Key Clinical Message
Although myopotential oversensing by a dedicated bipolar lead is rare, an epicardial lead on a dilated ventricle might contribute to its sensitivity. Myopotential oversensing was notified by the Lead Integrity Alert in this case. We should be aware of this possibility for the management of such patients.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, dedicated bipolar lead, epicardial lead, implantable cardioverter defibrillator, Lead Integrity Alert, myopotential oversensing
Introduction
Myopotential oversensing is a unique type of oversensing of extrinsic electric potential from skeletal muscle activity. The risk factors for myopotential oversensing are integrated bipolar use and keen sensitivity due to the automatic gain control function 1, 2, 3. Because of widespread use of dedicated bipolar leads for sensing ventricular signals in implantable cardioverter defibrillator (ICD) patients, myopotential oversensing is now rare. The myopotential oversensing rarely causes a notification by the Lead Integrity Alert (LIA) algorithm, which was originally developed for early detection of lead system problems 4. We describe a case of myopotential oversensing in an ICD patient with a dedicated epicardial bipolar lead that caused notification by LIA.
Case Report
A 67‐year‐old man visited the ICD clinic for an emergency examination because his ICD made a ringing noise. He had arrhythmogenic right ventricular cardiomyopathy with left ventricular (LV) involvement and reduced LV ejection fraction of 40% diagnosed 19 years previously. Refractory ventricular tachycardia (VT) originating from the right ventricle (RV) occurred 17 years previously and had been treated with a combination of optimal medication, radiofrequency catheter ablation, and single‐chamber ICD. A dedicated single‐coil active fixation lead was implanted at the RV endocardially (6932 Sprint™; Medtronic Inc., Minneapolis, MN, USA). Two epicardial leads (5071 Epicardial screw‐in™; Medtronic Inc.) using a Y‐connector (5866‐38M™; Medtronic Inc.) were sutured on the surface of the RV as a dedicated bipolar sensing lead. This was performed using thoracotomy because there was no proper place to implant the RV lead endocardially. Seventeen years had passed without any VT or ventricular fibrillation (VF) episodes requiring intensive treatment, except for twice ICD generator exchanges to finally Secure VR™ (model D234 VRC; Medtronic Inc.) because of battery exhaustion.
Nineteen years after his first ICD implantation, he was referred to our clinic because his ICD was making a ringing noise. Chest radiograph was used to reveal large cardiomegaly with a cardiothoracic ratio of 78% (Fig. 1A and B) and no signs of lead fraction or dislodgement. Twelve‐lead electrocardiogram showed atrial fibrillation with RV pacing (Fig. 1C). Emergency examination revealed that the ICD rang because of the LIA. The number of intervals to detect (NID) VF extended from 24/32 to 30/40, in accordance with the LIA algorithm. However, the measurement results of the device and leads were all within acceptable ranges: RV pacing threshold was 2.1 V at 0.5 msec; sensed R wave was 4.4 mV; and pacing lead impedance was 323 ohm. According to the examination data, two of three LIA criteria were met: (i) sensing integrity counter (SIC) had been counted 53 times within three consecutive days and (ii) nonsustained VT (NSVT) had occurred more than twice during 1 month. Stored intracardiac electrogram showed that the very high‐frequency, low‐amplitude, and short‐duration potential caused LIA, suggesting myopotential oversensing (Fig. 2A). Fortunately, only short improper pacing inhibition and no inappropriate shock delivery had occurred. Usual provocation maneuvers for myopotential oversensing such as Valsalva maneuver, left arm rotation, or palm apposition were unsuccessful. Because the causal episodes were seen at 4:09 am and 1:23 am (Fig. 2B), we questioned the patient carefully about what he did at these times. He told us that he was asleep at those times and that he often turns over using his left arm during sleep. We let him turn his upper body from the prone position using his left arm, and the noise and accompanying oversensing were successfully provoked (Fig. 3A and B), leading to the diagnosis of myopotential oversensing. We gave him instructions both to avoid the prone position and using his left arm to turn over during sleep. The incidence of NSVT and SIC decreased, and he was free from inappropriate shock delivery and improper pacing inhibition for 12 months until he died due to progressive heart failure.
Figure 1.
