Abstract
Introduction:
The term facial acanthosis nigricans (FAN) lacks definition of precise clinical and histopathological features. We present a descriptive study of patients with FAN to define pigmentary patterns and estimate the prevalence of obesity and insulin resistance in these cases.
Materials and Methods:
It is a prospective study that included all patients with classical AN of the neck and/or other areas with facial acanthosis nigricans described as brown-to-black macular pigmentation with blurred ill-defined margins, found on the zygomatic and malar areas. The body mass index (BMI) and waist circumference (WC) of the included patients were used as parameters of obesity. Homeostatic Model of Assessment of Insulin Resistance (HOMA2 IR) was used as a parameter to evaluate insulin resistance. Histopathological features of the 6 skin biopsies that were possible were reviewed.
Results:
Among the 102 included individuals, the patterns of facial pigmentation seen in addition to the classic pattern involving zygomatic and malar areas were a hyperpigmented band on the forehead in 59.80%, periorbital darkening in 17.64%, perioral darkening in 12.74%, and generalized darkening in 9.8% of cases. 85.29% of the males and 100% of the females were found to be obese. Varying degrees of insulin resistance was noted in 82.34% of the individuals. Six biopsies available for evaluation showed changes such as mild epidermal hyperplasia with prominent basal melanin, however, without the typical papillomatosis seen in AN of the flexures.
Conclusion:
We document an increased prevalence of obesity and insulin resistance in patients presenting with FAN and its presentations in addition to the classical description. We propose that FAN can be considered a cutaneous marker of insulin resistance and that HOMA2 IR can serve as a parameter of insulin resistance in such cases.
Keywords: Facial acanthosis nigricans, insulin resistance, obesity
INTRODUCTION
Facial acanthosis nigricans (FAN) is a term that is commonly used synonymously and interchangeably with metabolic melanosis and metabolic melasma by Indian dermatologists. This label of FAN is given to brown-to-black macular pigmentation with blurred ill-defined margins, commonly found on the zygomatic and malar areas with varying degrees of textural changes ranging from mild roughness to frank verrucous appearance of the affected areas [Figure 1].[1] At times, it is also labeled, somewhat inaccurately, as frictional hyperpigmentation or pigmented contact dermatitis. This reiterates the fact that the entity we call FAN lacks precise definition of clinical and histopathological features. Studies correlating its presence with documented insulin resistance are scarce. We present a descriptive study of patients with FAN in an attempt to define the patterns of pigmentation of FAN and estimate the prevalence of obesity and insulin resistance in these cases.
Figure 1.

Classical facial acanthosis nigricans
MATERIALS AND METHODS
The aims of this study were (1) to study the clinical patterns of FAN,(2) to estimate the prevalence of obesity using body mass index (BMI) and waist circumference (WC) as parameters in patients with FAN, and (3) to estimate the prevalence of insulin resistance (IR) and its degree in patients with FAN using Homeostatic Model of Assessment of Insulin Resistance (HOMA2 IR) as a parameter.
Inclusion criteria
We included patients with acanthosis nigricans (AN) of the neck, with classical facial acanthosis nigricans on the face described as brown-to-black macular pigmentation with blurred ill-defined margins, on the zygomatic and malar areas with varying degrees of textural changes.
Exclusion criteria
Patients having a defined clinical entity responsible for facial pigmentation such as melasma, pigmented contact dermatitis, lichen planus pigmentosus, erythema dyschromicum perstans, poikiloderma of civatte, pigmentary dermacation lines, post inflammatory pigmentation, topical/systemic drug induced pigmentation, congenital/nevoid and familial causes etc., were excluded from the study.
Patients with known diabetes mellitus were excluded from the study.
Study design
This was a prospective study conducted at two dermatology outpatient clinics with majority cases contributed by the first author. The study was conducted over a period of 2 years between March 2013 to March 2015. One hundred and two consecutive patients who fulfilled the inclusion criteria and provided consent for use of the clinical photographs were included in the study. Each patient was subjected to an in-depth history taking with regards to the onset, duration and progress of the pigmentation, associated aggravating factors such as excessive exposure to sunlight, atopy, friction, occupational or personal use of chemicals, and cosmetics and medications applied onto the face. Systemic comorbidities and medications along with relevant past, personal, and family history with particular emphasis on associated comorbidities such as diabetes, hypertension, dyslipidemias and coronary artery disease were noted. Menstrual and reproductive history was noted in females.
