Abstract
Background
In Egypt, liver flukes, Fasciola spp. (Digenea: Fasciolidae), have a serious impact on the farming industry and public health. Both Fasciola hepatica and Fasciola gigantica are known to occur in cattle, providing the opportunity for genetic recombination. Little is known on the identity and genetic variability of Fasciola populations in sheep.
Methods
This study was performed to determine the prevalence of liver flukes in sheep in Menofia Province as a representative area of the delta region in Egypt, as measured by postmortem examination of slaughtered animals at three abattoirs. The identity and genetic variability of Fasciola spp. in slaughtered animals were determined by PCR-sequence analysis of the nuclear ribosomal internal transcribed spacer 1 (ITS1) and the mitochondrial NADH dehydrogenase subunit 1 (nad1) genes.
Results
Physical inspection of the liver indicated that 302 of 2058 (14.7%) slaughtered sheep were infected with Fasciola spp. Sequence analysis of the ITS1 and nad1 genes of liver flukes from 17 animals revealed that 11 animals were infected with F. hepatica, four with F. gigantica, and two with both species. Seventy eight of 103 flukes genetically characterized from these animals were F. hepatica, 23 were F. gigantica, and two had ITS1 sequences identical to F. hepatica but nad1 sequences identical to F. gigantica. nad1 sequences of Egyptian isolates of F. gigantica showed pronounced differences from those in the GenBank database. Egyptian F. gigantica haplotypes formed haplogroup D, which clustered in a sister clade with haplogroups A, B and C circulating in Asia, indicating the existence of geographic isolation in the species.
Conclusions
Both F. hepatica and F. gigantica are prevalent in sheep in Egypt and an introgressed form of the two occurs as the result of genetic recombination. In addition, a geographically isolated F. gigantica population is present in the country. The importance of these observations in epidemiology of fascioliasis needs to be examined in future studies.
Keywords: Fasciola, Genotype, ITS1, nad1, Hybridization, Egypt
Background
Fascioliasis is a foodborne disease caused by infection with liver flukes of the genus Fasciola and occurs in a wide range of mammalian hosts worldwide [1, 2]. Liver flukes reside in the bile duct of the definitive hosts, resulting in sever hepatic damage and associated health consequences [3]. In developing countries such as African nations, Fasciola infections have been recognized as a major constraint to animal farming [1, 4], contributing to the impeded economic development [5]. In addition, the incidence rate of human fascioliasis is high in areas of high animal infections [6]. The World Health Organization estimates that at least 2.4 million people in more than 70 countries are affected by fascioliasis (http://www.who.int/foodborne_trematode_infections/fascioliasis/en/). Being a multifactorial disease with limited drugs available for treatment, effective measures are urgently needed to control this important foodborne and zoonotic disease [7].
Fasciola hepatica (Linnaeus, 1758) and Fasciola gigantica (Cobbold, 1856) are the causative agents of the disease in both humans and animals. The distribution of the two species of Fasciola appears to be geographically associated. F. hepatica is common in temperate zones especially Europe, Americas and Australia, while F. gigantica is the known species in tropical regions of Africa and Asia. Both species overlap in occurrence in subtropical areas [1, 2, 8–11]. Fasciola spp. have the ability to self-fertilize, cross-fertilize and in some cases undergo parthenogenesis [12]. Hybridization between the two Fasciola species has been documented, leading to the emergence of intermediate forms with mixed phenotypic characteristics and genetic structure [13–15]. Fasciola flukes of abnormal ploidy (triploid and mixoploid) have been reported; they are parthenogenetic with no evidence of sperm production [14, 16, 17].
Molecular analyses play a pivotal role in the identification of Fasciola spp. [18–20], resolving morphometric discrepancies associated with species identifications, especially those related to intermediate forms. Molecular analyses of the intermediate forms have detected individuals that have divergent copies of the nuclear ribosomal genes derived from both Fasciola species (the hybrid form), as well as individuals with nuclear DNA of one species while mitochondrial DNA of the other species (the introgressed form) [8, 14, 17, 21]. Noteworthy, all analyzed Fasciola flukes in Japan and South Korea are aspermic [21–23] and have mixed F. hepatica and F. gigantica sequences by analysis of the nuclear phosphoenolpyruvate carboxykinase and DNA polymerase delta genes, suggesting that the flukes are descendants of hybridization between the two species [24].
Egypt is one of the fascioliasis-endemic areas in the world [25]. The disease burden is high in several species of livestock [26] as well as humans [27]. Both species of Fasciola are present in cattle in Egypt, and the occurrence of the hybrid form has been reported [8]. Thus far, the introgressed form of Fasciola spp. has not been detected.
In contrast to cattle, no studies are available on the molecular identity of Fasciola spp. in sheep in Egypt. The present study was conducted to determine the occurrence rate of Fasciola spp. in sheep as measured by postmortem examination of slaughtered animals at abattoirs. In addition, the identity and genetic variability of Fasciola spp. derived from slaughtered animals were examined by PCR-sequence analysis of the nuclear ribosomal internal transcribed spacer 1 (ITS1) and the mitochondrial NADH dehydrogenase subunit 1 (nad1) gene.
Methods
Specimen collection
Livers from 2058 slaughtered adult sheep were collected during August 2012 to August 2014 during post-mortem inspection by veterinary officers at Shebein El Kom, Ashmoun and El Shouhada abattoirs in El Menofia Province (90 km East of Cairo), Egypt. The inspected sheep included 783 animals at Shebein El Kom, 1219 at Ashmoun, and 56 at El Shouhada abattoirs. The livers were physically inspected for the presence of Fasciola worms. Flukes from each infected individual were collected in plastic containers, washed in physiological saline and fixed in 95% ethanol. A total of 5–7 worms from 17 infected individuals were used in molecular analysis, resulting in 103 worms genetically characterized. Randomly selected individuals (11 of each Fasciola species) from genetically characterized worms (i.e. hologenophore specimens according to Astrin et al. [28]) were lightly pressed between two glass slides and used in morphometric analysis.
