Abstract
Gene electrotransfer is an effective approach for delivering plasmid DNA to a variety of tissues. Delivery of molecules with electric pulses requires control of the electrical parameters to achieve effective delivery. Since discomfort or tissue damage may occur with high applied voltage, the reduction of the applied voltage while achieving the desired expression may be an important improvement. One possible approach is to combine electrotransfer with exogenously applied heat. Previous work performed in vitro demonstrated that increasing temperature before pulsing can enhance gene expres sion and made it possible to reduce electric fields while maintaining expression levels. In the study reported here, this combination was evaluated in vivo using a novel electrode device designed with an inserted laser for application of heat. The results obtained in this study demonstrated that increased temperature during electrotransfer increased expression or maintained expression with a reduction in applied voltage. With further optimization this approach may provide the basis for both a novel method and a novel instrument that may greatly enhance translation of gene electrotransfer.
Keywords: Gene electrotransfer, Skin, Gene therapy, Heat, Electroporation
1. INTRODUCTION
A critical aspect for gene therapy is appropriate delivery. Non-viral delivery has a favorable safety profile; however, expression levels are typically low without the addition of physical, chemical or viral delivery methods. Physical approaches, including ultrasound, hydrodynamic pressure, and electrotransfer, enhance delivery to a variety of tissues and are increasingly evaluated for therapeutic applications [1-7]. Gene electrotransfer (GET; electroporation) uses electric pulses to transiently permeabilize cell membranes allowing entry of extracellular nucleic acids into the cell. The current challenge is to develop protocols that result in appropriate transfection efficiency without adverse effects [8, 9].
Skin is an excellent target for gene therapy [10-15] due to its easy accessibility for delivery and monitoring. GET of skin is a simple and direct method for gene therapy and can be accomplished in a minimally invasive way. Several approaches with potential therapeutic or prophylactic applications have been evaluated including wound healing [16-18], ischemia [19-21], infectious disease vaccines [22-25] and cancer vaccines and therapies [5, 8, 26]. To date, sixty-eight clinical trials are being enrolled, are ongoing, or are completed using GET (clinicaltrials.gov “electroporation”, “electrotransfer”). Most of these trials are designed to evaluate DNA vaccines against infectious agents or cancer or the effectiveness of cancer immunotherapy [27].
For optimal delivery GET electrodes must be designed based on the target tissue and/or the desired endpoint analysis, for example secreted protein, antibody production, or cell death. Therefore, important focus areas for GET research have been electrode development and the identification of “ideal” pulsing parameters. A variety of electrodes for in vivo use have been developed including needle, caliper, plate, and non-penetrating pin electrodes [23, 28, 29]. While each electrode type has its advantages and disadvantages, the surface electrodes are attractive because they allow for delivery of DNA non-invasively. A surface electrode design is well-suited for delivery to the skin. However, the skin is also well-adapted to serving as a barrier from intrusion. Our lab has developed two surface electrodes for gene delivery to the skin that provide high levels of transgene expression. The first was the 4 plate electrode (4PE) designed with 4 plates at 90° angles with a stopper to maintain a constant distance between electrodes [28].
This electrode worked well as it provided a fixed distance and allowed for directional control of the applied electric field in two perpendicular directions without the need for moving the electrodes. While an advantage of this design is the fixed distance, the treatment of larger areas also required an increase in the electrode gap. Since the applied voltage is based on the distance between the electrodes, higher voltages are required to achieve effective delivery. These voltages potentially cause cellular damage or discomfort [28, 30]. To minimize cellular damage or discomfort, a multi-electrode array (MEA) was designed. This applicator consisted of 16 flat pin electrodes 2mm apart in a 4X4 array [23, 29, 31]. The electrode covered the same tissue area as the 4PE but smaller electrode distances and an increased number of electrodes allowed for enhanced directional control and a reduction in applied voltage.
