2 consumption in the respiratory muscles and a decrease in blood oxygen saturation. Therefore, a global assessment of the patient was necessary, with simultaneous evaluation of gas exchanges (PaO2, PaCO2) and of the ventilatory “load” imposed to the native lung (pressure plateau, minute ventilation). Monitoring the behavior to progressive reloading of the native lung was helpful in indicating whether or not, and in this case how much, the patient was still dependent on extracorporeal support.
Regarding the discontinuation of the procedure, the extracorporeal support was reduced in parallel according to the improvement of native lung function, in terms of oxygenation and CO2 removal, which was tested every day.
Conclusion
In this patient, the awake ECMO strategy made weaning from IMV much easier than predicted. This case shows that ECMO is a feasible therapeutic option for weaning off from both sedation and IMV in obese patients. The present technological advancements, making ECMO easier to manage and safer, allow extracorporeal support to be considered as a reasonable alternative to mechanical ventilation in selected groups of patients combined with NIV 9.
Conflict of Interest
None declared.
Authorship
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
References
- 1. WPRO . Obesity. Updated August 2014. Fact sheet N°311. Available at http://www.wpro.who.int/mediacentre/factsheets/obesity/en/ (accessed 21 March 2016).
- 2. Stapleton, R. D. , and Suratt B. T.. 2014. Obesity and nutrition in ards. Clin. Chest Med. 35:655–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Extracorporeal Life Support Organization (ELSO) . Guidelines for Adult Respiratory Failure. Available via http://www.elso.org/Resources/Guidelines.aspx
- 4. Abhyankar, S. , Leishear K., Callaghan F. M., Demner Fushman D., and McDonald C. J.. 2012. Lower short‐ and long‐term mortality associated with overweight and obesity in a large cohort study of adult intensive care unit patients. Crit. Care 16:R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al‐Soufi, S. , Buscher H., Nguyen N. D., Rycus P., and Nair P.. 2013. Lack of association between body weight and mortality in patients on veno‐venous extracorporeal membrane oxygenation. Intensive Care Med. 39:1995–2002. [DOI] [PubMed] [Google Scholar]
- 6. Stapleton, R. D. , Dixon A. E., Parsons P. E., Ware L. B., and Suratt B. T.. 2010. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest 138:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt, M. , Bailey M., Sheldrake J., Hodgson C., Aubron C., Rycus P. T., et al. 2014. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am. J. Respir. Crit. Care Med. 189:1374–1382. [DOI] [PubMed] [Google Scholar]
- 8. Mols, G. , Loop T., Geiger K., Farthmann E., and Benzing A.. 2000. Extracorporeal membrane oxygenation: a ten‐year experience. Am. J. Surg. 180:144–154. [DOI] [PubMed] [Google Scholar]
- 9. MacLaren, G. , Combes A., and Bartlett R. H.. 2012. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 38:210–220. [DOI] [PubMed] [Google Scholar]