Morphometric analysis
Individual flukes were washed three times in PBS, stained in acetocarmine, and mounted in DPX medium [29]. Measurements, expressed in millimeters (mm) were made for 11 flukes of each Fasciola species, using a microscope equipped with a calibrated ocular micrometer (Leica Microsystems GmbH, Wetzlar, Germany). Six ratios were also calculated for each Fasciola type. Statistical analysis was conducted using the Student’s t-test implemented in SPSS 15.0 (SPSS, Chicago, Illinois), with values of P ≤ 0.05 at degree of freedom 20 considered significant.
DNA extraction and PCR analysis
Individual flukes fixed in ethanol were washed extensively with PBS. To avoid the inclusion of female genitalia that might contain foreign sperms, genomic DNA was extracted from a small portion of lateral margin of the posterior end using the FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA, USA). Some of the genetically characterized flukes were used in morphometric measurement described above. The complete nuclear ITS1 and partial mitochondrial nad1 genes in the extracted DNA were amplified using primers of Itagaki et al. [21]. PCR reactions were done in 50 μl volume consisted of 1 μl of genomic DNA, 5 μl 10× GeneAmp PCR buffer (Applied Biosystems, Foster City, CA, USA), 8 μl of dNTP (Promega, Madison, WI, USA), 3 μl MgCl2, 1.5 μl of each primer, 0.3 μl of GoTaq DNA polymerase (Promega) and 29.7 μl of molecular grade H2O. Each PCR consisted of 30 cycles of denaturation at 98 °C for 10 s, annealing at 56 °C (for ITS1) or 53 °C (for nad1) for 35 s, and extension at 68 °C for 50 s, with an initial denaturation step at 95 °C for 5 min and a final extension step at 68 °C for 10 min. PCR products were visualized by electrophoresis in 1.5% agarose gels.
DNA sequence analysis
PCR products were sequenced directly using the Big Dye® Terminator v3.1 Cycle Sequencing Kit and an ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequences obtained were assembled using the ChromasPro (version 1.5) software (http://www.technelysium.com.au/ChromasPro.html). The accuracy of data was confirmed by bi-directional sequencing. The obtained sequences from each genetic target were aligned with each other and reference sequences using ClustalX (http://www.clustal.org/) to determine the identity of Fasciola spp. Evolutionary relationship was inferred based on nad1 sequences using the Maximum Likelihood (ML) method implemented in MEGA7 (http://www.megasoftware.net/). The ML phylogenetic analysis was conducted using the Kimura 2-parameter model with 1000 bootstrap replicates. A nucleotide sequence from Paragonimus westermani (AF219379) was used as the outgroup to root the tree. Details of sequences from Egypt and other countries used in the construction of the phylogenetic tree are shown in Table 1. Additional phylogenetic analyses were conducted using the Maximum Parsimony implemented in MEGA7 and Bayesian Method implemented in MrBayes (http://mrbayes.sourceforge.net/).
Table 1.
Details of Fasciola nad1 sequences from Egypt and other countries used in phylogenetic analysis
| Accession number | Species | Host | Location | Reference |
|---|---|---|---|---|
| LC076235 (4 replicates) | F. hepatica | Sheep | Egypt | This study |
| LC076228 (10 replicates) | F. hepatica | Sheep | Egypt | This study |
| LC076258 (3 replicates) | F. hepatica | Cattle | Egypt | This study |
| LC076241 (4 replicates) | F. hepatica | Sheep | Egypt | This study |
| LC076240 (6 replicates) | F. hepatica | Sheep | Egypt | This study |
| LC076271 (51 replicates) | F. hepatica | Sheep | Egypt | This study |
| AB554188 | F. hepatica | Sheep | Egypt | [8] |
| LC070666 | F. hepatica | Cattle | Peru | [11] |
| KF111630 | F. hepatica | Sheep | Spain | [49] |
| KF111634 | F. hepatica | Sheep | Spain | [49] |
| KF111652 | F. hepatica | Sheep | Spain | [49] |
| KF111640 | F. hepatica | Sheep | Spain | [49] |
| AB207156 | F. hepatica | Cattle | Ireland | [21] |
| AB554179 | F. hepatica | Sheep | Egypt | [8] |
| AB554180 | F. hepatica | Sheep | Egypt | [8] |
| AB554190 | F. hepatica | Buffalo | Egypt | [8] |
| AB477359 | F. hepatica | Cattle | China | [50] |
| AB554185 | F. hepatica | Sheep | Egypt | [8] |
| AB554181 | F. hepatica | Sheep | Egypt | [8] |
| AB554183 | F. hepatica | Sheep | Egypt | [8] |
| AB554186 | F. hepatica | Sheep | Egypt | [8] |
| AB554194 | Fasciola sp. | Buffalo | Egypt | [8] |
| LC076204 (3 replicates) | F. gigantica | Sheep | Egypt | This study |
| LC076199 (6 replicates) | F. gigantica | Sheep | Egypt | This study |
| LC076218 (16 replicates) | F. gigantica | Sheep | Egypt | This study |
| AB554162 | F. gigantica | Cattle | Egypt | [8] |
| AB554167 | F. gigantica | Cattle | Egypt | [8] |
| AB554165 | F. gigantica | Cattle | Egypt | [8] |
| AB554154 | F. gigantica | Buffalo | Egypt | [8] |
| AB554156 | F. gigantica | Buffalo | Egypt | [8] |
| LC012900 | F. gigantica | Cattle | India | [20] |
| LC128314 | F. gigantica | Buffalo | India | [53] |
| AB894337 | F. gigantica | Buffalo | Nepal | [52] |
| AB894370 | F. gigantica | Capra | Bangladesh | [57] |
| AB604007 | F. gigantica | Ruminant | Myanmar | [51] |
| LC012899 | F. gigantica | Cattle | India | [20] |
| LC012897 | F. gigantica | Cattle | India | [20] |
| LC127275 | F. gigantica | Ruminant | Indonesia | [54] |
| LC127277 | F. gigantica | Ruminant | Indonesia | [54] |
| LC127264 | F. gigantica | Ruminant | Indonesia | [54] |
| AB385616 | F. gigantica | Cattle | Vietnam | [17] |
| AB603724 | F. gigantica | Cattle | Thailand | [44] |
| AB983822 | F. gigantica | Cattle | Zambia | a |
| AB983824 | F. gigantica | Cattle | Zambia | a |
| AB983823 | F. gigantica | Cattle | Zambia | a |
| AF219379 | P. westermani | a |
aGenBank (unpublished data)
Representative sequences from this study were deposited in the GenBank database under accession numbers LC076108–LC076196 for ITS1 and LC076197–LC076285 for nad1.
