Abstract
Primary cutaneous anaplastic large cell lymphoma (pcALCL) forms 9% of the cutaneous T-cell lymphomas. It usually presents as solitary reddish brown ulcerating nodule or indurated plaque. Sometimes, it mimics other dermatological diseases such as eczema, pyoderma gangrenosum, pyogenic granuloma, morphea, and squamous cell carcinoma. Our case presented with large pyogenic granuloma like lesion with regional lymphadenopathy. Since pcALCL is rare, one can misdiagnose such cases and therefore high index of suspicion is necessary.
Keywords: Anaplastic large cell lymphoma, cutaneous T-cell lymphoma, pyogenic granuloma
INTRODUCTION
Cutaneous lymphomas form the second most common group of extranodal non-Hodgkin's lymphoma. T-cell lymphomas comprise 65% of the cases, whereas B-cell lymphomas form 25% of the cutaneous lymphomas. The remaining 10% are of combined nature and difficult to distinguish.[1] Primary cutaneous anaplastic large cell lymphoma (pcALCL) forms 9% of the cutaneous T-cell lymphomas.[2] It was initially described in 1985 by Stein et al.[2] There are three types of anaplastic large cell lymphoma: pcALCL, primary systemic anaplastic lymphoma kinase (ALK)-positive ALCL, and primary systemic ALK-negative ALCL.[3] The diagnosis of primary cutaneous ALCL is made only if the lesions are limited to the skin for at least 6 months after the initial identification. Therefore, regular follow up of these patients is important to rule out any systemic disease.[4]
CASE REPORT
A 35-year-old man presented with a complaint of asymptomatic red raised nodule over chin for last 15 days. It was associated with a single enlarged submental lymph node. The patient underwent excision of the nodule at a local hospital. After a few days, the lesion increased in size and progressed to form an erythematous nodule, which used to bleed easily. There were no symptoms of fever, weight loss, and loss of appetite. There was no history of preceding insect bite, trauma, and drugs. His personal and family history was noncontributory. His general physical examination and systemic examination was unremarkable. On cutaneous examination there was a single well-defined exophytic erythematous nodule of size 4 × 3 cm approximately, with a glistening surface [Figure 1]. Lesion used to bleed easily on touch and was nontender. In view of these clinical findings, possibilities of pyogenic granuloma and cutaneous lymphoma were kept. Complete hemogram, routine biochemistry, HIV test, and chest X-ray were within normal limit. Fine-needle aspiration cytology (FNAC) from the lesion revealed cellular smears comprising of large atypical lymphoid cells in isolation. Skin biopsy showed ulceration of the lining epithelium. The dermis showed diffuse infiltrate of large atypical lymphoid cells with pleomorphic round to irregular horse shoe-shaped nuclei, finely granular chromatin, multiple nucleoli, and moderate cytoplasm. In addition there were increased apoptotic cells, neutrophils, and erythrophagocytosis [Figure 2]. Immunohistochemical staining showed positivity for CD45 and CD30 [Figures 3 and 4]. The CD45 positivity confirmed the lymphoid origin of atypical cells. CD30 is positive in ALCL. Keeping in view the histopathology, and CD45 and CD30 positivity, the diagnosis of ALCL was made. Ultrasonography of neck revealed regional cervical lymphadenopathy involving levels I–III; largest lymph node measuring 0.4 × 0.3 cm. FNAC from lymph node was suggestive of nonspecific reactive hyperplasia. Bone marrow biopsy and computed tomography scan of chest and abdomen were normal. Final diagnosis of pcALCL was made. The patient was started on CHOP regimen, which consisted of cyclophosphamide, doxorubicin, vincristine, and prednisolone. After first cycle of chemotherapy there was a significant reduction in the size of the lesion [Figure 5].
Figure 1.
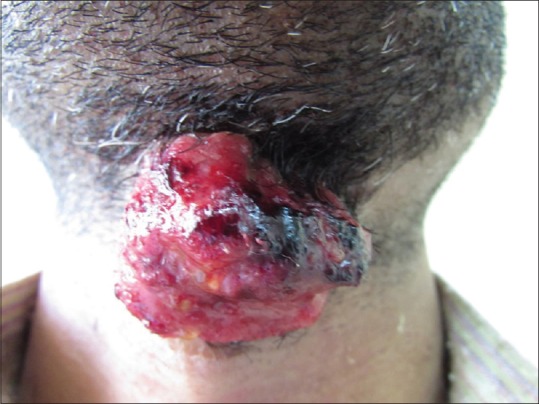
Exophytic erythematous nodule mimicking a large pyogenic granuloma in the submandibular region
Figure 2.