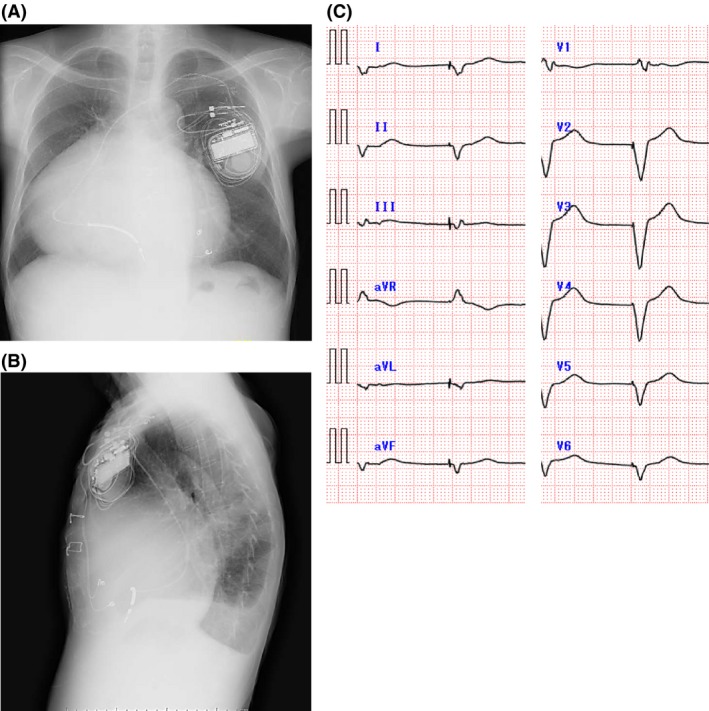
Posteroanterior (A) and lateral (B) views of chest radiograph and 12‐lead electrocardiogram image (C) during emergency examination in the ICD clinic just after the LIA alert.
Figure 2.
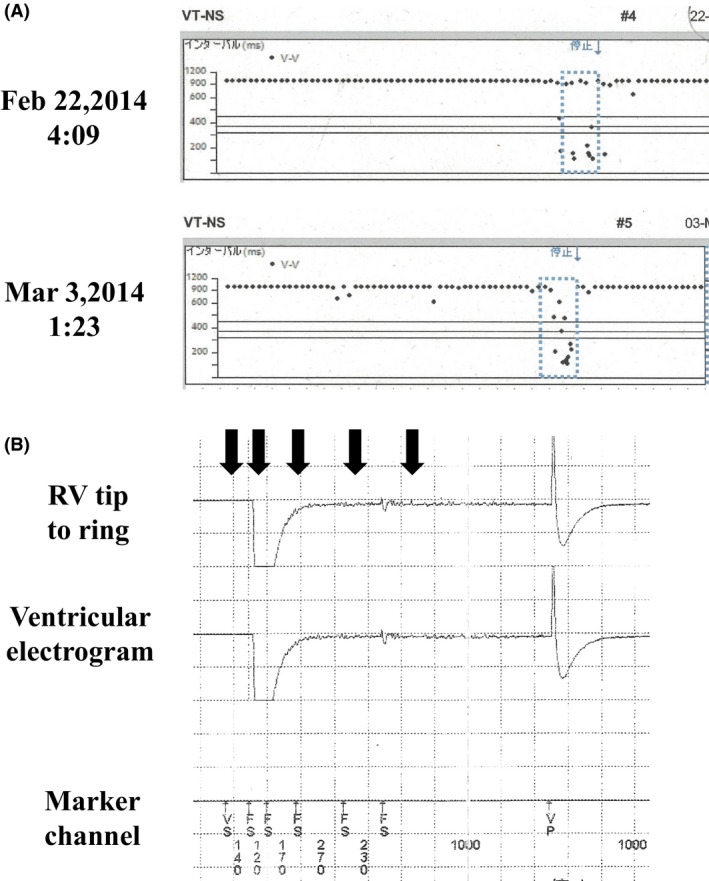
Interval plots and intracardiac electrogram image of LIA. (A) The interval plots at 4:09 am on February 22, 2014 (upper), and at 1:23 am on March 3, 2014 (lower). (B) The intracardiac electrocardiogram image at 1:23 am on March 3, 2014. High‐frequency and low‐amplitude potentials (black arrows) were detected as NSVT, which caused LIA.