Despite many successes of GET and the development of an increasing number of GET-based therapies, further improvement is needed, for example by reducing the necessary applied voltage and by increasing the ability to control expression. While GET is generally a non-thermal process, the administration of low heat from an exogenous source could potentially enhance delivery. Changes in both skin and core body temperature have been demonstrated to affect vasodilation and constriction. Additionally, local warming of tissue increases vasodilation, reaching a maximum blood flow in the skin at 42°C. This effect is maintained for up to one hour after heating [32-34]. The use of heat to enhance delivery is based on the hypothesis that permeability of cell membranes can be affected by temperature [35]. For example, hyper-thermia has been used to enhance delivery of chemotherapeutics [36-39]. Increased temperature can influence the efficiency of electrotransfer while decreasing the temperature to 4°C reduces delivery [35].
The goal of the current study was to determine if thermal assisted gene electrotransfer (TAGET) could enhance trans-gene expression and/or allow for a reduction of applied voltages in vivo. We hypothesized that moderate heating prior to GET in vivo would allow the use of lower applied voltages while maintaining gene expression without adverse effects on the tissue.
2. MATERIALS AND METHODS
2.1. Animals
Animals used for this study were 6-8 weeks old Female Hartley guinea pigs weighing approximately 200-250g. The animals were housed at the Old Dominion University animal facility and procedures approved by the Old Dominion Institutional Animal Care and Use Committee.
2.2. Plasmids
Endotoxin free preparations at 2mg/ml in physiological saline of gWizLuc,encoding firefly luciferase and gWizGFP green fluorescence protein (GFP) were purchased (Aldevron, Fargo, ND) in this study. Plasmids were prepared endotoxin free by Aldevron (proprietary fermentation technology and DNA purification) and suspended at 2mg/ml in physiological saline.
2.3. Heat Application
Animals were anesthetized with O2 containing 2.5-3.0% isoflurane. Both left and right flanks were shaved using Wahl Shears and extraneous hair removed by cleansing with water. To determine the intradermal skin temperature from exogenous heating, an 18G needle was inserted intradermally into the left or right flank then replaced with a thermo-couple temperature probe (Omega, Stamford, CT, USA). Baseline tissue temperature readings were recorded. Temperature measurements during heat application and GET were collected by positioning the electrode array on the skin above the temperature probe. Exogenous laser heat was applied at 1, 2 or 3 amps and temperature measured in real time.
2.4. Electrode Design
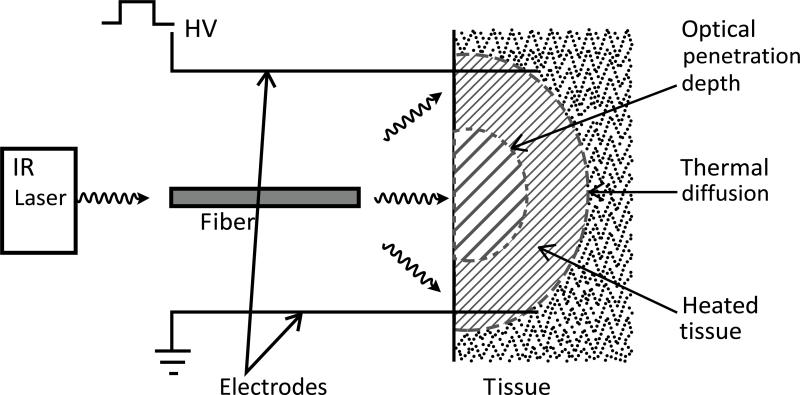
Electric pulses were administered utilizing a specially constructed applicator. The electric pulse delivery system had an integrated infrared radiation emitter Fig. (1) to heat the tissue radiatively. The emitter consisted of an optical fiber centered between 4 pins with rounded ends spaced 5 mm apart that acted as electrodes. The optical fiber has advantages over other nonionizing radiation emitters: its small diameter (< 1 mm) and dielectric material can be easily integrated into an existing electrical pulse delivery system. The fiber was connected to an infrared semiconductor laser which provided up to 8W of radiative power at a wavelength of approximately 1 m. Adjusting the distance of the fiber to the tissue irradiated a well-defined circular area of the tissue.
Fig. (1).
TAGET Device. Schematic of an optical fiber that was connected to an infrared laser and inserted into the device. The fiber is situated centrally between 4 electrodes. The fiber is also placed 1 cm above a 3 mm opening. This gap and opening allows for an increased spot size of the light as it exits into the space between the opening and the tissue which is set at 5 mm.
