Abstract
Obesity is a risk factor for stroke and is consequently one of the most common co-morbidities found in patients. There is therefore an identified need to model co-morbidities preclinically to allow better translation from bench to bedside. In preclinical studies, both diet-induced and genetically obese rodents have worse stroke outcome, characterised by increased ischaemic damage and an altered inflammatory response. However, clinical studies have reported an ‘obesity paradox’ in stroke, characterised by reduced mortality and morbidity in obese patients. We discuss the potential reasons why the preclinical and clinical studies may not agree, and review the mechanisms identified in preclinical studies through which obesity may affects stroke outcome. We suggest inflammation plays a central role in this relationship, as obesity features increases in inflammatory mediators such as C-reactive protein and interleukin-6, and chronic inflammation has been linked to worse stroke risk and outcome.
Keywords: Obesity, adiposity, stroke, inflammation, blood–brain barrier
Obesity as a stroke co-morbidity
The detrimental effects of obesity on overall health are well understood, including an increased risk of developing hypertension and cardiovascular disease, and higher all-cause mortality.1 However, despite 35.5% of first-ever stroke patients being classified as clinically obese (EUROASPIRE III survey),2 we do not fully understand whether or how obesity affects stroke outcome. Stroke is already the leading cause of adult disability, accounting for 7% of the total disability-adjusted life years lost and 12% of total deaths in Europe.3,4 As most people affected by a stroke are over the age of 65, its prevalence and associated disabilities will likely increase due to the population living longer. As well as an ageing population, worldwide obesity has more than doubled since 1980, and in 2014, over 600 million adults were classified as obese (World Health Organisation (WHO) 2015).
Obesity is usually defined by body mass index (BMI, calculated as body weight in kg divided by square of height in m), with the WHO classifying adults with a BMI ≥ 25 kg/m2 as overweight and a BMI ≥ 30 kg/m2 as obese. Obesity can then further divided based on BMI into class 1 (30–34.9), class 2 (35–39.0) or class 3 (≥40) obesity. Several studies have identified that obesity is a risk factor for both ischaemic and haemorrhagic stroke in several ethnic populations and in both sexes.5,6 A linear relationship between increasing BMI and stroke risk has been reported for ischaemic stroke, though a J-shaped or no correlation is often reported for haemorrhagic stroke.7–14 Obesity is also a strong risk factor for the development of other risk factors for stroke including hypertension, diabetes and dyslipidaemia. However, even after adjustment for these potential co-founding factors, obesity has been found to be an independent risk factor for stroke.5
Currently, the only treatment for stroke is thrombolysis using tissue plasminogen activator (tPA) or by mechanical retrieval. However, since tPA can only be given up to 4.5 h post-stroke due to reduced efficacy and increased risk of haemorrhage, only a minority of stroke suffers benefit from tPA treatment.15 Furthermore, clinical evidence suggests that recanalisation of cerebral arteries after tPA therapy is reduced in obese stroke patients, suggesting a reduced efficacy of tPA.16 Similar resistance to thrombolysis has been reported in patients with the metabolic syndrome,17,18 of which obesity is a part. A potential explanation is that patients weighing over 100 kg may be underdosed, as the maximum recommended dose of tPA is 0.9 mg/kg (Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008).19 Indeed, worse outcome at 3 months in tPA-treated patients has been reported in patients who were >100 kg compared with patients who were <100 kg.20,21
In both obese and non-obese stroke patients, there is therefore an urgent need for new stroke treatments. Unfortunately, despite hundreds of stroke treatments showing efficacy in preclinical trials, most have been unsuccessful in the clinic. It is now believed that a key factor for this lack of translation from ‘bench to bedside’ is the failure of preclinical studies to consider fully the underlying status of a typical stroke patient. As age is the strongest risk factor for stroke, patients are usually elderly, and present with co-morbid diseases such as hypertension, vascular disease (e.g. atherosclerosis), diabetes, infection or metabolic syndrome/obesity. Several of these co-morbidities may co-exist in the same patient, and it is rare for a stroke to occur in an otherwise healthy individual (Figure 1).
Figure 1.
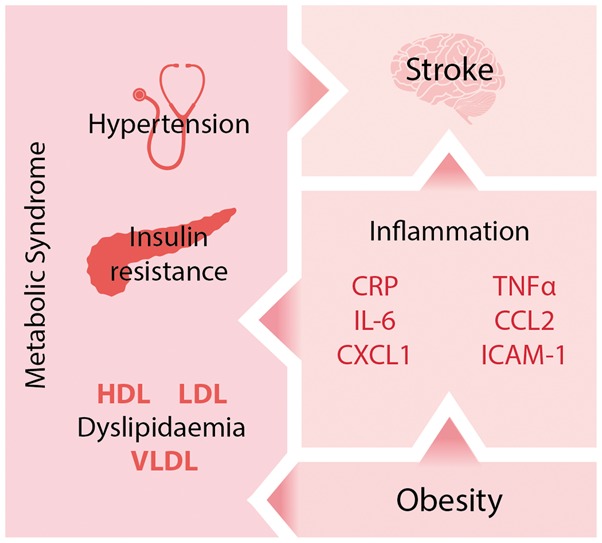
Obesity develops alongside other aspects of the metabolic syndrome and leads to chronic ‘low grade inflammation’. Systemic inflammation is known to affect stroke outcome, as are other aspects of the metabolic syndrome. CRP: C reactive protein; CCL2: chemokine (C-C motif) ligand 2; CXCL2: chemokine (C-X-C motif) ligand 2; ICAM-1: intracellular adhesion molecule 1; HDH: high-density lipoprotein; IL-6: interleukin-6; LDL: low-density lipoprotein; TNFα: tumour necrosis factor α; VLDL: very low-density lipoprotein.
Obesity and chronic inflammation in stroke risk
Inflammation and immune mechanisms are well established factors in stroke risk and outcome, in both patients and animals.22,23 Obesity has classically been associated with disruption of pathways controlling lipid and glucose metabolism, however recent evidence has shown that obesity also has an inflammatory component (Figure 1). Measures of obesity, such as BMI, correlate with several markers of inflammation in patients. In particular, circulating concentrations of C-reactive protein (CRP) and interleukin (IL)-6 have been shown repeatedly to correlate positively with obesity24–29 in addition to tumour necrosis factor alpha (TNFα),29 monocyte chemoattractant protein 1 (MCP-1/CCL2),30 interleukin-8 (IL-8)30 and soluble intracellular adhesion molecule 1 (ICAM-1).31 This positive correlation between makers of inflammation and adiposity has led to suggestions that obesity is an inflammatory condition. However, the profile of immune activation found in obesity is quite different to that found in infection or injury, where the immune system is acutely activated to remove harmful stimuli. In obesity, the concentrations of proinflammatory markers are relatively low by comparison, but are maintained over long periods. Consequently, obesity has been described as resulting in “low-grade chronic inflammation”. This peripheral inflammatory response is thought to originate within adipose tissue, which becomes dysfunctional and inflamed during obesity.32 The important endocrine role of adipose tissue is highlighted by the growing family of adipose tissue-derived protein factors, collectively called adipokines (e.g. leptin and adiponectin). Besides having an established role in immune regulation and energy balance, adipokines have been found to regulate an expanding array of physiological functions, including haemostasis, lipid and glucose metabolism, blood pressure, insulin sensitivity and angiogenesis.32,33 The inflammation associated with obesity may lead to progressively increased stroke risk, since elevated concentrations of IL-6 and CRP in the plasma are both risk factors for stroke.34–37 In support of this, in obese and overweight patients, the BMI-associated cardiovascular disease (combined stroke and ischaemic heart disease) risk decreases substantially when adjusted for CRP, suggesting that inflammation is important in mediating the risk of stroke associated with obesity.38
Stroke outcome in obese patients: The obesity paradox
Despite obesity being a risk factor for stroke, several studies have reported a protective effect of obesity on stroke outcome in patients. In these studies, patients are grouped by BMI on admission, and the presence of other stroke risk factors assessed. As would be expected, obese and overweight BMI groups have higher incidence of other risk factors including (but not limited to) hypertension, diabetes and dyslipidaemias. The relative impact of each factor on functional outcome is then assessed using multivariate statistics. When the contribution of other factors is adjusted for, higher BMI has been associated with reduced long-term all-cause mortality after stroke when compared with normal BMI groups in several populations.39–47 Furthermore, increased BMI has also been associated with other improved outcomes post-stroke, including a reduced risk of recurrent stroke,44,48 reduced morbidity,42 improved functional recovery49 and reduced short-term mortality.40,48,50
However, not all studies have confirmed the existence of an obesity paradox in stroke. For example, Ryu et al. found no association between increased BMI and long-term mortality after ischaemic stroke.51 Kim et al. found that higher BMI was associated with milder strokes on admission, and so when initial stroke severity was adjusted for there was no evidence of an obesity paradox in their cohort.52 In a study in patients receiving intravenous thrombolysis, body weight >100 kg was associated with increased mortality at 3 months post-stroke.21 The common use of long-term all-cause mortality as an end-point has also been criticised, for example when the Danish Stroke Register was reanalysed to only include deaths confirmed as occurring acutely due to the index stroke, there was no evidence of an obesity paradox.53 There is therefore still a lack of consensus about the obesity paradox in the literature; whether it predicts a currently unclear biological phenomenon, or is instead due to sampling or other methodological bias. A full discussion of these arguments is beyond the scope of this review, and have been previously discussed elsewhere.54–56 Overall, the clinical studies investigating stroke outcome in obese patients have focused on whether obesity has a positive or negative effect on long-term morbidity and mortality. However, the actual mechanisms through which obesity is proposed to have a beneficial or detrimental effect have undergone little to no investigation in clinical studies.