Results
Occurrence of liver flukes
Postmortem examinations of slaughtered sheep indicated that 302 of 2058 (14.7%) animals had Fasciola flukes in the bile duct. Among the three abattoirs, Fasciola infection rates were 9.8% (188 of 783) at Shebein El Kom, 17.8% (217 of 1219) at Ashmoun, and 14.8% (8 of 56) at El Shouhada.
Fasciola species at nuclear ITS1 locus
Sequences of ~ 639 bp containing the complete ITS1 sequence and partial 18S and 5.8S rRNA gene sequences were generated from 103 Egyptian flukes. Alignment of the sequences obtained showed the presence of 6 polymorphic sites, indicative the presence of both F. hepatica and F. gigantica. There were no nucleotide deletions between the two Fasciola species and, upon close examinations of the trace files from the sequencing reactions, there were no mixed peaks at any of the 6 polymorphic sites. No intra-species sequence diversity was observed at this genetic locus.
Fasciola species and haplotypes at mitochondrial nad1 locus
Sequence analysis of the mitochondrial nad1 gene showed considerable genetic diversity in the form of single nucleotide polymorphism (SNPs), resulting in the identification of multiple haplotypes. A total of 42 variable sites (leading to 30 amino acid changes) representing 19 haplotypes were detected in F. hepatica, compared to 9 variable sites (leading to 6 amino acid changes) representing 7 haplotypes in F. gigantica.
In a ML analysis of the nad1 sequences, all F. gigantica haplotypes from this study clustered with those derived from several hosts from Egypt, forming a distinct haplogroup (designated as haplogroup D). This haplogroup clustered in a sister clade with other haplogroups (A, B and C) in Asia and the haplogroup in Zambia (designated as haplogroup E) with high bootstrap value (> 90) (Fig. 1). There were about 96–99% similarities in nad1 sequences of F. gigantica between Egypt and other countries. In contrast, there were no geographic or host segregation in F. hepatica, as nad1 sequences from this study were distributed in several clusters across the tree (Fig. 1). Similar tree structures were obtained in the Maximum Parsimony and Bayesian analyses of these sequences (data not shown).
Fig. 1.
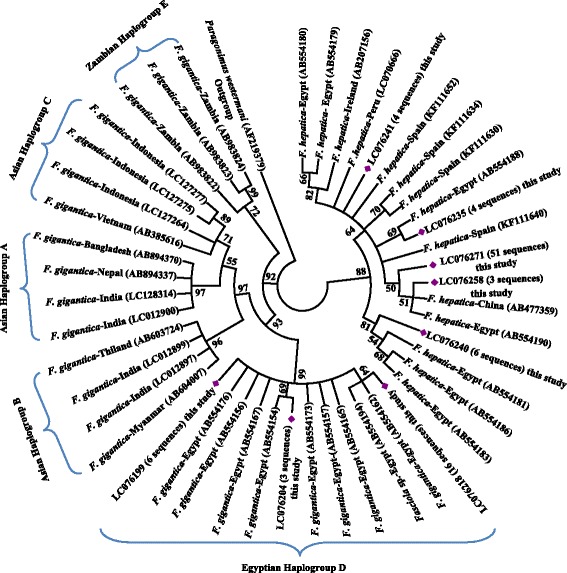
Phylogenetic relationships of Fasciola spp. from Egypt compared to reference nad1 sequences in the GenBank database based on the Maximum Likelihood analysis. Sequences obtained in this study are marked with a red diamond (see Table 1 for details on sequences used in the tree construction)
Distribution of Fasciola species
Results of ITS1 and nad1 sequence analyses showed that 11 of the 17 fascioliasis cases characterized genetically were infections of F. hepatica, 4 were of F. gigantica, and 2 had mixed infection of both Fasciola species. At the individual worm level, 78 of the 103 fluke sequenced were F. hepatica and 23 were F. gigantica. The remaining two worms (Worm IDs 38314 and 38318) derived from the same sheep showed ITS1 sequences identical to F. hepatica and mitochondrial nad1 sequences identical to F. gigantica, representing an introgressed form of the two species.
