Abstract
Salidroside (SDS) has been reported to have anti-inflammatory properties. The objective of this study was to investigate the protective effect of SDS on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. BALB/c mice were pretreated with SDS 1 hour before intranasal instillation of LPS. Seven hours after LPS administration, the myeloperoxidase in histology of lungs, lung wet/dry ratio, and inflammatory cells in the bronchoalveolar lavage fluid (BALF) were determined. The levels of pro-inflammatory cytokines, tumor necrosis factor α (TNF-α), interleukin-1β (IL 1β), and IL-6 in the BALF were measured by enzyme-linked immunosorbent assay. The expression of Toll-like receptor 4 (TLR4), inhibitor of nuclear factor-kappa B (IκB-α), and nuclear factor-kappa B (NF-κB) p65 was detected by Western blot. The SDS reduced the inflammatory cells in BALF, decreased the wet/dry ratio of lungs, attenuated the LPS-induced histological alterations in the lung, and inhibited the production of TNF-α, IL-1β, and IL-6. Western blot showed that SDS efficiently inhibited the phosphorylation of IκB-α, p65 NF-κB, and the expression of TLR4. These data show that the anti-inflammatory effects of SDS (at least 20 mg/kg) against LPS-induced ALI due to its ability to inhibit TLR4 mediated the NF-κB signaling pathways. The SDS may represent a novel strategy for treating LPS-induced ALI.
Keywords: salidroside, lipopolysaccharide, acute lung injury, nuclear factor-κB, Toll-like receptor 4
Introduction
As a form of acute respiratory distress syndrome, acute lung injury (ALI) is characterized by severe hypoxemia, pulmonary edema, and neutrophil accumulation in the lung, which is caused by the onset of pulmonary inflammation due to infection, shock, trauma, and burns, as well as other noncardiac disease, and it is associated with a mortality rate of 35% to 45%.1-3 Lipopolysaccharide (LPS) is a main component of the outer membrane of the gram-negative bacteria, which could induce a disturbance in the immune and inflammatory responses, and has been found to be one of the most important risk factor for ALI.4,5
Salidroside (SDS; Figure 1) is a phenylpropanoid glycoside extracted from Rhodiola rosea, which is a commonly used folk medicine for the treatment of high-altitude sickness, mountain malhypoxia, and anoxia.6 Previous studies have found that SDS has a broad spectrum of biological effects, including anti-inflammatory, antihypoxia, antitumor, antifibrosis, and antiaging effects.7-11 The SDS alleviated the pulmonary symptoms of paraquat-induced ALI by repressing inflammatory cell infiltration and the expression of transforming growth factor beta β1 resulting in delayed lung fibrosis12 and has protective effects against LPS-induced mastitis in mice.13 In this study, we investigated the protective effects of SDS in a LPS-induced mouse ALI model and elucidated the potential anti-inflammatory mechanism, and it may discover novel anti-inflammatory candidate agents for the prevention or treatment of LPS-induced ALI.
Figure 1.
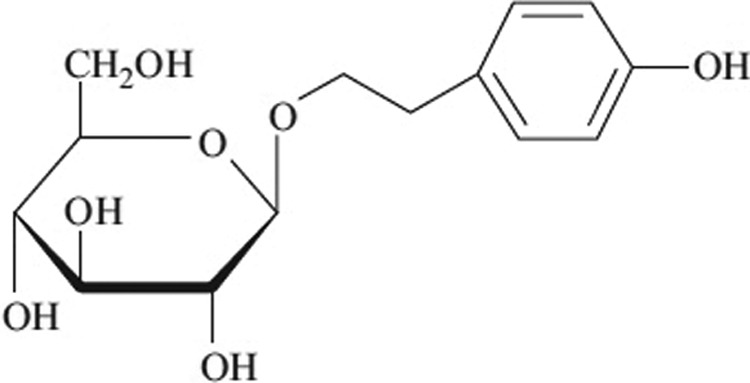
Chemical structure of salidroside.