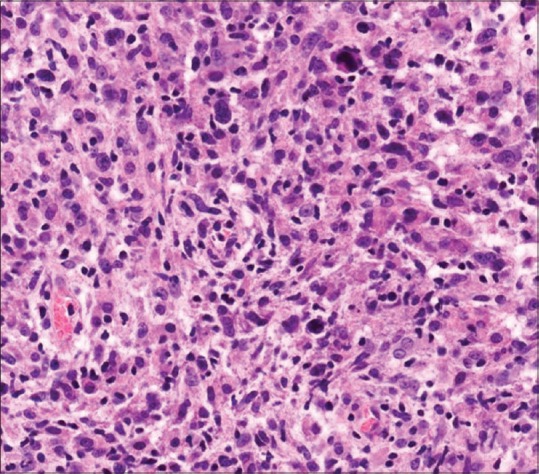
Photomicrograph showing diffuse infiltrate of large atypical lymphoid cells with pleomorphic round to irregular nuclei (H and E, ×40)
Figure 3.
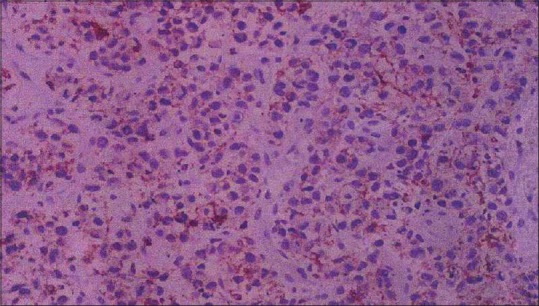
Tumor cells showing 1 + positivity for CD45
Figure 4.
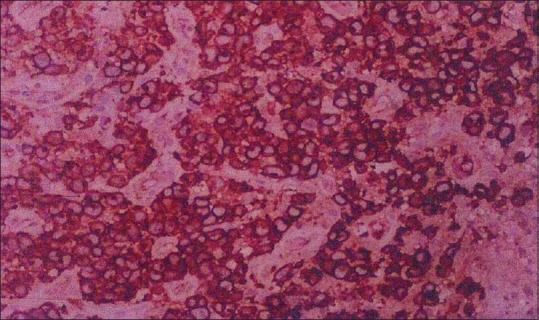
Tumor cells showing strong (4+) CD30 membranous positivity
Figure 5.
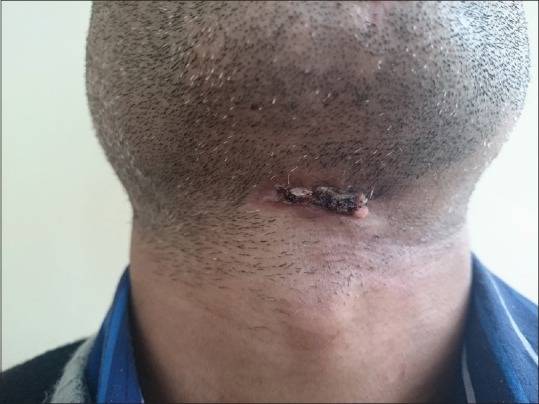
Improvement in lesion after first cycle of chemotherapy
DISCUSSION
Lymphoproliferative disorders of the primary cutaneous CD30+ type, of T-cell origin, consist of lymphomatoid papulosis, and CD30+ anaplastic large cell lymphoma.[5] pcALCL affects older age group with median age of 55 years. Male-to-female ratio is 1.5:1.[6] It commonly affects the trunk, extremities, and presents as solitary or multiple localized nodules with ulceration. But, sometimes it resembles other dermatological diseases like eczema, pyoderma gangrenosum, pyogenic granuloma, morphea, and squamous cell carcinoma.[7] Our case presented with a large pyogenic granuloma-like lesion. Pyogenic granuloma is a common benign vascular tumor, which appears as a smooth, lobulated, exophytic mass, exhibiting pink to reddish-purple color, which can bleed on slight manipulation.
Cutaneous CD30+ anaplastic large cell lymphoma (ALCL) is characterized by presence of CD30 expression in more than 75% of neoplastic cells.[3] Presence of cytogenetic abnormality, that is, nonrandom t(2; 5) (p23; q35) chromosomal translocation which involves fusion of anaplastic lymphoma kinase (ALK) with the nucleophosmin (NPM) gene is usually seen in systemic ALCL but only rarely in pcALCL.[3] pcALCL usually presents as a solitary papulonodular lesion, which sometimes shows ulceration.[8] Our case presented with a large pyogenic granuloma-like lesion. Cheng et al. reported a case of systemic ALCL with local cutaneous involvement in the form of nonhealing pyogenic granuloma-like lesion in the right axillary area.[8] However, in our patient, systemic involvement was absent. Rozza-de-Menezes et al.[9] reported a case of 57-year-old man who presented with asymptomatic lobulated erythematous nodule on the gingiva mimicking pyogenic granuloma. But the biopsy revealed anaplastic large cell lymphoma. There was no regional lymphadenopathy and systemic involvement; however, he was HIV positive. Our patient had a similar morphology of lesion but he was younger in age, immunocompetent, and had regional lymphadenopathy.