Experimental stroke in obese rodents
In contrast with the clinical outlook, there is clear consensus in preclinical studies that obesity worsens stroke outcome. This detrimental effect of obesity on outcome has primarily been demonstrated in obese rodents undergoing experimental cerebral ischaemia. In both mouse and rat models of obesity, obese rodents suffer increased ischaemic brain damage and have worse behavioural outcomes in comparison with control animals (Table 1). This unfavourable effect was initially observed in genetic models of obesity, such as the ob/ob mouse,57,63,78,80,84,86–88 db/db mouse74,75,77,91,92 and Zucker rat,76,79,83 which all become obese due to a deficiency in the satiety hormone leptin or a defective leptin receptor. Since leptin usually acts on the hypothalamus to limit feeding, these animals (ob/ob and db/db mice, and Zucker rats) rapidly gain weight because they are hyperphagic. The appearance of ischaemic damage is also more rapid in ob/ob mice.57 Worse stroke outcome has also been confirmed in diet-induced models in which animals become obese due to being fed a high-fat diet. These detrimental effects of a high-fat diet have been observed in rat,58,61,65,73,82,93 mouse60,64,66,88 and gerbil62,63,94 models of diet-induced obesity. A variety of models of cerebral ischaemia have also been used in these studies, including transient and permanent occlusion of the middle cerebral artery, common carotid artery ligation and exposure to a low-oxygen environment. In mice, it has recently been shown that the negative impact of diet-induced obesity on stroke outcome in mice is dependent on how long the obese phenotype is present and the severity of the initial stroke insult (length of ischaemia).60 It is also unclear whether the detrimental effects of obesity on outcome in these preclinical studies are permanent, or reversible with weight loss.
Table 1.
Summary of the studies that have reported outcome in obese animals, and the co-morbidities which they assessed.
| Study | Species, strain | Obesity model | Ischaemia model | Effect of obesity on ischaemia outcome | Co-morbidities assessed |
||||
|---|---|---|---|---|---|---|---|---|---|
| BW | Glu. | Ins. | Lip. | BP | |||||
| Haley and Lawrence57 | Mouse, ob/ob | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ||||
| Cao et al.58 | Rat, SD | 8-week HFD | tMCAo | ↑ Infarct volume | ↑ | ↑ | |||
| Deng et al.59 | Mouse, CD-1 | 10-week HFD (45%) | tMCAo | HFD-alone effect not assessed | ↑ | ↑ | |||
| Maysami et al.60 | Mouse, C57 | 3-/6-month HFD (60%) | tMCAo | ↑ Infarct volume (6 months only on diet) | ↑ | ↑ | ↔ | ||
| Wu et al.61 | Rat, SD | 3-month Western diet | tMCAo | ↑ Infarct volume | |||||
| Cheon et al.62 | Gerbil | 1-month HFD (60%) | Bilateral CCA | ↑ Neuronal loss | ↔ | ↑ | ↑ | ||
| Wu et al.61 | Rat, SD | 3-month Western diet | tMCAo | ↑ Infarct volume | |||||
| Yan et al.63 | Gerbil | 1-month HFD (60%) | Bilateral CAA | ↑ Neuronal loss | ↔ | ↑ | ↑ | ||
| Deng et al.64 | Mouse, C57 | 2.5-month HFD (45%) | tMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ||
| Yang et al.65 | Rat, SD | 3-month HFD (45%) | tMCAo | ↑ Neurological deficit | ↑ | ↔ | ↑ | ↑ | ↔ |
| Kim et al.66 | Mouse, C57 | 2-month HFD (36%) | tMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ||
| Herz et al.67 | Mouse, ApoE | 6-week Western diet | tMACAo | ↑ Infarct volume | |||||
| Dhungana et al.68 | Mouse, ApoE | 15-week Western diet | pMCAo | ↔ Infarct or neurological deficit | ↑ | ||||
| Kim et al.69 | Mouse, ApoE | 11-week HFD (21%) | tMCAo | HFD-alone effect not assessed | ↑ | ||||
| Pradillo et al.70 | Rat, cp/cp | Leptin receptor mutation | tMCAo | HFD-alone effect not assessed | ↑ | ||||
| Kawai et al.71,72 | Rat, Zucker | Leptin receptor mutation | tMCAO | HFD-alone effect not assessed | ↑ | ||||
| Langdon et al.73 | Rat, SD | 1- or 3-month Western diet | Endothelin injection | ↑ Infarct volume (3 months only on diet) | ↑ | ||||
| Kumari et al.74 | Mouse, db/db | Leptin receptor mutation | CCA ligation and hypoxia (8%) | ↑ Infarct volume | ↑ | ||||
| Tureyen et al.75 | Mouse, db/db | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ↑ | |||
| Ritter et al.76 | Rat, Zucker | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ↔ | |
| Chen et al.77 | Mouse, db/db | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ||||
| Kumari et al.78 | Mouse, ob/ob | Leptin mutation | CCA ligation and hypoxia (8%) | ↑ Infarct volume | ↑ | ↑ | |||
| Osmond et al.79 | Rat, Zucker | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ↑ | ↑ |
| McColl et al.80 | Mouse, ob/ob | Leptin mutation | tMCAO | ↑ Infarct volume | ↑ | ↔ | |||
| Arvanitidis et al.81 | Rat, SD | 1-month Western diet (40%) | Forebrain ischaemia | ↔ Neuronal loss | ↑ | ↔ | ↔ | ||
| Deutsch et al.82 | Rat, SD | 10-week HFD (36%) | pMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ↑ | |
| Osmond et al.83 | Rat, Zucker | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ↑ | ↑ |
| Valerio et al.84 | Mouse, ob/ob | Leptin mutation | pMCAo | ↑ Infarct volume | |||||
| Kim et al.85 | Mouse, ApoE | 2-month Western diet | tMCAo | ↑ Infarct volume | ↑ | ↑ | |||
| Terao et al.86 | Mouse, ob/ob | Leptin mutation | tMCAo | ↑ Infarct volume | ↑ | ↔ | ↔ | ||
| Mayanagi et al.87 | Mouse, ob/ob | Leptin mutation | tMCAo | ↑ Infarct volume | ↔ | ↑ | ↑ | ↑ | |
| Nagai et al.88 | Mouse, ob/ob & C57 | 15-week HFD (42%) for C57 | pMCAo | ↑ Infarct volume | ↑ | ↑ | ↑ | ||
| Tureyen et al.89 | Mouse, db/db | Leptin receptor mutation | tMCAo | ↑ Infarct volume | ↑ | ||||
| Kumari et al.90 | Mouse, db/db | Leptin receptor mutation | CCA ligation and hypoxia (8%) | db/db-alone effect not assessed | |||||
| Zhang et al.91 | Mouse, db/db | Leptin receptor mutation | CCA ligation and hypoxia (8%) | db/db-alone effect not assessed | ↑ | ↑ | |||
| Vannucci et al.92 | Mouse, db/db | Leptin receptor mutation | CCA ligation and hypoxia (8%) | ↑ Infarct volume | ↑ | ↑ | |||
An up arrow indicates an increase relative to non-co-morbid controls, a down arrow indicates a decrease, and double-headed arrow indicates no significant difference. A blank indicates the parameter was not reported. BW: body weight; BP: blood pressure; CCA: common carotid artery; Glu: blood glucose; HFD: high-fat diet (brackets indicate percentage of dietary calories which are fats); Ins: blood insulin; Lip: blood lipids; SD: Sprague Dawley; pMCAO: permanent MCAO; tMCAO: temporary MCAO.