Materials and Methods
The healthy male BALB/c mice (n = 72, 18-22 g; Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China) were randomly divided into 6 groups: the control group, LPS (0.5 mg/kg) group, SDS (10, 20, and 40 mg/kg; the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) + LPS group, and dexamethasone (DEX, 0.5 mg/kg) + LPS group, as described previously.14,15 Each group contained 12 mice. Before inducing acute lung inflammation, SDS was given by intraperitoneal (IP) injection, whereas DEX was administrated intragastrically as a positive control. Blank control and LPS group mice were given an equal volume of distilled water by IP, and 1 hour later, 10 µg of LPS in 50 µL phosphate-buffered saline (PBS) was instilled IN to induce lung injury. Blank control group mice were given a 50 µL PBS IN. All the mice were alive after 7-hour LPS treatment. The mice were killed by exsanguination at 7 hours after the administration of LPS. Collection of bronchoalveolar lavage fluid (BALF) was performed 3 times through a tracheal cannula with autoclaved PBS. The animal studies were approved by the First Affiliated Hospital of Zhejiang Chinese Medical University, China.
To minimize suffering, mice were sedated by ether anesthesia and euthanized, and the lungs were removed and the wet weight was determined. The lungs were then placed in an incubator at 60°C for 24 hours to obtain the dry weight, as described previously.14,15 The ratio of wet lung to dry lung was calculated to assess tissue edema. The fluid recovered from each sample was centrifuged at 3000 rpm for 15 minutes to pellet the cells. The cell pellets were resuspended in PBS for total cell counts using a hemocytometer. Cytospins were prepared for differential cell counts by staining with the Wright-Giemsa staining method. The levels of the pro-inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin (IL) 1β, and IL-6 in supernatant of BALF were measured by enzyme-linked immunosorbent assay (ELISA) kits (Research & Diagnostics Systems), according to the instructions recommended by the manufacturers. Protein concentrations in the supernatant of the BALF were quantified using the bicinchoninic acid (BCA) protein assay kit to evaluate vascular permeability in the airways of mice. All data are expressed as mean ± standard deviation (SD), and the differences were analyzed using 1-way analysis of variance (Dunnett t test) with SPSS 15.0. Statistical significance was accepted when P < .05.
The lungs were harvested at 6 hours after the injection of LPS and fixed in formalin for at least 24 hours before processing. The tissues were embedded in paraffin by standard tissue processing procedures, and evaluation of lung edema and inflammatory cell infiltration was examined with a light microscope. At 7 hours after the injection of LPS, lung tissues were harvested and frozen in liquid nitrogen immediately until homogenization. Proteins were extracted from the lungs and protein concentrations were determined by BCA protein assay kit, and equal amounts of protein were loaded per well on a 12% sodium dodecyl sulfate polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride membrane. The resulting membrane was blocked with Tris-buffered saline containing 0.05% Tween-20 (TBST), supplemented with 5% milk at room temperature for 2 hours. The specific primary antibody was incubated with the membrane overnight. The membrane was washed with TBST followed by incubation with the peroxidase-conjugated secondary antibody at room temperature for 1 hour. The immunoactive proteins were detected using an enhanced chemiluminescence Western blot detection kit, as described previously.14,15
Results and Discussion
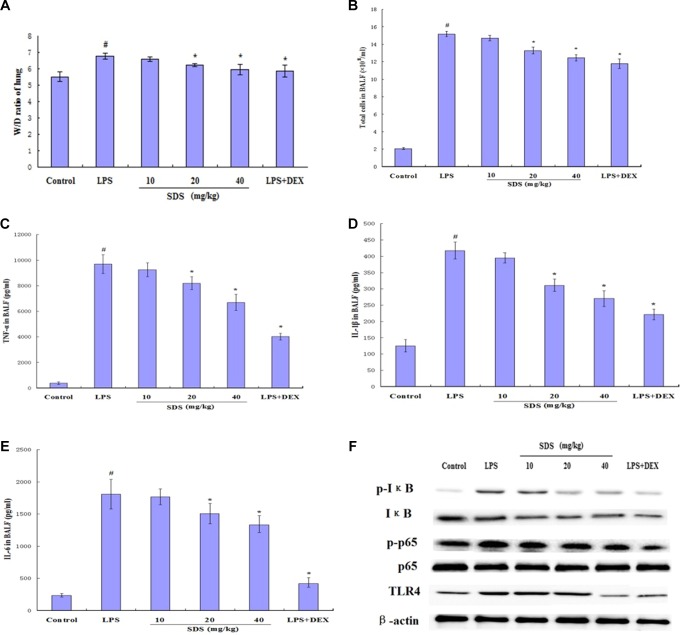
The results showed that LPS instillation significantly increased the lung wet/dry (W/D) ratio (Figure 2A). The increase was greater compared with that of the control group (P < .01). However, pretreatment with SDS (20 and 40 mg/kg) or DEX significantly decreased the lung W/D ratio (P < .05) compared to those in the LPS group. The BALF total protein concentration increased significantly in the LPS group, and pretreatment with SDS (20 and 40 mg/kg) or DEX significantly decreased the total protein concentration (P < .05) compared to those in the LPS group (Figure 2B).