Morphometric characteristics
Morphometric measurements showed that F. gigantica was significantly longer and narrower than F. hepatica. Similarly, the ratios Maximum body width/Total length, Pharynx length/Total length and Oesophagus length/Total length differed significantly between the two fluke species (Table 2).
Table 2.
Morphometric measurements (n = 11 for each species) of Fasciola hepatica and Fasciola gigantica from sheep in Egypt. Data are presented as the range followed by the mean in parentheses
| Parameter | Fasciola hepatica | Fasciola gigantica | t-value | P-value (2-tailed) |
|---|---|---|---|---|
| Total length | 29.60–20.80 (25.34) | 34.80–26.80 (29.9) | -4.18 | 0.0001* |
| Maximum width | 10.80–7.20 (9.65) | 10.80–7.80 (8.75) | 2.44 | 0.0242* |
| Shoulder breadth | 7.60–4.80 (5.80) | 6.00–4.00 (5.42) | 1.14 | 0.2671 |
| Oral cone length | 3.60–2.00 (2.85) | 3.20–2.20 (2.73) | 0.67 | 0.5102 |
| Oral sucker (L × W) | 0.63–0.40 × 1.08–0.60 (0.53 × 0.76) | 0.64–0.40 × 0.92–0.60 (0.51 × 0.79) | -0.65 | 0.5253 |
| Ventral sucker (L × W) | 1.20–0.80 × 1.32–0.92 (1.09 × 1.18) | 1.32–0.88 × 1.32–1.00 (1.12 × 1.21) | -0.65 | 0.5211 |
| Pharynx length | 0.80–0.60 (0.65) | 0.80–0.60 (0.65) | 0.23 | 0.8173 |
| Oesophagus length | 1.20–1.00 (1.10) | 1.28–0.80 (1.09) | 0.15 | 0.8842 |
| Maximum width/Total length | 0.36–0.34 (0.38) | 0.31–0.29 (0.29) | 6.26 | 0.0001* |
| Pharynx length/Total length | 0.03–0.03 (0.03) | 0.02–0.02 (0.02) | 3.36 | 0.0032* |
| Pharynx length/Oral cone length | 0.22–0.3 (0.23) | 0.25–0.27 (0.24) | -0.47 | 0.6443 |
| Oesophagus length/Total length | 0.04–0.05 (0.04) | 0.04–0.03 (0.04) | 3.41 | 0.0031* |
| Oesophagus length/Oral cone length | 0.33–0.50 (0.39) | 0.40–0.36 (0.40) | -0.40 | 0.6962 |
| Pharynx length /Oesophagus length | 0.67–0.6 (0.59) | 0.63–0.75 (0.60) | -0.13 | 0.8981 |
Abbreviations: L length, W width
*P ≤ 0.05 (significant differences revealed by Student’s t-test)
Discussion
Sheep and goat farming is a key element in sustained economic development in developing countries [30]. Fascioliasis is a serious challenge to small ruminant farming worldwide because of the high occurrence of infection and the associated morbidity and mortality. A temporal increase in the incidence of Fasciola infection in sheep has been recorded over the last few decades [31, 32], and has been linked to global climate changes [33] and changes in irrigation systems, favoring the life-cycle of lymnaeid vectors. The present study showed an occurrence of Fasciola worms in 14.7% of sheep examined at three abattoirs in Egypt. This is lower than infection rates of 30–40% previously reported in sheep in Egypt by stool examinations [26, 34]. Prevalence rates among studies can be affected by diagnostic techniques used, age of the animals examined, time and location of the investigation [35]. Elsewhere in Africa, Fasciola spp. were found in 10.8% of slaughtered sheep in Algeria [36] and 23% of slaughtered sheep in Chad [37]. Similarly, F. hepatica and F. gigantica were reported in 20–26% of slaughtered sheep in Ethiopia [38].
Results reported in the present study indicate that morphometric measurements differed significantly between F. hepatica and F. gigantica in five indices including total body length, maximum width, as well as ratios Maximum body width/Total length, Pharynx length/Total length and Oesophagus length/Total length. Comparable results were previously reported on Fasciola flukes from different hosts [39–41]. Traditional microscopic measurements are simple and may be helpful in morphometric characterization of Fasciolids [39]. Therefore, this technique is a valuable tool in discriminating the two common Fasciola species in areas with low occurrence or no recorded intermediate forms, including Egypt and other African countries. However, in countries such as Japan, Vietnam and Korea, liver flukes cannot be classified as F. hepatica or F. gigantica using morphometrics because of the presence of a variety of intermediate forms [21, 22].
Molecular characterizations have identified a higher occurrence of F. hepatica (11/17) than F. gigantica (4/17), with mixed infections of both species in two slaughtered sheep. The higher prevalence of F. hepatica was reported by Moghaddam et al. in Iran [42], and mixed infections of the two species were previously reported in cattle in Egypt [8]. Elsewhere in Africa, F. hepatica is the dominant species (64%) in cattle in South Africa, although F. gigantica (99%) is more commonly seen in cattle in neighboring Zimbabwe [43]. In Asia, flukes recovered from cattle in Thailand were exclusively F. gigantica in one study [44], although Bui et al. [35] concluded that sheep in Vietnam are more susceptible to F. hepatica than to F. gigantica. The distribution of Fasciola species could be affected by environmental and host-related factors.
Results of the present study have shown a higher haplotype diversity of the mitochondrial nad1 gene in F. hepatica than in F. gigantica. This is largely in agreement with previous reports on genetic variability in mitochondrial genes nad1 and cytochrome oxidase 1 of F. hepatica by other researchers [45–50]. Cwiklinski et al. [12] concluded that the F. hepatica genome is highly heterogeneous. In contrast, in the previous report on bovine Fasciola spp. collected from Cairo, Egypt, F. gigantica was shown to have higher genetic diversity than F. hepatica [8]. Such a discrepancy between studies might be attributed to differences in the number of flukes of both species characterized.