Clinical findings in each patient with regards to the location of the pigmentation, laterality, color, and textural changes were listed. Associated dermatological disorders were also recorded. BMI was calculated by weight in kilograms divided by the square of body height in meters. Asian Indians are more predisposed to developing insulin resistance and tend to manifest cardiovascular risk factors at lower levels of BMI, as compared to other ethnic groups, and have a higher percentage of body fat and abdominal obesity at lower or similar BMI levels as compared to Caucasians. Because this study comprised an Indian population, the consensus statement for diagnosis and treatment recommendations for Obesity and Metabolic Syndrome in Asian Indians[2] was used to classify the patients. The patients were classified on the basis of their BMI as normal: 18.0–22.9 kg/m2, overweight: 23–24.9 kg/m2, and obese >25 kg/m2.
The waist circumference of each patient was measured on standing using flexible tape measured from the narrowest part of the torso, as seen from anterior view (midway between the lowest rib and iliac crest). As per the consensus statement for diagnosis and treatment recommendations for obesity and metabolic syndrome in Asian Indians,[2] the risk of developing type 2 diabetes, hypertension and cardiovascular disease is higher in men with WC more than or equal to 36 inches and in females with WC more than or equal to 32 inches.
Each patient was subjected to fasting blood sugar, and fasting blood insulin levels and HOMA2 IR calculation were determined using a computer generated software. We used HOMA2 IR as a measuring tool in our study because it is a validated, sensitive, and a specific marker of insulin resistance. It is a simple test based on fasting glucose and insulin measurements and shares good correlation with the gold standard clamp tests without the potential risks of hypoglycemia.[3] The patients were classified on the basis of HOMA2 IR readings as normal <2, borderline 2–2.2, moderate 2.2–3, and severe >3.
Additional investigations such as a pelvic ultrasound, hormonal, and biochemical tests were performed in relevant cases to diagnose polycystic ovarian disease (PCOD). Histopathological features of the 6 skin biopsies of facial pigmentation, that were possible. were reviewed.
Data was statistically described in term of range, mean, standard deviation (±SD), mode of frequencies (number of cases) and relative frequencies (% of cases). All statistical calculations were performed usingSPSS version 20 (Statistical Package for Social Science SPSS, Inc. Chicago, IL, USA).
RESULTS
One hundred and two patients fulfilled the inclusion criteria within the study period. The male-to-female ratio was found to be 2.9:1 (76 males and 26 females). The selected patients were in the age group of 16–58 years. The average age of the male patients with such pigmentation was 37.57 ± 10.1 years (range: 16–58 years) and that of the female patients was 31 ± 8 years (range: 17–38 years). The duration of the pigmentation was 7 months to 8 years.
Twenty-two (21.5%) patients gave a history of being exposed to sunlight for more than 2 hours per day. Six patients (5.88%) had history of atopy, and 27 (26.47%) patients gave history of rubbing the pigmented areas often. Cosmetic use such as hair dye was observed in 14 (13.72%) patients.
Systemic comorbidities such as hypertension were found in 50 (49.01%), dyslipidemias in 52 (50.98%), and ischemic cardiac disease in 4 (3.92%) cases. Seventy-eight percent of females with pigmentation fulfilled the criteria for diagnosis of PCOD.
Following patterns of facial pigmentation were seen in addition to the classical pigmentation on the zygomatic and malar areas [Figure 1].
-
a)
Associated continuous or discontinuous horizontal band on the forehead with varying intensity of pigmentation was seen in 61 (59.80%) cases. The band merged with the zygomatic pigmentation to form a C shape when seen from the lateral view [Figure 2a]. Textural changes ranged from mild roughness to frank verrucous change [Figure 2b]
-
b)
Periorbital darkening was seen to be associated in 18 (17.64%) cases. The darkening was not only attributable to the pigmentation but also to the laxity, accentuated wrinkling, and shadow effect seen in that area [Figure 3a]
-
c)
Perioral darkening was seen to be associated in 13 (12.74%) individuals [Figure 3b]. In certain individuals, the darkening was accentuated over the sulcus alaris nasi and sulcus mentolabialis. We would like to clarify that we are not referring to isolated periorbital pigmentation. We have noticed accentuated periorbital pigmentation as an association with pigmentation of other areas zygomatic area/horizontal band/perioral/generalised pigmentation
-
d)
Generalized pigmentation of the face was seen in 10 (9.8%) cases [Figure 3c].
Figure 2.