2.5. Gene Delivery
Injection sites were marked to ensure accuracy of data collection. Gene delivery and TAGET were assessed after intradermal injections of 50 μl of plasmid to multiple sites on both left and right flanks. The electrode array was positioned over the injection area with or without exogenous heating. The heat was applied as determined in the first round of experiments at 1 amp for 30 seconds. The tissue remained at 43°C for approximately 10 seconds, allowing adequate time for pulsing at a range of voltages from 50-125V for luciferase experiments and 50-100V for GFP experiments. Based on our previously published results with a similar electrode, pulse number, time between pulses and pulse length were maintained at 8 pulses, 150ms, and 150ms, respectively [23, 29, 31].
During the course of these experiments we evaluated laser amperage, duration of thermal assistance and applied voltage. Gene expression levels were measured for all conditions by in vivo bioluminescent imaging or fluorescent imaging. Visual assessment of the skin was used to determine the extent of external tissue damage from the addition of moderate heat.
2.6. In Vivo Bioluminescent Imaging
On days 1, 2, 5, 7, 10, and 14, animals were anesthetized with O2 containing 2.5-3.0% isoflurane followed by injections of 50 μl of 15 mg/ml D-luciferin (Gold Biotechnology, St. Louis, MO) at the plasmid injection sites. The animals were confined in an anesthesia chamber for two minutes then transferred to the IVIS® Spectrum (Perkin Elmer, Akron, OH), imaging chamber under constant anesthesia (O2 containing 2.5% isoflurane). Regions of interest (ROI) were selected on the image to encompass the entirety of each injection site independently and compared to untreated control ROIs. After background correction bioluminescence results were represented as average total flux, photons/sec (p/s).
2.7. GFP Expression and Analysis
Forty-eight hours after plasmid delivery, the animals were euthanized and the treated areas were excised, fixed in 4% paraformaldehyde for 1-2 hours, equilibrated in optimum cutting temperature (OCT) compound, snap frozen using dry ice and stored at −80°C. Samples were sectioned on a cryostat and mounted in Vectashield® mounting media with 4', 6-diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA) was used for nuclear staining. Slides were imaged on an Olympus BX51 fluorescence microscope. The analysis of the tissues was performed in a blinded fashion. Two members of the research team performed the evaluation independently without knowledge of the specific treatment protocol for the sample being evaluated.
2.8. Statistical Analysis
Temperature and luciferase measurements are represented as the mean ± SD. Comparisons between specific voltage with or without the addition of thermal component were performed by Student T-Test. Significance was assumed at p<0.05.
3. RESULTS AND DISCUSSION
3.1. Electrode Design, Development and Temperature Determination
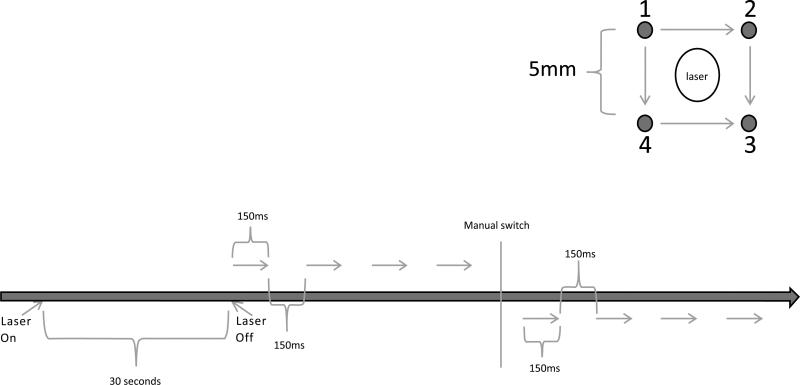
An in vivo surface array was designed that incorporated features from both the 4PE and MEA [23, 28-31]. This new array consisted of four electrodes with 5mm length and 5mm gap. Centered within the array was an infrared laser which sat flush to the electrode handle and applied a focused beam of light to the skin (Fig. 1). The electric pulses were administered in 2 sets of four pulses orthogonally. As shown in the schematic (Fig. 2), the pulses were administered simultaneously from the electrodes for four pulses (i.e., from 1 and 4 to 2 and 3) then using a manual switch the direction was rotated 90° and pulses administered for four additional pulses (1 and 2 to 4 and 3). Fig. (2) provides the full final protocol for administration of TAGET (not to scale). The total time required for 1 treatment was approximately 45 seconds.