There are apparent genetic differences in mitochondrial sequences of F. gigantica between Egypt and other countries. Phylogenetic analysis of nad1 sequences suggests that haplotypes of F. gigantica found in Asian countries can be categorized into three haplogroups: A, B and C [20, 44, 51, 52]. Haplogroup A is found in Indian subcontinent, including Nepal and Bangladesh [20, 52], while all three haplotypes are found in Southeast Asia, including Thailand and Myanmar [44, 51, 53, 54]. Our analysis suggested that Egyptian haplotypes of F. gigantica formed a monophyletic clade sister to those from Asia to the exclusion of Zambian samples (Fig. 1). In agreement with this, Ai et al. [55] described the separation of Chinese haplotypes from Niger ones.
In the present study, we identified two flukes with nuclear ITS1 data matching those of F. hepatica and mitochondrial nad1 data matching those of F. gigantica. This might have been caused by the occurrence introgression. Hybrid forms between F. hepatica and F. gigantica have been reported in several Asian countries [21, 22, 56, 57] and Egypt [8]. Le et al. [14] and Blair [58] defined the hybrid form as the F1 offspring of a mating between the two Fasciola species, carrying mitochondrial genome of the maternal parent and nuclear rRNA genes of both parents. In contrast, the introgressed form is the offspring of the back-crossing of hybrids with one parent species, which homogenizes the ribosomal array to one species and mitochondrial genome to other species (paternal introgression) or both ribosomal and mitochondrial arrays (maternal introgression) to the same species. The fact that the parasite can survive for many years in the definitive host and both Fasciola species have high infection rates in ruminants in some areas has apparently facilitated the occurrence of hybrid and introgressed forms. Hybrid and/or introgressed forms might play an important role in genetic diversity of Fasciola spp. [59], leading to potential emergence of more virulent forms. The existence of these two recombinant forms in Egypt needs confirmation using the newly developed genotyping tool targeting nuclear phosphoenolpyruvate carboxykinase and DNA polymerase delta genes [24].
Conclusions
The present study revealed a common occurrence of F. hepatica and F. gigantica in sheep in the middle delta region of Egypt, the existence of an introgressed form of the two species in some animals, and genetic differences in F. gigantica between Egypt and other areas. This and the previous identification of the hybrid form of F. hepatica and F. gigantica indicate that genetic recombination may play a significant role in shaping the population structure of Fasciola spp. in areas with high prevalence of both Fasciola spp. This and its epidemiologic implications warrant future studies.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Funding
This study was supported in part by National Natural Science Foundation of China (No. 31110103901), Kafr El Sheikh University, University of Sadat City, and Centers for Disease Control and Prevention. The funding bodies played no direct role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Nucleotide sequences, including those representing all sequence types, from this study were deposited in the GenBank database under accession numbers LC076108 - LC076196 for ITS1 and LC076197–LC076285 for nad1. The dataset analyzed in the current study is available from the corresponding author on reasonable request.
Authors’ contributions
SA, YF and LX conceived and designed the experiments; SA AELK and SZ performed the experiments, SA, YF and LX analyzed the data; SA, YF and LX wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Institutional Committees of the Post-graduate Studies and Research at Kafr El Sheikh University and University of Sadat City, Menofia, Egypt. Flukes were collected from slaughtered animals during post-mortem inspection by veterinary officers. Formal consent and permission for research use of the flukes were obtained from the attending abattoir veterinarians. No experiment was conducted on live animals.
Abbreviations
- ITS1
Internal transcribed spacer 1
- nad1
NADH dehydrogenase subunit 1
- PCR
Polymerase chain reaction
Contributor Information
Said Amer, Email: mssamer5@yahoo.com.
Ahmed ElKhatam, Email: elkhtama@yahoo.com.
Shereif Zidan, Email: shrifzidan@yahoo.com.
Yaoyu Feng, Email: yyfeng2015@sina.com.
Lihua Xiao, Email: lxiao@cdc.gov.