Besides an increase in ischaemic damage, obese rodents often show an increase in blood–brain barrier (BBB) permeability and an increased incidence of haemorrhagic transformation,57,60,61,64,74,77,80,86,88,93 although the latter is not always observed in diet-induced rodents.60 This enhanced microvascular damage in obese animals likely contributes to their worse ischaemic damage due to the neurotoxic effects of both BBB breakdown and haemorrhagic transformation. Perhaps owing to the important role of the BBB in brain ion and water homoeostasis, an increase cerebral oedema is seen in obese rodents.61,64,66,75,76,86,92 White matter damage is also increased in obese rodents post-stroke.77 Importantly, cerebral oedema, BBB breakdown and haemorrhagic transformation are all indicators of poor prognosis in patients.95–98
Effects of obesity on neuroinflammation in experimental stroke
The chronic low-grade inflammation resulting from obesity may affect stroke outcome by modulating central nervous system (CNS) inflammation prior to stroke. Obese patients often show increased levels of pro-inflammatory cytokines in the plasma, including CRP, IL-6, TNFα and CCL2/MCP-1.28–30 Circulating cytokines may access the brain parenchyma directly, or transduce the inflammatory signal over the BBB indirectly via receptors on the endothelial cells of the cerebrovasculature or by stimulation of afferent nerve fibres. However, the systemic elevation of inflammatory mediators in obesity is likely not great enough to cause an overt inflammatory response in the CNS. Instead, the low-level systemic inflammation found in obesity may prime cells to subsequent inflammatory stimuli, for example leukocytes and platelets in the circulation.86,99 Mice that become obese after being fed a high-fat diet show increased activation of astrocytes and microglia, and increases in inflammatory mediators in the brain, principally due to NF-κB activation.100–104 However, these responses could be due to dietary fats, rather than the resulting obesity. Increases in basal inflammation may therefore alter the reactivity of cells within the CNS to subsequent ischaemic stimuli, changing the subsequent inflammatory response.
Despite showing increased ischaemic damage, less microglial reactivity and reduced expression of inflammatory cytokines have been reported in the brains of both obese db/db, ob/ob and high-fat fed mice after cerebral ischemia.66,78,86,90,91 This may represent a failure to mount an appropriate inflammatory response, which may prevent the appropriate transition from acute inflammation to repair and recovery. Indeed, db/db mice show delayed and diminished expression of growth factor and other markers of repair in the brain after stroke.90 In contrast, other studies have shown cytokine and chemokine expression levels are increased in ischaemic brain tissue of obese rats and mice.58,60,75,76 This heightened central inflammatory response could be due to the increased damage in the obese rodents, although elevated expression of inflammatory mediators is observed as early as 2.5 h after reperfusion.76
Effects of obesity on peripheral inflammatory response to experimental stroke
Stroke triggers a peripheral immune response by both humoural and neural routes, featuring lymphocyte release from immune organs (bone marrow, spleen and thymus), release of acute-phase proteins from the liver, increased inflammatory mediators in several organs, and sustained increases in other circulating markers of inflammation.105–107 Since this post-stroke peripheral response occurs as central damage is evolving, this process is thought to amplify the post-ischaemic inflammatory reaction and resulting damage. In obesity, the post-stroke peripheral immune response appears to be increased, as plasma IL-6, CCL2/MCP-1 and CXCL-1 (KC) significantly increase post-stroke in obese mice.60,86 Inhibition of CCL2/MCP-1 using a blocking antibody reduces the damage in ob/ob mice, suggesting the peripheral inflammatory response may contribute to damage in obesity.86 Similarly, both rosuvastatin (a statin) and darglitazone (a PPAR-γ agonist) were shown to be anti-inflammatory and neuroprotective in ob/ob, but not ob/− mice.78,87
Obesity exacerbates microvascular disruption in experimental stroke
Evidence from rodent studies suggests that obesity exacerbates inflammatory disruption of cerebral microvessels, worsening stroke outcome. This is seen experimentally as an increase in BBB permeability, increased incidence of haemorrhagic transformation and worse cerebral oedema. This increase in BBB permeability in obese mice is seen within 4 h of reperfusion, where it appears to be mediated by an increase in transcytotic endothelial vesicles, rather than a loss of tight junction integrity.57 Vascular complications occurring later after reperfusion may be mediated by an upregulation of the matrix metalloproteinase (MMP) family of proteases. Increases in expression of both MMP-2 and MMP-9 have been found in the ischaemic hemispheres of obese rodents,64,74,77,80,82 and greater BBB breakdown, haemorrhagic transformation and ischaemic damage are absent in high-fat fed MMP-9 knock out mice64 suggesting a causative role of MMP-9 in the detrimental effects of obesity. MMP-9 is expressed by cerebral vessels after stroke in ob/ob mice,80 but MMP-9 may also originate from adherent leukocytes, particularly neutrophils that release large quantities of MMP-9 upon degranulation.108 In support of this, increased adhesion of neutrophils and other leukocytes are found in cerebral vessels of obese rodents post-stroke,76,86 as well as increased neutrophil recruitment in the parenchyma.60,74 Furthermore, the total number of circulating leukocytes is increased in obese people, including increases in monocyte and neutrophil abundance and oxidative burst activity109–114 and also in obese mice.60,115 Obese mice also show increased vascular inflammation after stroke, characterised by increased vascular ICAM-1 expression, which may mediate neutrophil recruitment.58,75,76 These data suggest that obesity exacerbates inflammatory processes converging at the brain microvasculature endothelium, resulting in leukocyte recruitment and BBB breakdown. However, it is currently unclear whether these effects are mediated by changes within or external to the vasculature. For example, obesity may result in changes to the vasculature that subsequently worsens the vascular response to stroke. Alternatively, they may be caused by the convergence of other non-vascular causes at the BBB.