Figure 2.
Effects of salidroside (SDS) on the lung wet/dry (W/D) ratio of lipopolysaccharide (LPS)-induced acute lung injury (ALI) mice (A), the number of total cells (B), the production of inflammatory cytokine tumor necrosis factor-α (TNF-α; C), interleukin (IL) 1β (D), and IL-6 (E) in the bronchoalveolar lavage fluid (BALF) of LPS-induced ALI mice. The SDS pretreatment inhibited LPS-induced activation of nuclear factor-kappa B (NF-κB) with Western blot (F). #P < .05 versus control group and *P < .05 versus LPS group.
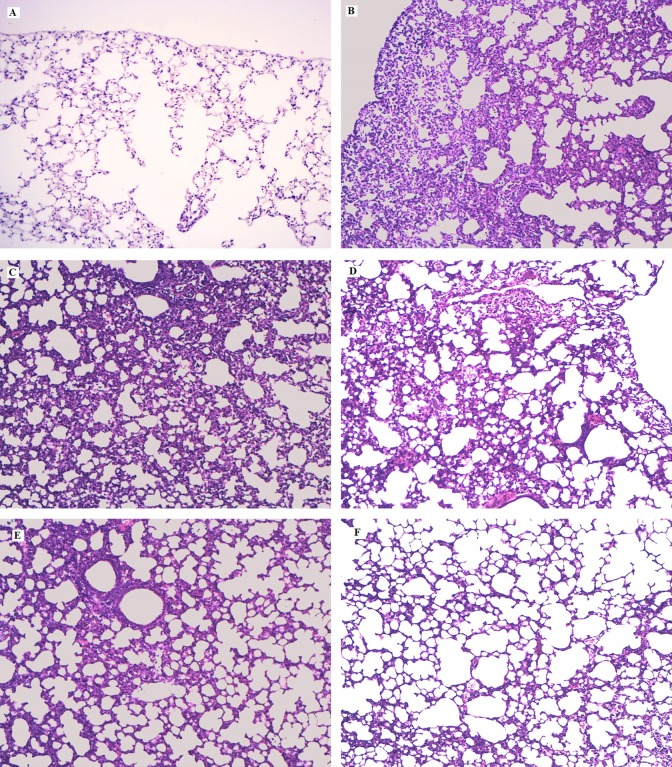
The results showed that the concentrations of TNF-α, IL-1β, and IL-6 in BALF by ELISA were significantly increased after LPS administration (Figure 2C-E). Both SDS and DEX significantly reduced TNF-α, IL-1β, and IL-6 production compared to those in the LPS group. In this study, SDS significantly inhibited the production of TNF-α, IL-1β, and IL-6 in BALF, and the results are consistent with those of Guan et al16 who showed that the SDS (120 mg/kg) possesses a protective effect on LPS-induced ALI in mice. As shown in Figure 3, LPS-induced pathological changes were significantly attenuated by SDS and DEX treatment.
Figure 3.
Effects of salidroside (SDS) on histopathological changes in lung tissues in lipopolysaccharide (LPS)-induced acute lung injury (ALI) mice. Mice were given an intraperitoneal injection of SDS 1 hour prior to an instilled intranasal administration of LPS (0.5 mg/kg). Lungs (n = 6) from each experimental group were processed for histological evaluation at 7 hours after LPS challenge. A, Control group; (B) LPS group, (C-E) SDS (10, 20, and 40 mg/kg) + LPS group; (F) dexamethasone (DEX) + LPS group (hematoxylin and eosin staining, magnification ×200).