References
- 1.Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–78. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Vázquez AA, Lounnas M, Sánchez J, Alba A, Milesi A, Hurtrez-Boussès S. Genetic and infective diversity of the liver fluke Fasciola hepatica (Trematoda: Digenea) from Cuba. J Helminthol. 2016;14:1–7. doi: 10.1017/S0022149X15001029. [DOI] [PubMed] [Google Scholar]
- 3.Gajewska A, Smaga-Kozłowska K, Wiśniewski M. Pathological changes of liver in infection of Fasciola hepatica. Wiad Parazytol. 2005;51:115–23. [PubMed] [Google Scholar]
- 4.Mekroud A, Titi A, Benakhla A, Rondelaud D. The proportion of liver excised in Algerian abattoirs is not a good indicator of Fasciola hepatica infections in local cattle breeds. J Helminthol. 2006;80:319–21. [PubMed] [Google Scholar]
- 5.Espinoza JR, Terashima A, Herrera-Velit P, Marcos LA. Human and animal fascioliasis in Peru: impact in the economy of endemic zones. Rev Peru Med Exp Salud Publica. 2010;27:604–12. doi: 10.1590/S1726-46342010000400018. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafi K, Bargues MD, O’Neill S, Mas-Coma S. Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Travel Med Infect Dis. 2014;12:636–49. doi: 10.1016/j.tmaid.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Knubben-Schweizer G, Torgerson PR. Bovine fasciolosis: control strategies based on the location of Galba truncatula habitats on farms. Vet Parasitol. 2015;208:77–83. doi: 10.1016/j.vetpar.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Amer S, Dar Y, Ichikawa M, Fukuda Y, Tada C, Itagaki T, et al. Identification of Fasciola species isolated from Egypt based on sequence analysis of genomic (ITS1 and ITS2) and mitochondrial (NDI and COI) gene markers. Parasitol Int. 2011;60:5–12. doi: 10.1016/j.parint.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Ashrafi K, Valero MA, Peixoto RV, Artigas P, Panova M, Mas-Coma S. Distribution of Fasciola hepatica and F. gigantica in the endemic area of Guilan, Iran: relationships between zonal overlap and phenotypic traits. Infect Genet Evol. 2015;31:95–109. doi: 10.1016/j.meegid.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Beesley NJ, Cwiklinski K, Williams DJ, Hodgkinson J. Fasciola hepatica from naturally infected sheep and cattle in Great Britain are diploid. Parasitol. 2015;142:1196–201. doi: 10.1017/S0031182015000499. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa-Seki M, Ortiz P, Cabrera M, Hobán C, Itagaki T. Molecular characterization and phylogenetic analysis of Fasciola hepatica from Peru. Parasitol Int. 2016;65:171–4. doi: 10.1016/j.parint.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Cwiklinski K, O’Neill SM, Donnelly S, Dalton JP. A prospective view of animal and human fasciolosis. Parasite Immunol. 2016. doi: 10.1111/pim.12343. [DOI] [PMC free article] [PubMed]
- 13.Agatsuma T, Arakawa Y, Iwagami M, Honzako Y, Cahyaningsih U, Kang SY, et al. Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica. Parasitol Int. 2000;49:231–8. doi: 10.1016/S1383-5769(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 14.Le TH, De NV, Agatsuma T, Thi Nguyen TG, Nguyen QD, McManus DP, et al. Human fascioliasis and the presence of hybrid/introgressed forms of Fasciola hepatica and Fasciola gigantica in Vietnam. Int J Parasitol. 2008;38:725–30. doi: 10.1016/j.ijpara.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Itagaki T, Ichinomiya M, Fukuda K, Fusyuku S, Carmona C. Hybridization experiments indicate incomplete reproductive isolating mechanism between Fasciola hepatica and Fasciola gigantica. Parasitol. 2011;138:1278–84. doi: 10.1017/S0031182011000965. [DOI] [PubMed] [Google Scholar]
- 16.Terasaki K, Noda Y, Shibahara T, Itagaki T. Morphological comparisons and hypotheses on the origin of polyploids in parthenogenetic Fasciola sp. J Parasitol. 2000;86:724–9. doi: 10.1645/0022-3395(2000)086[0724:MCAHOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Itagaki T, Sakaguchi K, Terasaki K, Sasaki O, Yoshihara S, Van Dung T. Occurrence of spermic diploid and aspermic triploid forms of Fasciola in Vietnam and their molecular characterization based on nuclear and mitochondrial DNA. Parasitol Int. 2009;58:81–5. doi: 10.1016/j.parint.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Lotfy WM, Brant SV, DeJong RJ, Le TH, Demiaszkiewicz A, Rajapakse RP, et al. Evolutionary origins, diversification, and biogeography of liver flukes [Digenea, Fasciolidae] Am J Trop Med Hyg. 2008;79:248–55. [PMC free article] [PubMed] [Google Scholar]
- 19.Walker SM, Prodöhl PA, Hoey EM, Fairweather I, Hanna RE, Brennan G, et al. Substantial genetic divergence between morphologically indistinguishable populations of Fasciola suggests the possibility of cryptic speciation. Int J Parasitol. 2012;42:1193–9. doi: 10.1016/j.ijpara.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Ichikawa-Seki M, Mohanta UK, Singh TS, Shoriki T, Sugiyama H, et al. Molecular phylogenetic analysis of Fasciola flukes from eastern India. Parasitol Int. 2015;64:334–8. doi: 10.1016/j.parint.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Itagaki T, Kikawa M, Sakaguchi K, Shimo J, Terasaki K, Shibahara T, et al. Genetic characterization of parthenogenetic Fasciola sp. in Japan on the basis of the sequences of ribosomal and mitochondrial DNA. Parasitol. 2005;131:679–85. doi: 10.1017/S0031182005008292. [DOI] [PubMed] [Google Scholar]
- 22.Itagaki T, Kikawa M, Terasaki K, Shibahara T, Fukuda K. Molecular characterization of parthenogenic Fasciola sp. in Korea on the basis of NA sequences of ribosomal ITS1 and mitochondrial NDI gene. J Vet Med Sci. 2005;67:1115–8. doi: 10.1292/jvms.67.1115. [DOI] [PubMed] [Google Scholar]
- 23.Terasaki K, Moriyama-Gonda N, Noda Y. Abnormal spermatogenesis in the common liver fluke (Fasciola sp.) from Japan and Korea. J Vet Med Sci. 1998;60:1305–9. doi: 10.1292/jvms.60.1305. [DOI] [PubMed] [Google Scholar]
- 24.Shoriki T, Ichikawa-Seki M, Suganuma K, Naito I, Hayashi K, Nakao M, et al. Novel methods for the molecular discrimination of Fasciola spp. on the basis of nuclear protein-coding genes. Parasitol Int. 2016;65:180–3. doi: 10.1016/j.parint.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich CF, Kabaalioglu A, Brunetti E, Richter J. Fasciolosis. Z Gastroenterol. 2015;53:285–90. doi: 10.1055/s-0034-1385728. [DOI] [PubMed] [Google Scholar]
- 26.Haridy FM, El-Sherbiny GT, Morsy TA. Some parasitic flukes infecting farm animals in Al-Santa Center, Gharbia Governorate, Egypt. J Egypt Soc Parasitol. 2006;36:259–64. [PubMed] [Google Scholar]
- 27.Mekky MA, Tolba M, Abdel-Malek MO, Abbas WA, Zidan M. Human fascioliasis: a re-emerging disease in Upper Egypt. Am J Trop Med Hyg. 2015;93:76–9. doi: 10.4269/ajtmh.15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astrin JJ, Zhou X, Misof B. The importance of biobanking in molecular taxonomy, with proposed definitions for vouchers in a molecular context. Zookeys. 2013;365:67–70. doi: 10.3897/zookeys.365.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguti S. Systema helminthum: the digenetic trematodes of vertebrates. New York: Interscience publisher; 1958. [Google Scholar]
- 30.World Bank. Sheep and goats in developing countries: their present and potential role. ISSN. 1983;02537494. http://www-wds.worldbank.org/servlet/WDSContentServer/WDSP/IB/1999/12/02/000178830_98101904140949/Rendered/PDF/multi_page.pdf).