Whether stroke leads to enhanced microvascular disruption in obese humans is much less clear. No study has yet compared BBB leakage in obese versus non-obese patients, or in patients with similar co-morbidities. However, in patients followed up within a week of ischaemic stroke, the risk of risk of haemorrhagic transformation was decreased in obese patients (BMI ≥ 25 kg/m2).116 Furthermore, in patients receiving intravenous thrombolysis after stroke, obesity had no effect on the development of haemorrhagic transformation.117,118 This is in contrast to genetically obese mice deficient in leptin (ob/ob) or its receptor (db/db), and diet-induced obese mice, which show an increased incidence of haemorrhagic transformation after experimental stroke.57,64,77,80
Obesity affects cerebral vessel structure and tone
Obesity has effects on the structure and responsiveness of the cerebral vasculature that are likely to negatively affect stroke outcome. In high-fat fed and genetically obese rodents the middle cerebral arteries undergo remodelling that features a decrease in the lumen diameter and an increase in the thickness of the vascular wall, increasing vascular resistance.64,82,83 This structural remodelling seems dependent on the activity of MMPs 2 and 9, and features increased deposition of collagen 1 in the vascular wall.64,82 Obesity also affects the production of and response to factors that affect vascular tone. In particular, cerebral arteries from obese animals show increased vasoconstriction in response to 5-hydroxtryptamine (5-HT), potassium chloride (KCl) and endothelin-1 (ET-1), and reduced vasodilation in response to acetylcholine.83,93,119 However, other authors reported no effect of high-fat diet on vascular responsiveness, or attributed it to co-morbid hypertension.79,82 These changes may have a functional impact on cerebral blood flow, for example obese mice show an altered response in cerebral blood flow after whisker stimulation.93,120
Similar findings have also been made in the peripheral blood vessels of obese patients. Obesity is associated with increased intima-media thickness and decreased elasticity in arteries, impaired endothelium-mediated vasodilation and increased endothelin-1 activity.121–124 Although these observations have not been made directly in cerebral vessels, obese patients show other hallmarks indicative of cerebrovascular dysfunction. For example, increasing BMI is associated with lower cerebral blood flow velocity, increased cerebrovascular resistance and decreased cerebral blood flow.125–127 Therefore, both preclinical and clinical data suggest that cerebrovascular dysfunction characterised by increased vascular resistance and impaired autoregulation may contribute to worsened stroke outcome or risk in obesity.
The relationship between altered adipokines in obesity and stroke
Plasma levels of adiponectin are high in healthy adults, but reduce in correlation with increasing adiposity.128,129 Adiponectin has a variety of anti-inflammatory actions, and has recently been shown to be neuroprotective in stroke.130,131 In humans, increased levels of plasma adiponectin correlate with decreased ischaemic stroke damage, and conversely, low levels are associated with increased mortality after stroke.132 Similar observations have been made in rodent models of transient focal ischaemia, with adiponectin administration decreasing infarct size and neurological deficit,130,131 and adiponectin-deficiency increasing damage.133 The neuroprotective effects of adiponectin appear to be primarily mediated via the ischaemic cerebrovascular endothelium where adiponectin selectively localises post-stroke. The mechanisms of this localisation are not fully understood since adiponectin is not synthesised locally and does not permeate an intact BBB,134 but may involve adhesion to collagens of the injured endothelium and passage over the ischaemically damaged endothelial barrier.131,133 The resulting accumulation of adiponectin may have a variety of beneficial effects. Adiponectin appears to promote BBB integrity by reducing BBB permeability, microvascular MMP-9 expression and parenchymal leukocyte accumulation.131 Furthermore, adiponectin induces endothelial nitric oxide synthase (eNOS) activation and consequentially increases cerebral blood flow during ischaemia.130 Finally, adiponectin has been shown to decrease expression of pro-inflammatory cytokines, purportedly by inhibiting NF-κB, possibly due to activation of adiponectin receptors.131,134 A similar decrease in inflammatory cytokine expression was found in cultured brain endothelial cells treated with adiponectin.134 Through these actions, adiponectin has been extensively shown to protect the vascular endothelium in the periphery.135 Therefore, a loss of these protective effects may explain increases in stroke damage and microvascular complications in obese mice where adiponectin levels are chronically decreased. In support, recent data demonstrate that obesity exacerbates experimental ischaemia by increasing apoptosis of adiponectin-expressing neurones.61
The adipokine leptin has also been investigated for its role in stroke outcome, with several authors reporting seemingly conflicting conclusions. Since leptin circulates at levels proportional to body fat, obesity results in an increase in plasma leptin concentration. However, because these high concentrations are sustained, obesity may result in desensitisation to central leptin signalling.136 Using lean mice, Zhang et al.137 reported that leptin dose-dependently reduced infarct volume and neurological deficits after transient focal ischaemia, suggesting leptin was neuroprotective. Furthermore, in leptin-deficient obese ob/ob mice administration of leptin was protective in permanent focal ischaemia84 but detrimental in transient focal ischaemia.86 In addition, a lack of any significant effect of leptin was reported in both lean and obese ob/ob mice.80 No conclusive explanation has been given for these conflicting reports, however differences in dose regimes, adiposity and reperfusion may be involved, suggesting that leptin’s role is more complicated than being merely pro- or anti-inflammatory.
Obesity and other co-morbidities
Obesity is a component of the metabolic syndrome, and so is often accompanied by hyperglycaemia, hypertension and dyslipidaemia in patients that may all affect stroke outcome (Figure 1). Type-2 diabetes increases stroke risk and mortality,138 with acute hyperglycaemia resulting in greater infarcts and an increased risk of HT in rodents.75,139 Hypertension is the most important modifiable risk factor for stroke, and itself increases HT risk.140 Importantly, the pathological evolution of these conditions is intimately linked to obesity,141 so that disassociating the specific impact of obesity on health outcomes is difficult. This is the case in patients, but also in preclinical models of obesity that often develop different aspects of the metabolic syndrome dependent on their age, diet and genetic background (Table 1). For example, leptin-deficient ob/ob mice, db/db mice lacking the leptin receptor and mice fed a high fat diet all not only become obese, but eventually develop other aspects of the metabolic syndrome to some extent.142,143 Studies in obese rats have shown that presence of hypertension is critical in whether the detrimental effect of obesity on stroke outcome is observed.79,83 In contrast, the relationship between obesity and a worsened stroke outcome has been confirmed in mice without hypertension or hyperglycaemia.60,80,86,93 However, the lack of clinically relevant end-points (increased plasma glucose and blood pressure) does not mean their causative pathologies are not present and developing. This means that although animal models of obesity mimic the situation in obese people, the possible contribution from other co-morbidities should be considered when attempting to study specifically the effects of obesity on stroke. This is especially important when considering effects of obesity on the cerebral microvasculature where the deleterious effects on stroke outcome of both diabetes and hypertension are focused.140,144 However, similar considerations must be made for studies that have previously attributed poor outcome in animal models solely to the presence of diabetes or hypertension, when animals are often also obese.
Reconciling preclinical and clinical outlooks
Despite the well-established link between obesity and stroke risk, several clinical studies have reported that obese and overweight stroke patients have improved mortality and morbidity. This is in stark contrast to the consensus of preclinical studies, which have clearly shown that obesity worsens stroke outcome in rodents. There are many potential reasons for this disparity, which will be discussed, including differences in experimental design, models, aims and outcomes between preclinical and clinical studies.
Common limitations of preclinical experimental studies
The preclinical studies assessing the effects of obesity on stroke outcome are all experimental, and have several limitations in common. Whereas the clinical studies typically assess outcome in terms of functional recovery or mortality at weeks or years post-stroke, the majority of preclinical studies have focused on the evolution of the ischaemic lesion within 48 h of reperfusion. This focus on short-term outcomes may explain why no obesity paradox is found in preclinical studies. For example, a hypothesised reason that obesity is protective in stroke patients is that the metabolic reserves present in obesity protects against post-stroke weight loss, and associated muscle wasting.145 The metabolic consequences of stroke have been poorly studied pre-clinically in general, and even less so in the context of obesity. In preclinical studies, stroke is usually surgically induced in obese mice, rather than waiting for spontaneous strokes to occur. Therefore, if the obesity paradox is primarily due to the effects of obesity on risk, for example increasing the risk of a stroke earlier in life, then this would not be detectable in current animal studies. A further problem is that animals are almost all young and male. This tight control of variables, such as sex and age, is useful in establishing causal links in experimental studies, but does not reflect the clinical situation. A final disadvantage of preclinical studies is their exclusive use of rodents, and whether rodent physiology can accurately model human stroke. This question has been discussed in detail elsewhere.146,147
Animal models of obesity and their co-morbidities
Worse stroke outcome has been reported in both genetically obese and diet-induced obese rodents, yet both models also develop other pathologies alongside their increased adiposity (Table 1). As discussed, the resulting phenotype in both models is not solely increased adiposity, but more similar to metabolic syndrome found in patients. This is an accurate recapitulation of the clinical situation, as individual patients are rarely solely obese, but often present with other co-morbidities, including hypertension, diabetes and dyslipidaemias.40,42–44,46–48,50 However, due to the presence of these other co-morbidities, it becomes difficult to attribute the worse outcome in rodent models as being solely due to obesity. This is in contrast to clinical studies that can quantify the specific contribution of obesity to stroke outcome by applying multivariate statistics to large, heterogeneous patient populations. By this reasoning, worse stroke outcome in obese rodents may not be due to their adiposity, but their development of dyslipidaemia, diabetes or vascular disease. However, the same may be true of the individual stroke patient, whose outcome will be determined by a constellation of common co-morbidities.