The Toll-like receptor 4 (TLR4) recognizes LPS and activation of TLR4 by LPS-induced nuclear factor-kappa B (NF-κB) activation.17 Normally, NF-κB is present in its inactive cytoplasmic form bound to the inhibitor of NF-κB (IκB).18 In this study, the expression of TLR4, IκB-α, and NF-κB p65 was detected by Western blot analysis (Figure 2F). The results showed that LPS significantly increased the expression of TLR4 and the phosphorylation of IκB-α and NF-κB p65. However, these results showed that pretreatment with SDS decreased the expression of TLR4 and the phosphorylation of IκB-α and NF-κB p65.
Conclusion
In conclusion, our data show that the protective effects of SDS (at least 20 mg/kg) on ALI induced by LPS may be attributed to the inhibition of inflammatory cytokines. The results also showed that LPS stimulation dramatically increased the phosphorylation of IκBα and NF-κB p65 protein, and LPS-induced IκBα degradation and NF-κB p65 activation were significantly blocked by pretreatment with SDS. These data suggest that SDS may represent a novel strategy for preventing and treating LPS-induced ALI.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Scientific Research Fund of Zhejiang Province Chinese Medicine (2012ZB039).
References
- 1. Blank R, Napolitano LM. Epidemiology of ARDS and ALI. Crit Care Clin. 2011;27(3):439–458. [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306(8):L709–L725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lalu MM, Moher D, Marshall J, et al. Efficacy and safety of mesenchymal stromal cells in preclinical models of acute lung injury: a systematic review protocol. Syst Rev. 2014;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayeux PR. Pathobiology of lipopolysaccharide. J Toxicol Environ Health. 1997;51(5):415–435. [DOI] [PubMed] [Google Scholar]
- 5. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(8):1334–1349. [DOI] [PubMed] [Google Scholar]
- 6. Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293–302. [PubMed] [Google Scholar]
- 7. Qian EW, Ge DT, Kong SK. Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis. J Nat Prod. 2012;75(4):531–537. [DOI] [PubMed] [Google Scholar]
- 8. Skopinska-Rozewska E, Malinowski M, Wasiutynski A, et al. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol J Vet Sci. 2008;11(2):97–104. [PubMed] [Google Scholar]
- 9. Mao GX, Wang Y, Qiu Q, et al. Salidroside protects human fibroblast cells from premature senescence induced by H(2)O(2) partly through modulating oxidative status. Mech Ageing Dev. 2010;131(11-12):723–731. [DOI] [PubMed] [Google Scholar]
- 10. Song B, Huang G, Xiong Y, et al. Inhibitory effects of salidroside on nitric oxide and prostaglandin E(2) production in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Med Food. 2013;16(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 11. Tang H, Gao L, Mao J, et al. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-beta1/Smad-2/-3 pathways. Cell Stress Chaperones. 2016;21(2):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Z, Ding L, Wu L, Xu L, Zheng L, Huang X. Salidroside alleviates paraquat-induced rat acute lung injury by repressing TGF-β1 expression. Int J Clin Exp Pathol. 2014;7(12):8841–8847. [PMC free article] [PubMed] [Google Scholar]
- 13. Li D, Fu Y, Zhang W, et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-κB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm Res. 2013;62(2):9–15. [DOI] [PubMed] [Google Scholar]
- 14. Tang Y, Chen Y, Chu Z, Yan B, Xu L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2014;723:494–500. [DOI] [PubMed] [Google Scholar]
- 15. Liu Z, Yang Z, Fu Y, et al. Protective effect of gossypol on lipopolysaccharide-induced acute lung injury in mice. Inflamm Res. 2013;62(5):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan S, Xiong Y, Song B, et al. Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol Immunotoxicol. 2012;34(4):667–672. [DOI] [PubMed] [Google Scholar]
- 17. Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198(7):1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanarek N, Ben-Neriah Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol Rev. 2012;246(1):77–94. [DOI] [PubMed] [Google Scholar]