- 31.Martínez-Valladares M, Robles-Pérez D, Martínez-Pérez JM, Cordero-Pérez C, Famularo Mdel R, Fernández-Pato N, et al. Prevalence of gastrointestinal nematodes and Fasciola hepatica in sheep in the northwest of Spain: relation to climatic conditions and/or man-made environmental modifications. Parasit Vectors. 2013;6:282. doi: 10.1186/1756-3305-6-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen A, Frankena K, Bødker R, Toft N, Thamsborg SM, Enemark HL, et al. Prevalence, risk factors and spatial analysis of liver fluke infections in Danish cattle herds. Parasit Vectors. 2015;8:160. doi: 10.1186/s13071-015-0773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosco A, Rinaldi L, Musella V, Amadesi A, Cringoli G. Outbreak of acute fasciolosis in sheep farms in a Mediterranean area arising as a possible consequence of climate change. Geospat Health. 2015;9:319–24. doi: 10.4081/gh.2015.354. [DOI] [PubMed] [Google Scholar]
- 34.Morsy TA, Salem HS, Haridy FM, Rifaat MM, Abo-Zenadah NY, Adel el-Kadi M. Farm animals’ fascioliasis in Ezbet El-Bakly (Tamyia Center) Al-Fayoum Governorate. J Egypt Soc Parasitol. 2005;35:825–32. [PubMed] [Google Scholar]
- 35.Bui TD, Doanh PN, Saegerman C, Losson B. Current status of fasciolosis in Vietnam: an update and perspectives. J Helminthol. 2016;90:511–22. doi: 10.1017/S0022149X15000929. [DOI] [PubMed] [Google Scholar]
- 36.Mekroud A, Benakhla A, Vignoles P, Rondelaud D, Dreyfuss G. Preliminary studies on the prevalences of natural fasciolosis in cattle, sheep, and the host snail (Galba truncatula) in north-eastern Algeria. Parasitol Res. 2004;92:502–5. doi: 10.1007/s00436-004-1072-1. [DOI] [PubMed] [Google Scholar]
- 37.Jean-Richard V, Crump L, Abicho AA, Naré NB, Greter H, Hattendorf J, et al. Prevalence of Fasciola gigantica infection in slaughtered animals in south-eastern Lake Chad area in relation to husbandry practices and seasonal water levels. BMC Vet Res. 2014;10:81. doi: 10.1186/1746-6148-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sissay MM, Uggla A, Waller PJ. Prevalence and seasonal incidence of nematode parasites and fluke infections of sheep and goats in eastern Ethiopia. Trop Anim Health Prod. 2007;39:521–31. doi: 10.1007/s11250-007-9035-z. [DOI] [PubMed] [Google Scholar]
- 39.Ashrafi K, Valero MA, Panova M, Periago MV, Massoud J, Mas-Coma S. Phenotypic analysis of adults of Fasciola hepatica, Fasciola gigantica and intermediate forms from the endemic region of Gilan. Iran Parasitol Int. 2006;55:249–60. doi: 10.1016/j.parint.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Periago MV, Valero MA, El Sayed M, Ashrafi K, El Wakeel A, Mohamed MY, et al. First phenotypic description of Fasciola hepatica/Fasciola gigantica intermediate forms from the human endemic area of the Nile Delta. Egypt Infect Genet Evol. 2008;8:51–8. doi: 10.1016/j.meegid.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Yakhchali M, Malekzadeh-Viayeh R, Imani-Baran A, Mardani K. Morphological and molecular discrimination of Fasciola species isolated from domestic ruminants of Urmia city. Iran Iran J Parasitol. 2015;10:46–55. [PMC free article] [PubMed] [Google Scholar]
- 42.Moghaddam AS, Massoud J, Mahmoodi M, Mahvi AH, Periago MV, Artigas P, et al. Human and animal fascioliasis in Mazandaran province, northern Iran. Parasitol Res. 2004;94:61–9. doi: 10.1007/s00436-004-1169-6. [DOI] [PubMed] [Google Scholar]
- 43.Mucheka VT, Lamb JM, Pfukenyi DM, Mukaratirwa S. DNA sequence analyses reveal co-occurrence of novel haplotypes of Fasciola gigantica with F. hepatica in South Africa and Zimbabwe. Vet Parasitol. 2015;214:144–51. doi: 10.1016/j.vetpar.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Chaichanasak P, Ichikawa M, Sobhon P, Itagaki T. Identification of Fasciola flukes in Thailand based on their spermatogenesis and nuclear ribosomal DNA, and their intraspecific relationships based on mitochondrial DNA. Parasitol Int. 2012;61:545–9. doi: 10.1016/j.parint.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Morozova EV, Chrisanfova GG, Arkhipov IA, Semyenova SK. Polymorphism of the ND1 and CO1 mitochondrial genes in populations of liver fluke Fasciola hepatica. Russ J Genet. 2004;40:817–20. doi: 10.1023/B:RUGE.0000036534.01952.19. [DOI] [PubMed] [Google Scholar]