Fig. (2).
Experimental Setup and Delivery Protocol. Upper panel: The pulse sequence is outlined. All 4 electrodes are active for each pulse, but are applied in two perpendicular directions in sequence. Four pulses in one direction followed by four in a perpendicular direction. Lower panel: schematic of the delivery protocol to the skin.
In a previous in vitro study we evaluated the use of low and moderate heat conditions prior to application of GET to increase gene expression and determine if reduction in absolute voltage could achieve a reduction in cell mortality [40]. This study demonstrated that the application of moderate levels of heat could achieve increased gene expression, although utilizing maximal electric fields came at the expense of cell viability. Interestingly, we found that when moderate exogenous heat was applied prior to pulsing, similar gene expression levels could be achieved using about 30% lower electric fields. These increased gene expression levels were obtained with a significantly higher level of cell viability. An important observation from this work was that temperatures higher than 43°C resulted in increased cell death [40] while lower temperatures did not produce consistently higher gene expression. Therefore, 43°C was used for all in vivo experiments.
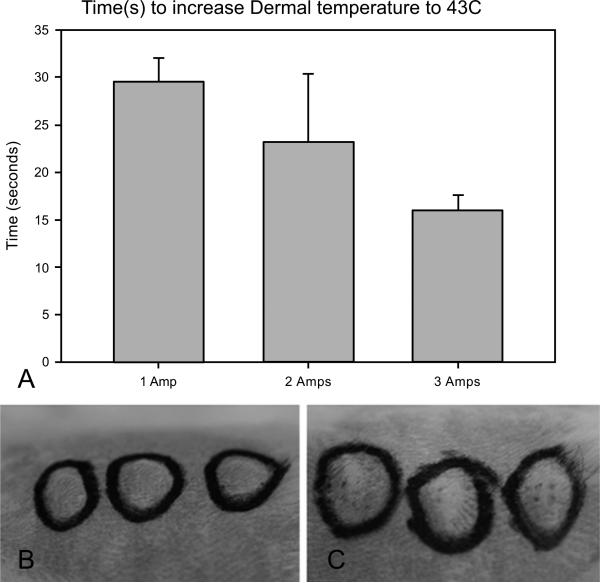
The first set of experiments was designed to determine the time it took to heat the tissue to 43°C. Using a temperature probe inserted laterally into the dermis of the guinea pig, the time to reach 43°C was measured from application of direct currents between 1 and 3 amps. Averages of 29 (+/−4), 23 (+/−7), and 17 (+/−2) seconds were measured for 1, 2, and 3 amps respectively (Fig. 3A). However, at both 2 and 3 amps the temperatures continued to rise quickly beyond 43°C. A consistent rise in heating was obtained by using 1 amp for 30 seconds. In addition, the temperature probe was left inserted in the skin after heating and application of TAGET to determine the increase in temperature at the end of delivery. Consistent with the previously published in vitro results and reports on the lack of heat generation from reversible electroporation, changes in temperature were less than 1°C. However, minimal visual damage that typically healed within 2-4 days was noted at the location of electrode contact (Fig. 3B, C).
Fig. (3).
Thermal Application Timing and Effect on Skin. (A) Comparison of various laser amperages and the time necessary to increase intradermal skin temperature to 43°C. Error is represented as standard deviation (SD). (B) Evaluation of tissue damage by visual analysis after application of heat and prior to pulsing. (C) Evaluation of tissue damage by visual analysis after application of heat and after pulsing.