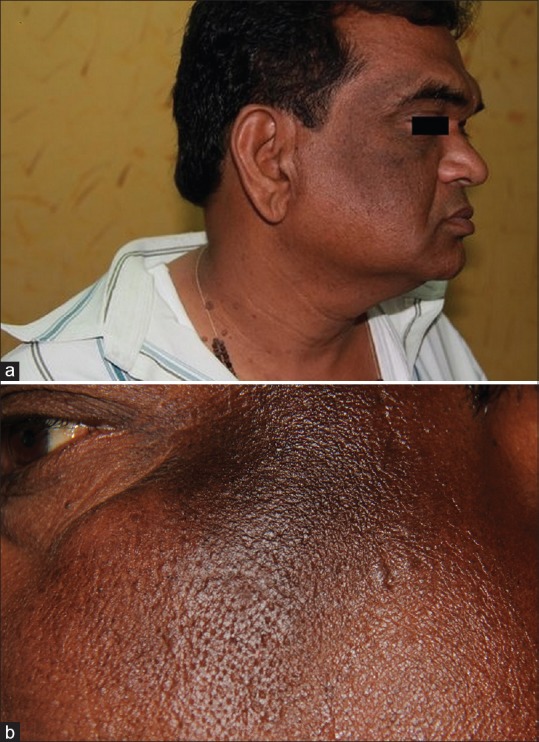
(a) Horizontal band on the forehead merging with the zygomatic pigmentation to form a C shape when seen from the lateral view. (b) Verrucous textural change of the affected area
Figure 3.
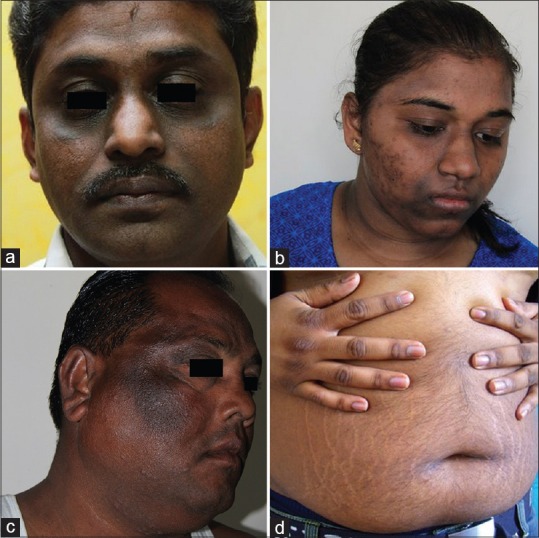
(a) Periorbital pigmentation with wrinkling. (b) Prominent perioral pigmentation with mild periorbital pigmentation, horizontal forehead band, and associated post-inflammatory pigmentation due to acne. (c) Generalized facial pigmentation in addition to the classical pigmentation. (d) Abdominal obesity with presence of acanthosis nigricans over the knuckles
AN of other body sites such as axillae, groins, and knuckles was noted in 88 (86.27%) of the cases [Figure 3d]. Acne with its complications such as post-inflammatory hyperpigmentation and scarring was seen in 12 (11.76%) cases. Acrochordons on the neck and/or on the face was the next most common cutaneous association seen in these cases. Seventy-four (72.54%) of the patients had a round obese face with excessive fat deposition around the cheeks and jaws [Figure 4].
Figure 4.
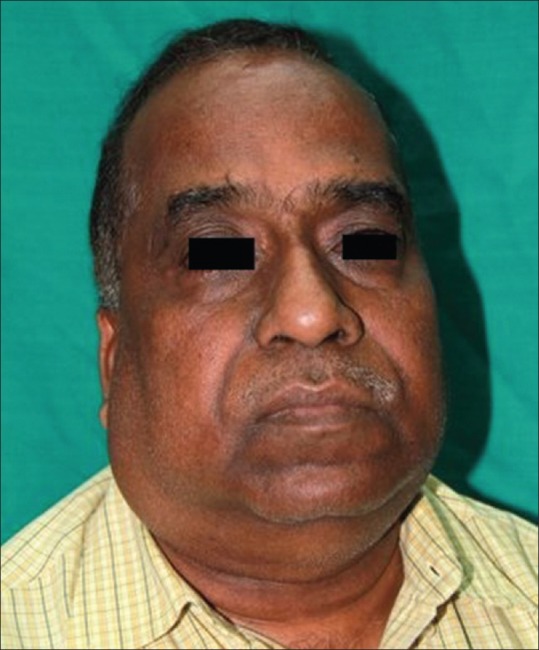
The “obese” face with deposition of fat on the cheeks and jowls
The average BMI and the WC of males were found to be 30.72 ± 4.9 kg/m2 (range: 22.5–39.9) and 37.6 inches, respectively [Figure 3d]. As per criteria followed, 87 (85.29%) of the males were obese, 8 (7.8%) were overweight, and 5 (4.9%) had a normal BMI. In contrast, the average BMI of the female patients was 32.58 kg/m2, which was higher than that of the males. Hundred percent of females belonged to the obese category with the average BMI being 32.5 ± 3.3 (range: 27.9–37.2). The average WC in the females was 34.2 inches.