- 46.Semyenova SK, Morozova EV, Chrisanfova GG, Gorokhov VV, Arkhipov IA, Moskvin AS, et al. Genetic differentiation in eastern European and western Asian populations of the liver fluke, Fasciola hepatica, as revealed by mitochondrial nad1 and cox1 genes. J Parasitol. 2006;92:525–30. doi: 10.1645/GE-673R.1. [DOI] [PubMed] [Google Scholar]
- 47.Walker SM, Johnston C, Hoey EM, Fairweather I, Borgsteede F, Gaasenbeek C, et al. Population dynamics of the liver fluke, Fasciola hepatica: the effect of time and spatial separation on the genetic diversity of fluke populations in the Netherlands. Parasitol. 2011;138:215–23. doi: 10.1017/S0031182010001149. [DOI] [PubMed] [Google Scholar]
- 48.Elliott T, Muller A, Brockwell Y, Murphy N, Grillo V, Toet HM, et al. Evidence for high genetic diversity of NAD1 and COX1 mitochondrial haplotypes among triclabendazole resistant and susceptible populations and field isolates of Fasciola hepatica (liver fluke) in Australia. Vet Parasitol. 2014;200:90–6. doi: 10.1016/j.vetpar.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Martínez-Valladares M, Rojo-Vázquez FA. Intraspecific mitochondrial DNA variation of Fasciola hepatica eggs from sheep with different level of anthelmintic resistance. Parasitol Res. 2014;113:2733–41. doi: 10.1007/s00436-014-3934-5. [DOI] [PubMed] [Google Scholar]
- 50.Peng M, Ichinomiya M, Ohtori M, Ichikawa M, Shibahara T, Itagaki T. Molecular characterization of Fasciola hepatica, Fasciola gigantica, and aspermic Fasciola sp. in China based on nuclear and mitochondrial DNA. Parasitol Res. 2009;105:809–15. doi: 10.1007/s00436-009-1459-0. [DOI] [PubMed] [Google Scholar]
- 51.Ichikawa M, Bawn S, Maw NN, Htun LL, Thein M, Gyi A, et al. Characterization of Fasciola spp. in Myanmar on the basis of spermatogenesis status and nuclear and mitochondrial DNA markers. Parasitol Int. 2011;60:474–9. doi: 10.1016/j.parint.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Shoriki T, Ichikawa-Seki M, Devkota B, Rana HB, Devkota SP, Humagain SK, Itagaki T. Molecular phylogenetic identification of Fasciola flukes in Nepal. Parasitol Int. 2014;63:758–62. doi: 10.1016/j.parint.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi K, Mohanta UK, Neeraja T, Itagaki T. Molecular characterization of Fasciola gigantica in Delhi, India and its phylogenetic relation to the species from South Asian countries. J Vet Med Sci. 2016;78(9):1529–1532. [DOI] [PMC free article] [PubMed]
- 54.Hayashi K, Ichikawa-Seki M, Allamanda P, Wibowo PE, Mohanta UK, Sodirun, et al. Molecular characterization and phylogenetic analysis of Fasciola gigantica from western Java, Indonesia. Parasitol Int. 2016;65:424–7. doi: 10.1016/j.parint.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Ai L, Weng YB, Elsheikha HM, Zhao GH, Alasaad S, Chen JX, et al. Genetic diversity and relatedness of Fasciola spp. isolates from different hosts and geographic regions revealed by analysis of mitochondrial DNA sequences. Vet Parasitol. 2011;181:329–34. doi: 10.1016/j.vetpar.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 56.Choe SE, Nguyen TT, Kang TG, Kweon CH, Kang SW. Genetic analysis of Fasciola isolates from cattle in Korea based on second internal transcribed spacer (ITS-2) sequence of nuclear ribosomal DNA. Parasitol Res. 2011;109:833–9. doi: 10.1007/s00436-011-2323-6. [DOI] [PubMed] [Google Scholar]
- 57.Mohanta UK, Ichikawa-Seki M, Shoriki T, Katakura K, Itagaki T. Characteristics and molecular phylogeny of Fasciola flukes from Bangladesh, determined based on spermatogenesis and nuclear and mitochondrial DNA analyses. Parasitol Res. 2014;113:2493–501. doi: 10.1007/s00436-014-3898-5. [DOI] [PubMed] [Google Scholar]
- 58.Blair D. Ribosomal DNA, variation in parasitic flatworms. In: Maule AG, Marks NJ, editors. Parasitic flatworms: Molecular biology, biochemistry, immunology and control. Wallingford: CABI; 2005. p. 96–123.
- 59.King KC, Stelkens RB, Webster JP, Smith DF, Brockhurst MA. Hybridization in parasites: consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015;11:e1005098. doi: 10.1371/journal.ppat.1005098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nucleotide sequences, including those representing all sequence types, from this study were deposited in the GenBank database under accession numbers LC076108 - LC076196 for ITS1 and LC076197–LC076285 for nad1. The dataset analyzed in the current study is available from the corresponding author on reasonable request.