Generalized or multifocal lesions are seen in about 20% of the patients.[2] Secondary regional lymphadenopathy is seen in approximately 25% of patients and it does not correlate with the prognosis of the disease.[10] In our patient, regional cervical lymphadenopathy was present. Systemic ALCL was ruled out as all the related investigations were within normal limit and there was no systemic lymphadenopathy. A five-year survival rate of pcALCL is more than 90% but cutaneous disease arising from systemic ALCL has worse prognosis with five-year survival rate of less than 45%. ALK expression in cutaneous lesions usually indicates systemic disease but rarely, it can be positive in pcALCL. Also negative expression of ALK does not rule out systemic disease as 20%–60% of systemic ALCL can be ALK negative.[11] Twenty-five percent of the cases of primary cutaneous anaplastic CD30+ large T-cell lymphoma can also remit spontaneously.[1]
CONCLUSION
ALCL can have varied cutaneous presentations. Being uncommon, it may be missed. As the cutaneous lesions can be the only manifestation in a case of systemic ALCL, clinicians should keep a high suspicion so that the disease can be detected at an early stage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tchernev G, Cardoso JC, Arseniev L, Okamoto H. Extrinsic apoptotic pathways: A new potential “target” for more sufficient therapy in a case of cutaneous anaplastic large CD30+ALK-T-cell lymphoma. Indian J Dermatol. 2011;56:87–91. doi: 10.4103/0019-5154.77563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfeld C, Khan I, Mahon B, Nelson BP, Rosen ST, Evens AM. Primary cutaneous and systemic anaplastic large cell lymphoma: Clinicopathologic aspects and therapeutic options. Oncology (Williston Park) 2010;24:574–87. [PubMed] [Google Scholar]
- 3.Kumaran MS, Jithendriya M, Nagaraj P, Tirumalae R, Jayaseelan E. Anaplastic lymphoma kinase-positive primary cutaneous anaplastic large cell lymphoma – Is it a new variant? Indian J Dermatol Venereol Leprol. 2012;78:354–7. doi: 10.4103/0378-6323.95454. [DOI] [PubMed] [Google Scholar]
- 4.Delsol G, Falini B, Muller-Hermelink HK, Campo E, Jaffe ES, Gascoyne RD, et al. Anaplastic large cell lymphoma ALK-positive. In: Swerdlow SH, Campo E, Harris NL, Jaffe E, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: World Health Organization IARC Press; 2008. pp. 312–6. [Google Scholar]
- 5.Sen S, Gangopadhyay A, Chatterjee U, Ray AC. Primary cutaneous CD30+anaplastic lymphoma: A case report and reappraisal. Indian J Dermatol. 2013;58:165. doi: 10.4103/0019-5154.108117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asha LK, Thomas D, Binitha MP, Nandakumar G. Primary cutaneous multifocal CD30+ anaplastic large cell lymphoma. Indian J Dermatol Venereol Leprol. 2006;72:376–8. doi: 10.4103/0378-6323.27758. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan J, Tomaszewski MM, Kao GF. Primary cutaneous CD30-positive anaplastic large cell lymphoma. Report of 27 cases. J Cutan Pathol. 1993;20:193–202. doi: 10.1111/j.1600-0560.1993.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YC, Lin CH, Chai CY, Lai CS. Primary axillary anaplastic large cell lymphoma mimicking pyogenic granuloma clinically. Kaohsiung J Med Sci. 2014;30:380–1. doi: 10.1016/j.kjms.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Rozza-de-Menezes RE, Jeronimo Ferreira S, Lenzi Capella D, Schwartz S, Willrich AH, de Noronha L, et al. Gingival anaplastic large-cell lymphoma mimicking hyperplastic benignancy as the first clinical manifestation of AIDS: A case report and review of the literature. Case Rep Dent 2013. 2013:852932. doi: 10.1155/2013/852932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shehan JM, Kalaaji AN, Markovic SN, Ahmed I. Management of multifocal primary cutaneous CD30+ anaplastic large cell lymphoma. J Am Acad Dermatol. 2004;51:103–10. doi: 10.1016/j.jaad.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Campo E, Chott A, Kinney MC, Leoncini L, Meijer CJ, Papadimitriou CS, et al. Update on extranodal lymphomas. Conclusions of the workshop held by the EAHP and the SH in Thessaloniki, Greece. Histopathology. 2006;48:481–504. doi: 10.1111/j.1365-2559.2006.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