3.2. Evaluation of In Vivo Luciferase Gene Expression After TAGET
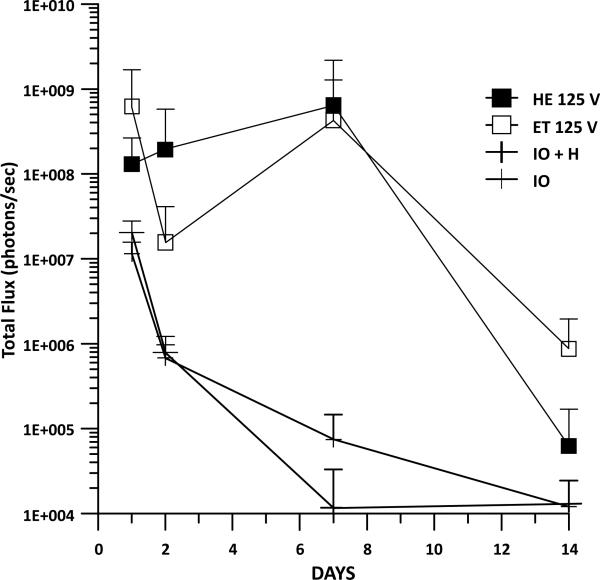
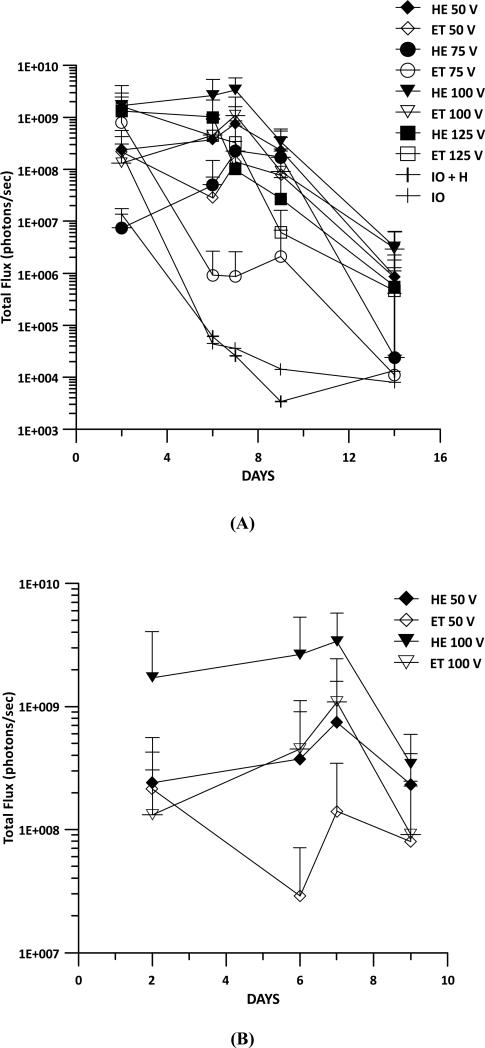
Luciferase expression was measured to determine whether the addition of heat prior to GET would enhance expression in vivo over time. Injection of DNA only, thermally assisted injection of DNA, and GET were included as controls in these experiments. Guinea pigs received an intradermal injection of 50 μl gWizLuc on Day 0 immediately followed by TAGET consisting of 30 seconds of heating at 1 amp at an applied voltage of 125V, 8 pulses for 150ms. Luciferin was injected into the same location as treatment on Days 1, 2, 7, and 14 and photon emissions measured on the IVIS Spectrum. No differences between the application of DNA + heat as compared to DNA alone were observed and as expected both had lower expression than DNA + GET (Fig. 4). Interestingly the greatest differences between TAGET and GET were noted at Day 2 (a 12 fold increase). On Day 7, the greatest difference (6-8000 fold) observed was between TAGET and DNA injection. At Day 14 TAGET groups decreased below the levels of GET.
Fig. (4).
Gene Expression Resulting from TAGET. Luciferase gene expression was evaluated during a 2 week time course following delivery with or without heat pretreatment and GET. 8 pulses of 150ms were administered with an applied voltage of 125 with heat (HE) or without heat (ET). This was compared to injection of plasmid DNA with heat (IO + H) or without heat (IO). Error is represented as SD.