The average HOMA2 IR in males was 3.5 ± 1.58 (range: 1.4–7) whereas in females it was 3.2 ± 1.34 (range: 1.2–5.5). Normal insulin levels were observed in 18 (17.64%) of the patients, 3 (2.94%) patients had borderline levels, whereas 17 (16.66%) and 64 (62.74%) of the patients had moderate and severe insulin resistance, respectively, as per the HOMA2 IR levels. The average fasting blood sugar level was 103.95 mg%.
Biopsies of the 6 patients that were available for evaluation showed mild epidermal hyperplasia with prominent melanin in the basal layer, however, without the typical papillomatosis seen in AN of the flexures. There were also increased numbers of large pigmented melanocytes at the dermoepidermal junction without any nest formation [Figure 5a]. Two cases also showed mild dermal fibroplasia and many scattered melanophages in the papillary dermis without any interface changes or significant dermal inflammatory infiltrate [Figure 5b].
Figure 5.
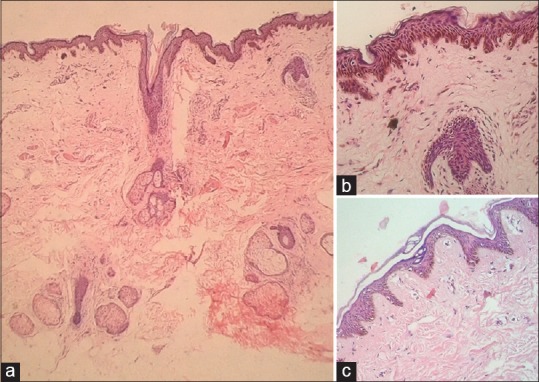
(a) Hematoxylin and eosin (H and E, ×20): scanner view showing lentigenous hyperplasia of rete with uniformly increased melanin in the basal layer with upper dermal fibroplasia and scattered melanophages without much inflammatory infiltrate. (b) (H and E, ×100) : Presence of epidermal hypermelanosis, few large melanocytes, a thickened granular layer, and upper dermal fibroplasia and scatter of melanophages. (c) (H and E, ×20): mild hyperplasia of rete with mildly increased melanin in the basal layer. No dermal inflammation or melanophages
DISCUSSION
Varied terminologies have been attributed to the pigmentation described as FAN in this article on account of lack of consensus. Studies from India describing the clinicohistopathological characteristics of FAN and its correlation with IR and other metabolic markers are lacking. A study by Sharquie et al.[4] among 30 Iraqi patients with FAN between 16 and 58 years of age showed a male preponderance (M:F: 29:1) with presence of pigmentation on the forehead in 92.3% (28 patients), temporal areas in 54% (17 patients), nasolabial folds in 57% (18 patients), and cheeks in 66.6% (22 patients) of the patients. As also observed in our study, they found an increased prevalence of obesity with 33.3% (10 patients) being overweight and 53.3% (16 patients) being obese. Increased WC was found in 83.3% (25 patients). Our study has, however, used parameters defined for Indian patients making it more relevant and customized to the Indian population. Another study by Sharquie et al.,[5] with the same cohort and additional controls, found that fasting serum triglyceride, total cholesterol, growth hormone, and serum leptin were statistically significantly high in patients with FAN in comparison with control individuals. Though an elaborate panel of metabolic markers was not performed in our study, the above mentioned observation does strengthen the association between FAN and IR.
A literature search also yielded a term maturational pigmentation (MH), an entity coined and described by Dr. Melvin Alexander, at the L’Oreal institute of Ethnic hair and skin International symposium in 2006.[6] We have found this condition to be similar to FAN. Dr. Alexander described the condition as dark brown-to-black pigmentation localized over the malar and zygomatic areas with blurry ill-defined borders, gradually merging with the surrounding normal skin. This pigmentation is very similar to what we have described as FAN. Other features of MH that are very similar to our findings were an adult onset, relative lack of symptoms, common occurrence of bilateral lesions, patients being overweight, lack of preceding trauma, and a negative family history. However, we would like to point out some notable differences from the study by Alexander et al.[6] We had a striking preponderance of males (2.9:1). We did not notice a significant association with allergies (7%) in contrast to 75% in their cases. Fifty percent of Dr. Alexander's patients had hyperglycemia and 36% showed hyperinsulinism in contrast to 82.34% of our patients in whom we documented IR by HOMA2 IR.[6] Known cases of diabetes milletus as mentioned earlier were excluded from our study.