Other considerations of obese models
Although both genetic and diet-induced obese models replicate the clinical situation found in obese patients, both have other drawbacks. Monogenic mutations leading to obesity are actually very rare in the general population and leptin has other immunological roles beyond regulation of energy balance.148,149 In diet-induced obesity, the constitution of the diet (principally the macronutrient ratio of fats, protein and carbohydrates) can affect the resulting phenotype. Use of an altered diet also adds an extra variable to consider, as effects on outcome may be due to the diet per se, rather the resulting obesity. Preclinical studies also use animals that are more homogenously and robustly obese than the patient population. Although this homogeneity makes interpretation of the contribution of obesity easier in experimental studies, it ignores the potentially protective effects of mild obesity (e.g. overweight). For example, mice fed a high-fat diet for 4 months show an average body weight increase of 60%.60 Assuming mice fed a control diet have a healthy BMI of 20–25, this translates to an increase in ‘mouse BMI’ to 32–40. In both mice and rats, the harmful effects of obesity on stroke outcome are not observed until a threshold level of obesity is obtained.60,73
Common limitations of clinical observational studies
The clinical studies assessing the impact of obesity on stroke outcome are almost universally observational – experimental studies in this setting are not possible as they would require purposefully exposing patients to either stroke and obesity. For similar reasons, observational studies are limited in their potential outcomes as non-invasive techniques are preferred. Furthermore, very acute events after the onset of ischaemia are difficult to study clinically as patients may not arrive at hospital until hours after symptom onset, and even then their care must be prioritised. Therefore, such findings of faster evolution of damage in obese rodents would be difficult to replicate in patients.
A further potential problem with observational studies is their potential for selection bias. A typical epidemiological study assessing how obesity affects stroke outcome will include all patients in their cohort that present with ischaemic stroke on hospital admittance. However, stratifying based on the presence of ischaemic stroke introduces collider-stratification bias, a type of selection bias.150,151 This results in non-causal relationships or associations between effects (factors that can affect the risk of stroke), for example between obesity and age. For this reason, the average age of patients in the obese groups is lower than in the non-obese groups of several studies reporting on the obesity paradox, with age often decreasing inversely with BMI and reaching a difference of 10 years in the most obese groups.42,47–51,53 This problem is not insurmountable, as in the case of known and commonly measured effects, such as age and traditional cardiovascular risk factors, their impact can be adjusted for statistically. However, there are many potential factors that may affect stroke incidence that are not commonly measured, so-called unmeasured effects. Similarly to age, stratification based on the presence of stroke will also create non-causal relationship between these unmeasured effects and obesity due to collider-stratification bias. As we cannot statistically adjust or account for the presence of unmeasured effects, they can distort the relationship between obesity and stroke outcome. This confounding of the stroke-mortality relationship by unmeasured confounding effects has been hypothesised to account for all of,151 or at least part of,152 the obesity paradox.
The existing clinical studies have primarily used BMI as a measure of obesity, but BMI may not always be the best indicator of adiposity. This is primarily because BMI does not discriminate between lean body mass and fat mass, and obesity is associated with an increase in both lean and fat mass. Other measures of obesity may more accurately reflect harmful adiposity, for example visceral/abdominal obesity rather than BMI is more strongly correlated with metabolic health.153,154 In support of this, obesity has a beneficial effect on vascular disease survival in obese diabetic patients when obesity is classified by BMI, but not when classified by waist circumference.155 Furthermore, people with elevated BMI are not always obese (i.e. have increased adiposity) and conversely people within normal body weight by BMI can still show a high percentage of body fat that is associated with higher risk of disease.156 The usefulness of BMI as a measure of adiposity may also decrease with age. In a cohort of patients with chronic heart failure with mean age 62–66, BMI misclassified body fat status 41% of the time, and was a better indicator of lean body mass than adiposity.157
Conclusion
Preclinical research has clearly shown that obesity worsens stroke outcome in rodents, suggesting a central role of pathological changes in the cerebrovasculature, including vascular inflammation and remodelling. In comparison, it is still unclear whether obesity worsens outcome in patients, and many preclinical findings cannot be replicated in the clinic. Future unification of preclinical and clinical findings will rely upon better alignment of research goals, with common outcomes that can be assessed in both fields. For example, studies assessing longer term functional outcomes in obese rodents could give insights into the obesity paradox in patients.
In this approach, preclinical studies should aim to make findings, which are verifiable in the clinic, and thereby give more confidence in translation potential of preclinical research.
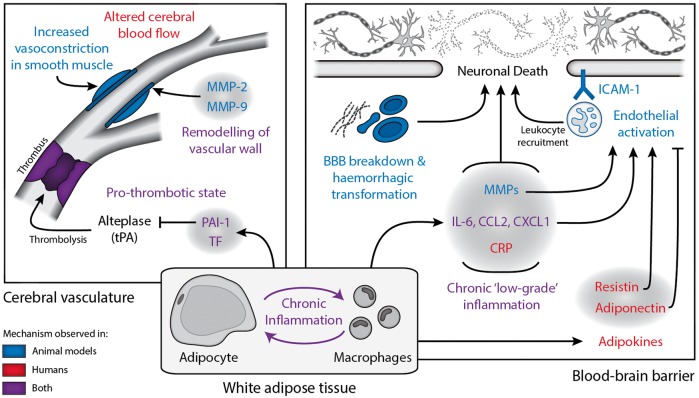
Despite this disparity between the preclinical and clinical outlooks, several key mechanisms that may mediate the interaction between stroke and obesity have been identified (Figure 2), though the potential interactions not fully discussed here are extensive. This complexity is due to the wide-ranging effects of stroke and obesity on physiology, spanning cardiovascular, neurological, immunological and metabolic systems. Another complication for both preclinical and clinical research is that obesity commonly occurs with other conditions such as hypertension and diabetes.
Figure 2.
Summary of potential mechanism by which obesity may affect stroke outcome. The effects of obesity on stroke outcome appear to converge at the cerebral vasculature and the blood–brain barrier, with both animal and human studies suggesting that inflammation is key in mediating these effects. Mechanisms coloured in blue have been studied in obese animals undergoing experimental stroke, red coloured items have been observed in obese patients and purple in both obese animals and patients. This figure is not exhaustive, as there are many hypothetical ways in which obesity may affect stroke, which have not yet been studied. BBB: blood-brain barrier; CRP: C reactive protein; CCL2: chemokine (C-C motif) ligand 2; CXCL2: chemokine (C-X-C motif) ligand 2; ICAM-1: intracellular adhesion molecule 1; IL-6: interleukin-6; MMP: matrix metalloproteinase; PAI-1: plasminogen activator inhibitor 1; TF: tissue factor; tPA: tissue plasminogen activator.
At least in the preclinical data, there is strong evidence that altered inflammatory processes are central in mediating the worse stroke outcome found in obese rodents. Furthermore, there is a clinically established role of inflammation in both stroke and obesity. Further work is therefore required to reconcile the preclinical and clinical outlooks and allow identification of mechanisms important in both rodents and men, hopefully translating into better care for obese stroke patients.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical Research Council UK and Kohn Foundation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors contributions
Both authors (MJH and CBL) contributed to the writing of the article.