While the addition of heat alone did not result in enhanced gene expression as compared to injection of DNA alone, the combination of heat pretreatment and GET did result in higher gene expression at specific time points. Therefore, we sought to determine the most appropriate GET voltage condition for enhancing gene expression with thermal assistance. The previous heating conditions were maintained, but the applied voltage varied from 50 to 125V (Fig. 5A). The highest gene expression was achieved at an applied voltage of 100V with or without the addition of heat. However, the addition of heat at 50V resulted in significantly (p<0.05) increased gene expression as compared to GET alone at Days 6 and 7 (Fig. 5B). Significant (p<0.05) gene expression increases were also noted at the same time points for 100V. TAGET at lower voltages resulted in similar gene expression levels as higher voltage GET conditions. For example expression levels achieved with TAGET at 50V were similar to levels following 100V GET (Fig. 5B).
Fig. (5).
Comparison of Various Applied Voltages with or without Heat Pretreatment. Luciferase gene expression was evaluated with GET administered at various applied voltages. Baseline values of IO and HIO were also determined. (A) Applied voltages were administered from 50-125 with (HE) or without (ET) heat pretreatment. (B) Enlarged graph highlighting differences at 50 and 100 volts with and without heat pretreatment. All errors are represented as SD.
3.3. Location of Gene Expression by TAGET
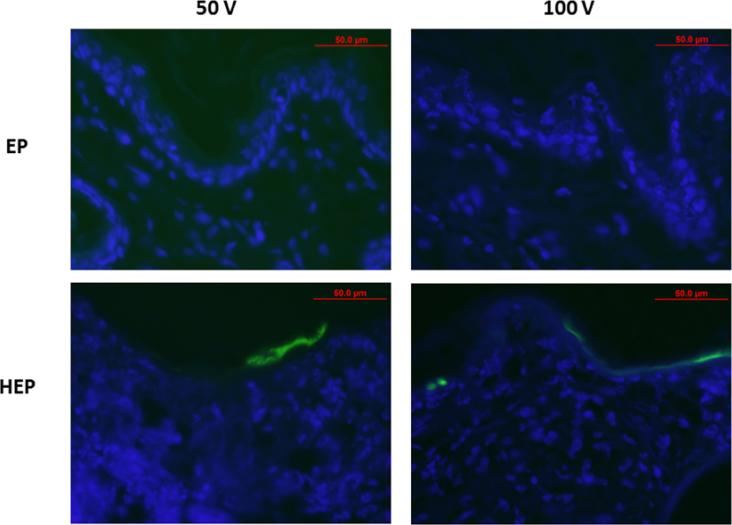
A disadvantage of surface electrodes is their inability to penetrate deep into the tissue without using high potentially damaging electric fields. The addition of heat prior to GET might facilitate delivery and might produce gene expression deeper within the tissue. To analyze this, gWizGFP plasmid was injected and the site treated with and without heat and with and without GET at 50 and 100V. After 48 hours, increased GFP protein expression was observed in TAGET groups (Fig. 6); however, deeper penetration into the dermis was not observed. As with GET, the use of TAGET appears to localize expression within the epidermis.
Fig. (6).
Evaluation of Distribution Following TAGET Delivery of gWizGFP. Plasmid encoding GFP was delivered by GET with or without heat pretreatment at applied voltage of 50 or 100. Tissue was harvested at 48 hours, sectioned, counter stained with Dapi, and fluorescent image analyzed.
CONCLUSION
In this study, we demonstrated a novel method of in vivo GET employing exogenous heat. The intradermal skin temperature was increased to 43°C using an infrared laser and electrical fields applied via electrodes. We previously demonstrated that the addition of exogenous heat to GET in vitro resulted in maximal gene expression with a reduction of applied voltage by 30% and an increase in cell viability [40]. This current study demonstrated that in vivo TAGET maintained gene expression levels while utilizing 50% lower applied voltage with only short-term adverse effects to the tissue. In addition to gene expression, our current study also demonstrated that TAGET did not result in temperature increases above those induced by the exogenous heating source.