Figure 3.
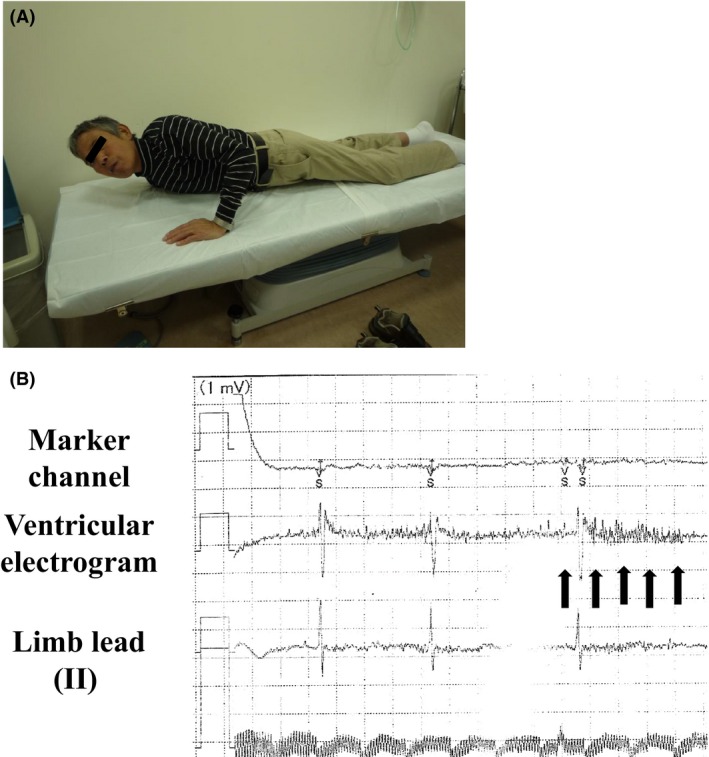
The provocation maneuver for myopotential oversensing. (A) Provocation. The patient turned his upper body from the prone position using his left arm. (B) Intracardial electrogram image during provocation. Characteristic potentials (black arrows) for the LIA were induced with improper pacing inhibition during the turning movement.
Discussion
This case demonstrated two issues: (i) myopotential oversensing could occur with an epicardial lead with dedicated bipolar sensing and (ii) LIA could be a result of myopotential oversensing.
Ventricular oversensing is a well‐known device‐related problem caused by lead malfunctioning or improper sensing of T waves, electromagnetic waves, or myopotential. The incidence of myopotential oversensing was reported as 1.5% in a large cohort of 518 ICD patients 5. Because myopotential oversensing could be managed noninvasively by reducing the maximum sensitivity for arrhythmia detection in many cases 6, proper diagnosis is important. One risk factor for myopotential oversensing is integrated bipolar use, with its large RV shock coil used as the anode for sensing 1, 2, 3. Requena et al. demonstrated the larger sensing scope of the integrated bipolar lead in comparison with the dedicated bipolar lead using their mathematical simulation model. They also demonstrated that the sensitivity measurement distribution for bipolar leads decreased inversely with γ cubed (γ 3), where γ is the distance from the cardiac current source to the lead system, implying the importance of lead location 7. In this case, because the sensing epicardial lead consisted of two leads connected by a Y‐connector, the sensing property was considered to be similar to that of a dedicated bipolar lead. In contrast, both the epicardial position itself and the patient's extremely dilated RV enabled greater sensitivity to myopotential. In addition, the function “automatic gain control” is more susceptible to detecting extracardiac far‐field signals because sensitivity increases faster and reaches maximum earlier in ICD patients highly dependent on RV pacing, like in this case 8. We considered that the contribution of the epicardial lead and ventricular dilatation might be risk factors for myopotential oversensing. We should keep in mind the possibility of myopotential oversensing when encountering similar cases, even though the sensing property of the lead is similar to that of the dedicated bipolar sensing lead.