References
- 1.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heuschmann PU, Kircher J, Nowe T, et al. Control of main risk factors after ischaemic stroke across Europe: data from the stroke-specific module of the EUROASPIRE III survey. Eur J Prev Cardiol 2015; 22: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 3.Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe: epidemiological update. Eur Heart J 2013; 34: 3028–3034. [DOI] [PubMed] [Google Scholar]
- 4.Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014; 35: 2950–2959. [DOI] [PubMed] [Google Scholar]
- 5.Strazzullo P, D’Elia L, Cairella G, et al. Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke 2010; 41: e418–e426. [DOI] [PubMed] [Google Scholar]
- 6.Ni Mhurchu C, Rodgers A, Pan WH, et al. Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol 2004; 33: 751–758. [DOI] [PubMed] [Google Scholar]
- 7.Rexrode KM, Hennekens CH, Willett WC, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA 1997; 277: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 8.Kurth T, Gaziano JM, Berger K, et al. Body mass index and the risk of stroke in men. Arch Intern Med 2002; 162: 2557–2562. [DOI] [PubMed] [Google Scholar]
- 9.Song YM. Body mass index and ischemic and hemorrhagic stroke: A prospective study in Korean men. Stroke 2004; 35: 831–836. [DOI] [PubMed] [Google Scholar]
- 10.Park JW, Lee S-Y, Kim SY, et al. BMI and stroke risk in Korean women. Obesity 2008; 16: 396–401. [DOI] [PubMed] [Google Scholar]
- 11.Lu M, Ye W, Adami HO, et al. Prospective study of body size and risk for stroke amongst women below age 60. J Int Med 2006; 260: 442–450. [DOI] [PubMed] [Google Scholar]
- 12.Jood K, Jern C, Wilhelmsen L, et al. Body mass index in mid-life is associated with a first stroke in men: A prospective population study over 28 years. Stroke 2004; 35: 2764–2769. [DOI] [PubMed] [Google Scholar]
- 13.Kurth T. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation 2005; 111: 1992–1998. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Tuomilehto J, Silventoinen K, et al. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med 2007; 167: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 15.Cheng NT, Kim AS. Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist 2015; 5: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deguchi I, Ohe Y, Fukuoka T, et al. Relationship of obesity to recanalization after hyperacute recombinant tissue-plasminogen activator infusion therapy in patients with middle cerebral artery occlusion. J Stroke Cerebrovasc Dis 2012; 21: 161–164. [DOI] [PubMed] [Google Scholar]
- 17.Dorado L, Arenillas JF, López-Cancio E, et al. Metabolic syndrome predicts refractoriness to intravenous thrombolysis in acute ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24: 2605–2612. [DOI] [PubMed] [Google Scholar]
- 18.Arenillas JF, Sandoval P, Perez de la Ossa N, et al. The metabolic syndrome is associated with a higher resistance to intravenous thrombolysis for acute ischemic stroke in women than in men. Stroke 2009; 40: 344–349. [DOI] [PubMed] [Google Scholar]
- 19.Brott TG, Haley EC, Levy DE, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 1992; 23: 632–640. [DOI] [PubMed] [Google Scholar]
- 20.Lou M, Selim M. Does body weight influence the response to intravenous tissue plasminogen activator in stroke patients? Cerebrovasc Dis 2009; 27: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarikaya H, Arnold M, Engelter ST, et al. Outcome of intravenous thrombolysis in stroke patients weighing over 100 kg. Cerebrovasc Dis 2011; 32: 201–206. [DOI] [PubMed] [Google Scholar]
- 22.Murray KN, Buggey HF, Denes A, et al. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci 2013; 53: 14–25. [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Lawrence CB, Rodriguez-Grande B, et al. The immune system in stroke: clinical challenges and their translation to experimental research. J Neuroimmune Pharmacol 2013; 8: 867–887. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999; 282: 2131–2135. [DOI] [PubMed] [Google Scholar]
- 25.Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972–978. [DOI] [PubMed] [Google Scholar]
- 26.Herder C, Schneitler S, Rathmann W, et al. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab 2007; 92: 4569–4574. [DOI] [PubMed] [Google Scholar]
- 27.Rexrode KM, Pradhan A, Manson JE, et al. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol 2003; 13: 674–682. [DOI] [PubMed] [Google Scholar]
- 28.Khaodhiar L, Ling P-R, Blackburn GL, et al. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr 2004; 28: 410–415. [DOI] [PubMed] [Google Scholar]
- 29.Maachi M, Piéroni L, Bruckert E, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord 2004; 28: 993–997. [DOI] [PubMed] [Google Scholar]
- 30.Kim C-S, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006; 30: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 31.Thompson AM, Zhang Y, Tong W, et al. Association of obesity and biomarkers of inflammation and endothelial dysfunction in adults in Inner Mongolia, China. Int J Cardiol 2011; 150: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guri AJ, Bassaganya-Riera J. Systemic effects of white adipose tissue dysregulation and obesity-related inflammation. Obesity (Silver Spring) 2011; 19: 689–700. [DOI] [PubMed] [Google Scholar]
- 33.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 2005; 33: 1078–1081. [DOI] [PubMed] [Google Scholar]
- 34.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001; 32: 2575–2579. [DOI] [PubMed] [Google Scholar]
- 35.Miwa K, Tanaka M, Okazaki S, et al. Association between interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk factors. Arterioscler Thromb Vasc Biol 2013; 33: 400–405. [DOI] [PubMed] [Google Scholar]
- 36.Ofstad AP, Gullestad L, Orvik E, et al. Interleukin-6 and activin A are independently associated with cardiovascular events and mortality in type 2 diabetes: the prospective Asker and Bærum Cardiovascular Diabetes (ABCD) cohort study. Cardiovasc Diabetol 2013; 12: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson CC, Smith AE, Yarnell JWG, et al. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly Study. Atherosclerosis 2010; 209: 551–557. [DOI] [PubMed] [Google Scholar]
- 38.Seven E, Husemoen LLN, Sehested TSG, et al. Adipocytokines, C-reactive protein, and cardiovascular disease: a population-based prospective study. PLoS One 2015; 10: e0128987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen TSOJ, Dehlendorff C, Petersen HG, et al. Body mass index and poststroke mortality. Neuroepidemiology 2008; 30: 93–100. [DOI] [PubMed] [Google Scholar]
- 40.Vemmos K, Ntaios G, Spengos K, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke 2011; 42: 30–36. [DOI] [PubMed] [Google Scholar]
- 41.Kim BJ, Lee SH, Jung KH, et al. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology 2012; 79: 856–863. [DOI] [PubMed] [Google Scholar]
- 42.Doehner W, Schenkel J, Anker SD, et al. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J 2013; 34: 268–277. [DOI] [PubMed] [Google Scholar]
- 43.Skolarus LE, Sanchez BN, Levine DA, et al. Association of body mass index and mortality after acute ischemic stroke. Circ Cardiovasc Qual Outcomes 2014; 7: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke 2015; 10: 99–104. [DOI] [PubMed] [Google Scholar]
- 45.Kazemi-Bajestani SMR, Ghayour-Mobarhan M, Thrift AG, et al. Obesity paradox versus frailty syndrome in first-ever ischemic stroke survivors. Int J Stroke 2015; 10: E75–E75. [DOI] [PubMed] [Google Scholar]
- 46.Wohlfahrt P, Lopez-Jimenez F, Krajcoviechova A, et al. The obesity paradox and survivors of ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 47.Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke 2009; 40: 2704–2708. [DOI] [PubMed] [Google Scholar]
- 48.Barba R, Marco J, Ruiz J, et al. The obesity paradox in stroke: impact on mortality and short-term readmission. J Stroke Cerebrovasc Dis 2015; 24: 766–770. [DOI] [PubMed] [Google Scholar]
- 49.Nishioka S, Wakabayashi H, Yoshida T, et al. Obese Japanese patients with stroke have higher functional recovery in convalescent rehabilitation wards: A retrospective cohort study. J Stroke Cerebrovasc Dis 2016; 25: 26–33. [DOI] [PubMed] [Google Scholar]
- 50.Hassan AE, Chaudhry SA, Jani V, et al. Is there a decreased risk of intracerebral hemorrhage and mortality in obese patients treated with intravenous thrombolysis in acute ischemic stroke? J Stroke Cerebrovasc Dis 2013; 22: 545–549. [DOI] [PubMed] [Google Scholar]
- 51.Ryu W-S, Lee S-H, Kim CK, et al. Body mass index, initial neurological severity and long-term mortality in ischemic stroke. Cerebrovasc Dis 2011; 32: 170–176. [DOI] [PubMed] [Google Scholar]