We did not demonstrate an increased depth of penetration for gene delivery. However, there were several parameters which were not evaluated. Throughout the current experimental design the heat level and duration at 43°C and pulse number, length, and interval were maintained. In the future, these parameters could be adjusted to assess the effect of their changes. GET parameters can be designed to increase penetration by changing electrode distance or electric field [28, 29]. The current electrode design could be adjusted to evaluate the combination of these factors by reducing the electrode distance or increasing the field.
While this method still requires optimization, the initial results suggest that TAGET could prove to be a valuable tool for obtaining better control over delivery and subsequent protein expression. In addition, to the overall reduction of applied voltage, targeted application of heat could aid in localization of plasmid expression. Future studies will evaluate alternate electrode designs, heat application and electrical parameters with the goal of optimizing the applications of in vivo TAGET.
ACKNOWLEDGEMENTS
Declared none.
Biography

R. Heller
Footnotes
CONFLICT OF INTEREST
Drs. R. Heller and K. Schoenbach are inventors on patents and patent applications which cover the technology that was reported in this manuscript. This research was supported in part by a research grant from the National Institutes of Health R01 EB018956 The funders had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript.
REFERENCES
- 1.Escoffre JM, Zeghimi A, Novell A, Bouakaz A. In vivo gene delivery by sonoporation: recent progress and prospects. Curr Gene Ther. 2013;13:2–14. doi: 10.2174/156652313804806606. [DOI] [PubMed] [Google Scholar]
- 2.Song S, Shen Z, Chen L, Brayman AA, Miao CH. Explorations of high-intensity therapeutic ultrasound and microbubble-mediated gene delivery in mouse liver. Gene Ther. 2011;18:1006–14. doi: 10.1038/gt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonamassa B, Hai L, Liu D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharm Res. 2011;28:694–701. doi: 10.1007/s11095-010-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potter H, Heller R. Transfection by electroporation. In: Ausubel FM, Brent R, Kingston RE, et al., editors. Current protocols in molecular biology. John Wiley & Sons; Hoboken, New Jersey: 2010. Chapter 9: Unit 9.3. [Google Scholar]
- 5.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther. 2010;10:312–7. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 6.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–9. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 7.Heller LC, Heller R. In vivo electroporation for gene therapy. Hum Gene Ther. 2006;17:890–7. doi: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- 8.Shirley SA, Lundberg CG, Li F, Burcus N, Heller R. Controlled gene delivery can enhance therapeutic outcome for cancer immune therapy for melanoma. Curr Gene Ther. 2015;15:32–43. doi: 10.2174/1566523214666141121111630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golzio M, Escoffre JM, Portet T, et al. Observations of the mechanisms of electromediated DNA uptake-from vesicles to tissues. Curr Gene Ther. 2010;10:256–66. doi: 10.2174/156652310791823461. [DOI] [PubMed] [Google Scholar]
- 10.Spirito F, Meneguzzi G, Danos O, Mezzina M. Cutaneous gene transfer and therapy: the present and the future. J Gene Med. 2001;3:21–31. doi: 10.1002/1521-2254(2000)9999:9999<::AID-JGM156>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Trainer AH, Alexander MY. Gene delivery to the epidermis. Hum Mol Genet. 1997;6:1761–7. doi: 10.1093/hmg/6.10.1761. [DOI] [PubMed] [Google Scholar]
- 12.Khavari PA, Rollman O, Vahlquist A. Cutaneous gene transfer for skin and systemic diseases. J Intern Med. 2002;252:1–10. doi: 10.1046/j.1365-2796.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 13.Geusens B, Strobbe T, Bracke S, et al. Lipid-mediated gene delivery to the skin. Eur J Pharm Sci. 2011;43:199–211. doi: 10.1016/j.ejps.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz PR. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therrien JP, Pfutzner W, Vogel JC. An approach to achieve long-term expression in skin gene therapy. Toxicol Pathol. 2008;36:104–11. doi: 10.1177/0192623307312705. [DOI] [PubMed] [Google Scholar]