While we did not notice laterality of pigmentation corresponding to the preferred side while sleeping as in Dr. Alexander's study, we believe that friction and exposure to sunlight contributes to the accentuation of FAN. The histopathological findings of increased numbers of large pigmented melanocytes in the epidermis and presence of fibroplasia with many scattered melanophages in the upper dermis without significant dermal inflammatory infiltrate or interface changes reflect rubbing effects or post-inflammatory changes due to the use of irritant or phototoxic topical medications [Figure 5b]. Whether photofrictional effects are the initiating trigger in predisposed individuals or occur as a secondary additional effect need to be investigated further.
Although we feel that FAN and MH are essentially different names of a single entity describing IR in individuals, often with an ’obese face’, we prefer to use the term FAN for the following reasons.
-
(1)
There was classical AN on the neck in all these cases, and of other anatomical sites in 88% of our cases
-
(2)
We have documented varying degrees of IR in 82.34% of the affected individuals
-
(3)
The mild hyperplasia of the blunt rete [Figure 5c] seen in our patients may signify an early change of AN, as has been noted by Sharquieet al. in their two studies, where they confirmed the histopathology findings of AN of the face to be similar to that of the neck and axilla (acanthosis with or without papillomatosis together with epidermal and dermal melanosis), however, milder.[5,6]
We have found an increased prevalence of obesity (87.5% in males and 100% in females) and IR (82.1%) in patients with FAN. An additional observation in our cases is the frequent occurrence (74%) of such pigmentation on an obese face with excessive fat deposition around the cheeks and jowls. The serendipitous discovery of a relevant paper by Sierra-Johnson et al.[7] documenting the obese face to correlate directly with insulin resistance and visceral fat deposition lends support to our observation of the obese face.
Though the pigmentation in AN has been popularly attributed to the thickening of the skin and the resultant velvety texture, increased melanin, presence of large epidermal melanocytes and dermal melanin have also been shown to be important causes of pigmentation in FAN as documented by Sharquie et al.[4,5] Histological findings in some of our cases concur with their observations[4] [Figure 5c]. They also document the severity of pigmentation of the face to be proportional to the degree of thickness and velvety texture of the epidermis. This can be explained by likening the epidermis to a storehouse of melanin. Thickening of the epidermis translates into an increased melanin store, and therefore a higher degree of pigmentation. There is documentation of elevated levels of growth hormone leading to increased fibroblast and keratinocyte activity, thus playing a role in the pathogenesis of AN.[8] The other possible cause of pigmentation is the pigment epithelium derived factor (PEDF).
PEDF, a multifunctional protein encoded by the SERPINF-1 gene, has been shown to induce insulin resistance by acute activation of proinflammatory serine/threonine kinases and stimulation of adipocyte lipolysis that results in ectopic lipid deposition.[9] There is a strong correlation between circulating PEDF and fasting insulin and HOMA IR. PEDF is abundant in stage I melanosomes and is known to increase pigment granules. It is thought that because AN is a clinical marker of IR, increased PEDF may be associated with AN.[10,11] This corroborates with the clinical observation that the pigmentation in these patients responds to weight loss and lifestyle modifications with little response to depigmenting agents and procedures.
We would like to highlight some of the shortcomings of the current study. A comparative analysis using a control group was not done. We were not able to perform facial biopsies in more patients due to its invasive nature and the patients’concerns regarding a possible scar on their faces. Additional investigations to document metabolic syndrome were not performed due to financial constraints of the patient in a resource poor country, where the majority of patients do not have health insurance. Grading of severity of FAN and its correlation with the metabolic derangements were not a part of this study.
CONCLUSION
The brown-to-black ill defined macular pigmentation on the zygomatic and malar areas with varying degrees of textural changes that we have called facial acanthosis nigricans is a common presentation, especially in overweight and obese individuals. Varied terminologies have been loosely applied to this presentation reiterating the fact that this entity needs further validation as regards its nomenclature, which is possible through description of the histopathological findings and confirmation of its association with insulin resistance and metabolic syndrome.