- 52.Kim Y, Kim CK, Jung S, et al. Obesity-stroke paradox and initial neurological severity. J Neurol Neurosurg Psychiatry 2015; 86: 743–747. [DOI] [PubMed] [Google Scholar]
- 53.Dehlendorff C, Andersen KK, Olsen TS. Body mass index and death by stroke: no obesity paradox. JAMA Neurol 2014; 71: 978–984. [DOI] [PubMed] [Google Scholar]
- 54.Katsnelson M, Rundek T. Obesity paradox and stroke: noticing the (fat) man behind the curtain. Stroke 2011; 42: 3331–3332. [DOI] [PubMed] [Google Scholar]
- 55.Doehner W, Audebert HJ. The impact of body weight on mortality after stroke: the controversy continues. JAMA Neurol 2015; 72: 126–127. [DOI] [PubMed] [Google Scholar]
- 56.Brzecka A, Ejma M. Obesity paradox in the course of cerebrovascular diseases. Adv Clin Exp Med 2015; 24: 379–383. [DOI] [PubMed] [Google Scholar]
- 57.Haley MJ, Lawrence CB. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab . Epub ahead of print 28 January 2016. DOI: 10.1177/0271678X16629976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao X-L, Du J, Zhang Y, et al. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res 2015; 233: 2753–2765. [DOI] [PubMed] [Google Scholar]
- 59.Deng J, Xiong L, Zuo Z. Trans-sodium crocetinate provides neuroprotection against cerebral ischemia and reperfusion in obese mice. J Neurosci Res 2015; 93: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maysami S, Haley MJ, Gorenkova N, et al. Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worse outcome after stroke. J Neuroinflamm 2015; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu M-H, Chio C-C, Tsai K-J, et al. Obesity exacerbates rat cerebral ischemic injury through enhancing ischemic adiponectin-containing neuronal apoptosis. Mol Neurobiol 2016; 53: 3702–3713. [DOI] [PubMed] [Google Scholar]
- 62.Cheon SH, Yan BC, Chen BH, et al. Accelerated and exacerbated effects of high dietary fat on neuronal damage induced by transient cerebral ischemia in the gerbil septum. Endocrinol Metab (Seoul) 2014; 29: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan BC, Park JH, Ahn JH, et al. Effects of high-fat diet on neuronal damage, gliosis, inflammatory process and oxidative stress in the hippocampus induced by transient cerebral ischemia. Neurochem Res 2014; 39: 2465–2478. [DOI] [PubMed] [Google Scholar]
- 64.Deng J, Zhang J, Feng C, et al. Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodelling and increase of ischaemic brain injury in mice. Cardiovasc Res 2014; 103: 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Chen Y, Zhang Y, et al. Sevoflurane postconditioning against cerebral ischemic neuronal injury is abolished in diet-induced obesity: role of brain mitochondrial KATP channels. Mol Med Rep 2014; 9: 843–850. [DOI] [PubMed] [Google Scholar]
- 66.Kim E, Tolhurst AT, Cho S. Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. J Neuroinflamm 2014; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herz J, Hagen SI, Bergmüller E, et al. Exacerbation of ischemic brain injury in hypercholesterolemic mice is associated with pronounced changes in peripheral and cerebral immune responses. Neurobiol Dis 2014; 62: 456–468. [DOI] [PubMed] [Google Scholar]
- 68.Dhungana H, Rolova T, Savchenko E, et al. Western-type diet modulates inflammatory responses and impairs functional outcome following permanent middle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J Neuroinflamm 2013; 10: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim E, Febbraio M, Bao Y, et al. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol 2012; 71: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pradillo JM, Denes A, Greenhalgh AD, et al. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab 2012; 32: 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawai H, Deguchi S, Deguchi K, et al. Protection against ischemic stroke damage by synergistic treatment with amlodipine plus atorvastatin in Zucker metabolic rat. Brain Res 2011; 1382: 308–314. [DOI] [PubMed] [Google Scholar]
- 72.Kawai H, Deguchi S, Deguchi K, et al. Synergistic benefit of combined amlodipine plus atorvastatin on neuronal damage after stroke in Zucker metabolic rat. Brain Res 2011; 1368: 317–323. [DOI] [PubMed] [Google Scholar]
- 73.Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience 2011; 182: 82–87. [DOI] [PubMed] [Google Scholar]
- 74.Kumari R, Willing LB, Patel SD, et al. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem 2011; 119: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tureyen K, Bowen K, Liang J, et al. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J Neurochem 2011; 116: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritter L, Davidson L, Henry M, et al. Exaggerated neutrophil-mediated reperfusion injury after ischemic stroke in a rodent model of type 2 diabetes. Microcirculation 2011; 18: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J, Cui X, Zacharek A, et al. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke 2011; 42: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumari R, Willing LB, Patel SD, et al. The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic ob/ob mouse. J Cereb Blood Flow Metab 2010; 30: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osmond JM, Mintz JD, Stepp DW. Preventing increased blood pressure in the obese Zucker rat improves severity of stroke. AJP: Heart Circ Physiol 2010; 299: H55–H61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McColl BW, Rose N, Robson FH, et al. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J Cereb Blood Flow Metab 2010; 30: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arvanitidis AP, Corbett D, Colbourne F. A high fat diet does not exacerbate CA1 injury and cognitive deficits following global ischemia in rats. Brain Res 2009; 1252: 192–200. [DOI] [PubMed] [Google Scholar]
- 82.Deutsch C, Portik-Dobos V, Smith AD, et al. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc Res 2009; 78: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osmond JM, Mintz JD, Dalton B, et al. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 2009; 53: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valerio A, Dossena M, Bertolotti P, et al. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke 2009; 40: 610–617. [DOI] [PubMed] [Google Scholar]
- 85.Kim E, Tolhurst AT, Qin LY, et al. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci 2008; 28: 4661–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Terao S, Yilmaz G, Stokes KY, et al. Inflammatory and Injury Responses to Ischemic Stroke in Obese Mice. Stroke 2008; 39: 943–950. [DOI] [PubMed] [Google Scholar]
- 87.Mayanagi K, Katakam PV, Gáspár T, et al. Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagai N, Van Hoef B, Lijnen HR. Plasminogen activator inhibitor-1 contributes to the deleterious effect of obesity on the outcome of thrombotic ischemic stroke in mice. J Thromb Haemost 2007; 5: 1726–1731. [DOI] [PubMed] [Google Scholar]
- 89.Tureyen K, Kapadia R, Bowen KK, et al. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem 2007; 101: 41–56. [DOI] [PubMed] [Google Scholar]
- 90.Kumari R, Willing LB, Krady JK, et al. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab 2007; 27: 710–718. [DOI] [PubMed] [Google Scholar]
- 91.Zhang L, Nair A, Krady K, et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest 2004; 113: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vannucci SJ, Willing LB, Goto S, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab 2001; 21: 52–60. [DOI] [PubMed] [Google Scholar]
- 93.Li W, Prakash R, Chawla D, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol 2013; 304: R1001–R1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S, Kim Da Sol, Kang S, et al. Ischemic hippocampal cell death induces glucose dysregulation by attenuating glucose-stimulated insulin secretion which is exacerbated by a high fat diet. Life Sci 2011; 88: 766–773. [DOI] [PubMed] [Google Scholar]
- 95.Lorberboym M, Lampl Y, Sadeh M. Correlation of 99mTc-DTPA SPECT of the blood-brain barrier with neurologic outcome after acute stroke. J Nucl Med 2003; 44: 1898–1904. [PubMed] [Google Scholar]
- 96.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008; 39: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 97.Brouns R, Wauters A, De Surgeloose D, et al. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur Neurol 2011; 65: 23–31. [DOI] [PubMed] [Google Scholar]
- 98.Alvarez-Sabin J, Maisterra O, Santamarina E, et al. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol 2013; 12: 689–705. [DOI] [PubMed] [Google Scholar]
- 99.Vachharajani V, Russell JM, Scott KL, et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation 2005; 12: 183–194. [DOI] [PubMed] [Google Scholar]