- 16.Byrnes CK, Malone RW, Akhter N, et al. Electroporation enhances transfection efficiency in murine cutaneous wounds. Wound Repair Regen. 2004;12:397–403. doi: 10.1111/j.1067-1927.2004.012409.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol. 2004;123:791–8. doi: 10.1111/j.0022-202X.2004.23309.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinstraesser L, Lam MC, Jacobsen F, et al. Skin electroporation of a plasmid encoding hCAP-18/LL-37 host defense peptide promotes wound healing. Mol Ther. 2014;22:734–42. doi: 10.1038/mt.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu G, Downey H, Guo S, et al. Prevention of distal flap necrosis in a rat random skin flap model by gene electro transfer delivering VEGF(165) plasmid. J Gene Med. 2014;16:55–65. doi: 10.1002/jgm.2759. [DOI] [PubMed] [Google Scholar]
- 20.Ferraro B, Cruz YL, Baldwin M, Coppola D, Heller R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010;17:763–9. doi: 10.1038/gt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther. 2009;17:651–7. doi: 10.1038/mt.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brave A, Gudmundsdotter L, Sandstrom E, et al. Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine. 2010;28:8203–9. doi: 10.1016/j.vaccine.2010.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donate A, Coppola D, Cruz Y, Heller R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PloS One. 2011 Apr 29;6:e19181. doi: 10.1371/journal.pone.0019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–23. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broderick KE, Shen X, Soderholm J, et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 18:258–65. doi: 10.1038/gt.2010.137. [DOI] [PubMed] [Google Scholar]
- 26.Gothelf A, Gehl J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr Gene Ther. 2010;10:287–99. doi: 10.2174/156652310791823443. [DOI] [PubMed] [Google Scholar]
- 27.Heller R, Heller LC. Gene electrotransfer clinical trials. Adv Genet. 2015;89:235–62. doi: 10.1016/bs.adgen.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Heller LC, Jaroszeski MJ, Coppola D, McCray An, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007;14:275–80. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heller R, Cruz Y, Heller LC, Gilbert R, Jaroszeski MJ. Electrically mediated delivery of plasmid DNA to the skin using a multi electrode array. Hum Gene Ther. 2010;21:357–62. doi: 10.1089/hum.2009.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rols MP, Delteil C, Serin G, Teissie J. Temperature effects on electrotransfection of mammalian cells. Nucleic Acids Res. 1994;22(3):540. doi: 10.1093/nar/22.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo S, Isreal AL, Basu G, Donate A, Heller R. Topical gene electrotransfer to the epidermis of hairless guinea pig by non-invasive multielectrode array. PLoS One. 2013 Aug 28;8(8):e73423. doi: 10.1371/journal.pone.0073423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:191–6. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- 33.Kellogg DL., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100:1709–18. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 34.Davison JL, Short DS, Wilson TE. Effect of local heating and vasodilation on the cutaneous venoarteriolar response. Clin Auton Res. 2004;14:385–90. doi: 10.1007/s10286-004-0223-x. [DOI] [PubMed] [Google Scholar]
- 35.Kanduser M, Sentjurc M, Miklavcic D. The temperature effect during pulse application on cell membrane fluidity and permeabilization. Bioelectrochemistry. 2008;74:52–7. doi: 10.1016/j.bioelechem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia. 2006;22:205–13. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 37.May JP, Li SD. Hyperthermia-induced drug targeting. Exp opinion on drug delivery. 2013;10:511–27. doi: 10.1517/17425247.2013.758631. [DOI] [PubMed] [Google Scholar]
- 38.Pace M, Millanta L, Polignano M, Gattai R, Macera Mascitelli E. Optimal procedure for thermal delivery in hyperthermic/chemotherapeutic treatments in the isolated perfusion of the the limbs. J Exp Clin Cancer Res. 2005;24(1):35–42. [PubMed] [Google Scholar]
- 39.Sarnaik AA, Sussman JJ, Ahmad SA, McIntyre BC, Lowy AM. Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. Recent Results Cancer Res. 2007;169:75–82. doi: 10.1007/978-3-540-30760-0_6. [DOI] [PubMed] [Google Scholar]
- 40.Donate A, Burcus N, Schoenbach K, Heller R. Application of increased temperature from an exogenous source to enhance gene electrotransfer. Bioelectrochemistry. 2015;103:120–3. doi: 10.1016/j.bioelechem.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]