Our study demonstrates a higher incidence of obesity and insulin resistance in patients with such pigmentation and also describes a few additional patterns we came across in association with the classical presentation of FAN on the zygomatic and malar areas, namely the horizontal forehead band, perioral, periorbital and the generalized facial pigmentation. We are unable to comment on whether the additional patterns in isolation represent FAN because they are also seen in situations unrelated to obesity and hyperinsulinism. However we have found them worthy of mention since they have been documented in association with the classical FAN and AN on the nape of neck in patients in who we have found metabolic abnormalities.
FAN being essentially a simple visual finding can serve the vital role of being one of the early indicators of IR, metabolic syndrome, and probably impending diabetes mellitus. A comprehensive biochemical work-up based on this simple visual finding can ensure more timely remedial measures such as lifestyle changes and pharmacotherapy. This is, therefore, of particular importance in India which is known to be an epicenter of metabolic syndrome and diabetes mellitus.[12]
At present the most common biochemical investigations used to assess glucose metabolism are fasting blood sugar levels and 2 hours post-meal sugar levels that only help in detecting diabetes mellitus or prediabetes (impaired glucose tolerance). This is potentially misleading because the tests do not specifically detect IR.[13] Hence, an elevated HOMA2 IR level can serve as marker for IR in such cases. In addition to the investigations performed in this study, measurement of blood pressure, post-glucose 2 hours plasma glucose levels, HbA1c levels, complete lipid profile, thyroid function tests, serum uric acid level, cardiovascular risk assessment, and polysomnographic sleep study are suggested in order to complete the evaluation for metabolic syndrome in these patients. Further studies are required to reach a consensus on the minimum biochemical investigations required in resource poor countries like India.
Future larger, histopathologically backed studies with a comparison group would help in validating the nomenclature, pathogenesis, and correlation of this oft seen but ill studied entity with metabolic syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Veysey E, Ratnavel R. Facial acanthosis nigricans associated with obesity. Clin Exp Dermatol. 2005;30:437–9. doi: 10.1111/j.1365-2230.2005.01767.x. [DOI] [PubMed] [Google Scholar]
- 2.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 3.Schwartz B, Jacobs D, Moran A, Steinberger J, Hong C, Sinaiko A. Measurement of Insulin Sensitivity in Children: Comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 4.Sharquie KE, Al-Ogaily SM. Acanthosis Nigricans as a Cause of facial melanosis (Clinical And histopathological Study) IOSR J Dent Med Sci. 2015;1:84–90. [Google Scholar]
- 5.Sharquie K, Noaimi A, Mahmood H, Al-Ogaily S. Clinical and Biochemical Evaluation of Facial Acanthosis Nigricans. JCDSA. 2015;5:231–7. [Google Scholar]
- 6.Alexander A. Maturational hyperpigmentation. In: Kelly A, Taylor S, editors. Dermatology for Skin of Color. 1st ed. New York: McGraw-Hill Medical; 2009. p. 344. [Google Scholar]
- 7.Sierra-Johnson J, Johnson BD. Facial fat and its relationship to abdominal fat: Amarker for insulin resistance? Med Hypotheses. 2004;63:783–6. doi: 10.1016/j.mehy.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Puri N. A study of pathogenesis of acanthosis nigricans and its clinical implications. Indian J Dermatol. 2011;56:678–83. doi: 10.4103/0019-5154.91828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.[Internet] [Last accessed on 2016 Jul 05]. Available from: http://9. www.healthcentre.org.uk .
- 10.Bell C. Pigment epithelium-derived factor: A not so sympathetic regulator of insulin resistance? Exerc Sport Sci Rev. 2011;39:187–90. doi: 10.1097/JES.0b013e31822673f0. [DOI] [PubMed] [Google Scholar]
- 11.Sabater M, Moreno-Navarrete JM, Ortega FJ, Pardo G, Salvador J, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95:4720–8. doi: 10.1210/jc.2010-0630. [DOI] [PubMed] [Google Scholar]
- 12.Joshi SR, Parikh RM. India--Diabetes capital of the world: Now heading towards hypertension. J Assoc Physicians India. 2007;55:323–4. [PubMed] [Google Scholar]
- 13.Meyer C, Pimenta W, Woerle H, Van Haeften T, Szoke E, Mitrakou A, et al. Different Mechanisms for Impaired Fasting Glucose and Impaired Postprandial Glucose Tolerance in Humans. Diabetes Care. 2006;29:1909–14. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]