- 100.Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 2010; 219: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nerurkar PV, Johns LM, Buesa LM, et al. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflamm 2011; 8: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thaler JP, Yi C-X, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012; 122: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knight EM, Martins IVA, Gümüsgöz S, et al. High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging 2014; 35: 1821–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 2013; 24: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab 2006; 26: 654–665. [DOI] [PubMed] [Google Scholar]
- 106.Chapman KZ, Dale VQ, Denes A, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab 2009; 29: 1764–1768. [DOI] [PubMed] [Google Scholar]
- 107.Denes A, McColl BW, Leow-Dyke SF, et al. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab 2011; 31: 1036–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gidday JM, Gasche YG, Copin J-C, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. AJP: Heart Circ Physiol 2005; 289: H558–68. [DOI] [PubMed] [Google Scholar]
- 109.Nieman DC, Henson DA, Nehlsen-Cannarella SL, et al. Influence of obesity on immune function. J Am Diet Assoc 1999; 99: 294–299. [DOI] [PubMed] [Google Scholar]
- 110.Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30). Am J Cardiol 2002; 89: 1441–1443. [DOI] [PubMed] [Google Scholar]
- 111.Dixon JB, O’Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg 2006; 16: 251–257. [DOI] [PubMed] [Google Scholar]
- 112.Herishanu Y, Rogowski O, Polliack A, et al. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol 2006; 76: 516–520. [DOI] [PubMed] [Google Scholar]
- 113.Nijhuis J, Rensen SS, Slaats Y, et al. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity 2009; 17: 2014–2018. [DOI] [PubMed] [Google Scholar]
- 114.Trottier MD, Naaz A, Kacynski K, et al. Functional capacity of neutrophils from class III obese patients. Obesity (Silver Spring) 2012; 20: 1057–1065. [DOI] [PubMed] [Google Scholar]
- 115.Trottier MD, Naaz A, Li Y, et al. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci USA 2012; 109: 7622–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim CK, Ryu W-S, Kim BJ, et al. Paradoxical effect of obesity on hemorrhagic transformation after acute ischemic stroke. BMC Neurol 2013; 13: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sarikaya H, Elmas F, Arnold M, et al. Impact of obesity on stroke outcome after intravenous thrombolysis. Stroke 2011; 42: 2330–2332. [DOI] [PubMed] [Google Scholar]
- 118.Seet RCS, Zhang Y, Wijdicks EFM, et al. Thrombolysis outcomes among obese and overweight stroke patients: an age- and National Institutes of Health Stroke Scale-matched comparison. J Stroke Cerebrovasc Dis 2014; 23: 1–6. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki M, Yamamoto D, Suzuki T, et al. High fat and high fructose diet induced intracranial atherosclerosis and enhanced vasoconstrictor responses in non-human primate. Life Sci 2006; 80: 200–204. [DOI] [PubMed] [Google Scholar]
- 120.Tucsek Z, Toth P, Tarantini S, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 2014; 69: 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czernichow S, Bertrais S, Blacher J, et al. Metabolic syndrome in relation to structure and function of large arteries: a predominant effect of blood pressure. A report from the SU.VI.MAX. Vascular Study. Am J Hypertens 2005; 18: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 122.Lind L, Siegbahn A, Ingelsson E, et al. A detailed cardiovascular characterization of obesity without the metabolic syndrome. Arterioscler Thromb Vasc Biol 2011; 31: e27–e34. [DOI] [PubMed] [Google Scholar]
- 123.Weil BR, Westby CM, Van Guilder GP, et al. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol 2011; 301: H689–H695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Campia U, Tesauro M, Cardillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacol 2012; 165: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Selim M, Jones R, Novak P, et al. The effects of body mass index on cerebral blood flow velocity. Clin Auton Res 2008; 18: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity (Silver Spring) 2011; 19: 1095–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Birdsill AC, Carlsson CM, Willette AA, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 2013; 21: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Halberg N, Schraw TD, Wang ZV, et al. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 2009; 58: 1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 2010; 314: 1–16. [DOI] [PubMed] [Google Scholar]
- 130.Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation 2008; 117: 216–223. [DOI] [PubMed] [Google Scholar]
- 131.Chen B, Liao W-Q, Xu N, et al. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res 2009; 1273: 129–137. [DOI] [PubMed] [Google Scholar]
- 132.Efstathiou SP, Tsioulos DI, Tsiakou AG, et al. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke 2005; 36: 1915–1919. [DOI] [PubMed] [Google Scholar]
- 133.Yatomi K, Miyamoto N, Komine-Kobayashi M, et al. Pathophysiological dual action of adiponectin after transient focal ischemia in mouse brain. Brain Res 2009; 1297: 169–176. [DOI] [PubMed] [Google Scholar]
- 134.Spranger J, Verma S, Göhring I, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 2006; 55: 141–147. [PubMed] [Google Scholar]
- 135.Lam KSL, Xu A. Adiponectin: protection of the endothelium. Curr Diab Rep 2005; 5: 254–259. [DOI] [PubMed] [Google Scholar]
- 136.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 2009; 297: E1247–E1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang F, Wang S, Signore AP, et al. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke 2007; 38: 2329–2336. [DOI] [PubMed] [Google Scholar]
- 138.Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke: epidemiology and possible mechanisms. Diabetes Care 2007; 30: 3131–3140. [DOI] [PubMed] [Google Scholar]
- 139.Ergul A, Elgebaly MM, Middlemore M-L, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol 2007; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veglio F, Paglieri C, Rabbia F, et al. Hypertension and cerebrovascular damage. Atherosclerosis 2009; 205: 331–341. [DOI] [PubMed] [Google Scholar]
- 141.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediat Inflamm 2010; 2010: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shafrir E, Ziv E. A useful list of spontaneously arising animal models of obesity and diabetes. AJP: Endocrinol Metab 2009; 296: E1450–E1452. [DOI] [PubMed] [Google Scholar]
- 143.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev 2010; 23: 270–299. [DOI] [PubMed] [Google Scholar]
- 144.Ergul A, Li W, Elgebaly MM, et al. Hyperglycemia, diabetes and stroke: focus on the cerebrovasculature. Vascul Pharmacol 2009; 51: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: Lessons from the obesity paradox. Stroke 2011; 42: 3646–3650. [DOI] [PubMed] [Google Scholar]
- 146.Sharp FR, Jickling GC. Modeling immunity and inflammation in stroke: differences between rodents and humans? Stroke 2014; 45: e179–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Becker KJ. Strain-related differences in the immune response: Relevance to human stroke. Transl Stroke Res 2016; 7: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol 2005; 174: 3137–3142. [DOI] [PubMed] [Google Scholar]
- 149.Farooqi IS, O’Rahilly S. 20 years of leptin: human disorders of leptin action. J Endocrinol 2014; 223: T63–T70. [DOI] [PubMed] [Google Scholar]
- 150.Ferreira I, Stehouwer CDA. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. J Hypertens 2012; 30: 2271–2275. [DOI] [PubMed] [Google Scholar]
- 151.Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol 2015; 25: 342–349. [DOI] [PubMed] [Google Scholar]
- 152.Sperrin M, Candlish J, Badrick E, et al. Collider bias is only a partial explanation for the obesity paradox. Epidemiology 2016; 27: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014; 233: 104–112. [DOI] [PubMed] [Google Scholar]
- 154.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients 2013; 5: 2019–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dallongeville J, Bhatt DL, Steg PG, et al. Relation between body mass index, waist circumference, and cardiovascular outcomes in 19,579 diabetic patients with established vascular disease: the REACH Registry. Eur J Prev Cardiol 2012; 19: 241–249. [DOI] [PubMed] [Google Scholar]
- 156.Oliveros E, Somers VK, Sochor O, et al. The concept of normal weight obesity. Prog Cardiovasc Dis 2014; 56: 426–433. [DOI] [PubMed] [Google Scholar]
- 157.Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc 2010; 85: 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]