The LIA algorithm was originally developed by Medtronic Inc. for early detection of lead system problems 4 and reduced inappropriate therapy in patients with lead fractures 9, 10. Although its usefulness was reported, the pseudopositive LIA, such as for T‐wave oversensing, R‐wave double count, electromagnetic interference, and, rarely, myopotential, was also reported. Interestingly, myopotential oversensing, which caused the LIA notification, was found only with the integrated bipolar lead. It was considered that this finding of myopotential oversensing with a dedicated bipolar sensing lead is rare. Because this case clearly demonstrated that myopotential oversensing with a dedicated bipolar sensing lead caused LIA notification, cardiologists should be aware of this and remember its possibility when a patient presents with LIA, even though the incidence is rare.
Conclusion
Myopotential oversensing could occur in patients with an epicardial lead and ventricular dilatation, even if the lead system includes a dedicated bipolar sensing property. LIA could be the first sign of this special type of device‐related problem.
Conflict of Interest
None declared.
Acknowledgments
The English editing and publishing fee were funded by the Clinical Research Division of Yokohama Medical Center. We thank Editage (www.editage.jp) for English language editing.
References
- 1. Schulte, B. , Sperzel J., Carlsson J., Dursch M., Erdogan A., Pitschner H. F., et al. 2001. Inappropriate arrhythmia detection in implantable defibrillator therapy due to oversensing of diaphragmatic myopotentials. J. Interv. Card. Electrophysiol. 5:487–493. [DOI] [PubMed] [Google Scholar]
- 2. Santos, K. R. , Adragao P., Cavaco D., Morgado F. B., Candeias R., Lima S., et al. 2008. Diaphragmatic myopotential oversensing in pacemaker‐dependent patients with CRT‐D devices. Europace 10:1381–1386. [DOI] [PubMed] [Google Scholar]
- 3. Swerdlow, C. D. , and Friedman P. A.. 2005. Advanced ICD troubleshooting: part I. Pacing Clin. Electrophysiol. 28:1322–1346. [DOI] [PubMed] [Google Scholar]
- 4. Ellenbogen, K. A. , Gunderson B. D., Stromberg K. D., and Swerdlow C. D.. 2013. Performance of lead integrity alert to assist in the clinical diagnosis of implantable cardioverter defibrillator lead failures: analysis of different implantable cardioverter defibrillator leads. Circ. Arrhythm. Electrophysiol. 6:1169–1177. [DOI] [PubMed] [Google Scholar]
- 5. Rauwolf, T. , Guenther M., Hass N., Schnabel A., Bock M., Braun M. U., et al. 2007. Ventricular oversensing in 518 patients with implanted cardiac defibrillators: incidence, complications, and solutions. Europace 9:1041–1047. [DOI] [PubMed] [Google Scholar]
- 6. Schulte, B. , Sperzel J., Schwarz T., Pitschner H. F., Strupp G., and Neuzner J.. 2000. Detection of ventricular fibrillation in implantable defibrillators with automatic gain control amplifiers: effects of programming sensitivity. Europace 2:160–162. [DOI] [PubMed] [Google Scholar]
- 7. Requena‐Carrión, J. , Väisänen J., Alonso‐Atienza F., Rojo‐Álvarez J., Hyttinen J., and García‐Alberola A.. 2007. Comparison of the scope of true and integrated bipolar leads in implantable cardioverter defibrillators. Comput. Cardiol. 34:233–236. [DOI] [PubMed] [Google Scholar]
- 8. Salukhe, T. V. , Wright I., Wright M., Kanagaratnam P., and O'Neill M. D.. 2008. A fortuitous syncope. The pitfalls of integrated bipolar defibrillator leads. Indian Pacing Electrophysiol. J. 8:312–316. [PMC free article] [PubMed] [Google Scholar]
- 9. Blanck, Z. , Axtell K., Brodhagen K., O'Hearn L., Albelo T., Ceretto C., et al. 2011. Inappropriate shocks in patients with Fidelis(R) lead fractures: impact of remote monitoring and the lead integrity algorithm. J. Cardiovasc. Electrophysiol. 22:1107–1114. [DOI] [PubMed] [Google Scholar]
- 10. Kneller, J. , Delacey W., Wood M. A., and Ellenbogen K. A.. 2012. Detection of a Riata insulation failure by the Medtronic Lead Integrity Alert. Europace 14:1215–1216. [DOI] [PubMed] [Google Scholar]


