Abstract
Noonan syndrome is a rasopathy caused by mutations in multiple genes encoding components of the RAS/MAPK pathway. Despite its variable phenotype, limited genotype-phenotype correlations exist. Noonan syndrome with loose anagen hair (NS-LAH) is characterized by its distinctive hair anomalies, developmental differences and structural brain abnormalities and is caused by a single recurrent missense SHOC2 mutation. SHOC2 forms a complex with protein phosphatase 1 (PP1C). Protein phosphatases counterbalance kinases and control activation of signaling proteins, such as the mitogen activated protein kinases of the RAS/MAPK pathway. Here we report four patients with de novo missense mutations in protein phosphatase 1 catalytic subunit beta (PPP1CB), sharing a recognizable phenotype. Three individuals had the recurrent PPP1CB c.146G>C, p.Pro49Arg mutation, the fourth had a c.166G>C, p.Ala56Pro change. All had relative or absolute macrocephaly, low-set and posteriorly angulated ears and developmental delay. Slow growing and/or sparse hair and/or an unruly hair texture was present in all. Three individuals had feeding difficulties requiring feeding tubes. One of two males had cryptorchidism, another had pectus excavatum. Short stature was present in three. A female with the recurrent mutation had a Dandy-Walker malformation and optic nerve hypoplasia. Mild ventriculomegaly occurred in all, cerebellar tonsillar ectopia was seen in two and progressed to Chiari 1 malformation in one individual. Based on the combination of phenotypic findings and PPP1CB’s effect on RAF dephosphorylation within the RAS/MAPK pathway, this novel condition can be considered a rasopathy, most similar to NS-LAH. Collectively, these mutations meet the standardized criteria for pathogenicity.
Keywords: Noonan syndrome, rasopathy, Loose anagen hair, PPP1CB, Dandy-Walker malformation, short stature, devolpmental day
INTRODUCTION
The rasopathies are a group of syndromic conditions resulting from germline mutations in genes affecting the RAS mitogen activated protein kinase (MAPK) pathway. Pathogenic mutations alter regulatory protein-protein interactions within the pathway, resulting in prolonged RAS/MAPK pathway activation with increased phosphorylation of downstream effectors, MEK and ERK. Noonan syndrome with loose anagen hair (NS-LAH)(MIM#607721), also known as Mazzanti syndrome, was reported clinically based on its distinctive hair findings in combination with growth hormone deficiency and other typical Noonan syndrome findings, such as short stature and pectus excavatum [Mazzanti et al., 2003]. Loose anagen hair presents with easily pluckable, sparse, thin and slow growing hair with an irregular texture caused by an abnormal hair bulb lacking inner and outer root sheaths. Noonan syndrome with loose anagen hair is caused exclusively by a single recurrent missense mutation in SHOC2, p.Ser2Gly [Cordeddu et al., 2009]. SHOC2 is an ubiquitously expressed leucine-rich repeat containing protein which interacts with the catalytic subunit of protein phosphatase 1 (PP1C), playing an essential role in RAS/MAPK pathway regulation [Rodriguez-Viciana et al., 2006; Young et al., 2013]. Protein phosphatase 1 is a serine/threonine specific phosphatase that counterbalances serine/threonine kinases, thereby controlling activation of signaling proteins, such as the mitogen activated protein kinases of the RAS/MAPK pathway. Protein phosphatase 1 has three catalytic subunits called alpha, beta and gamma. Here we report on four unrelated patients with de novo missense mutations in protein phosphatase 1 catalytic subunit beta (PPP1CB) (OMIM#600590), who share a recognizable phenotype consistent with a rasopathy and most similar to NS-LAH.
MATERIALS AND METHODS
As a part of ongoing clinical care and molecular research on rasopathies, patients with clinical and/or molecular diagnoses of Costello syndrome and related conditions are enrolled into a study approved by our institutional review board (A. I. duPont Hospital for Children #2005-051). In parallel, patients diagnosed with developmental disorders are enrolled into an ongoing molecular genetics study approved by the institutional review board at Seattle Children’s Hospital (#13291). Signed consents for publication of data and photographs were obtained from all families.
Patients 1, 2, and 3 were ascertained due to clinical features suggestive of a rasopathy, and exome sequencing was performed on each proband and parent trio on a clinical basis at GeneDx as detailed in Retterer et al. [2015]. Paternity and maternity were established through the trio exome analysis. Patient 4 was ascertained as a part of a larger study of genetic causes of Dandy-Walker malformation (DWM), and exome sequencing of this individual and her parents was performed by Beckman Coulter Genomics as follows: Genomic DNA isolated from peripheral blood was captured using the Agilent SureSelect V5 enrichment kit and sequenced on an Illumina HiSeq as 150-bp paired-end runs. Variants were annotated using Annovar [Wang et al., 2010] then filtered for de novo inheritance, absence from 1000 Genomes, the NHLBI Exome Sequencing Project Exome Variant Server (EVS), the Exome Aggregation Consortium (ExAC), dbSNP version 137, and predicted to be deleterious by CADD (>10) [Kircher et al., 2014].
Clinical Reports
Patient 1 (CS#375; LR12–049)
This boy (Figure 1) was born after an uncomplicated pregnancy to his 36-year-old Caucasian mother and 35-year-old Caucasian father. He had two older healthy sisters. An uncomplicated vaginal delivery occurred at term. His length was 52 cm (50th–75th centile); weight 3.52 kg (50th centile) and OFC was not recorded. Apgar values were 8 and 9, at one and five minutes, respectively. An undescended testicle (Table I) and distinctive facial features with prominent eyes, different from family members, were noted. Nasolacrimal duct stenosis required surgical intervention with unilateral silicone tube placement at age 4 years. Feeding difficulties, related to laryngomalacia and gastroesophageal reflux (GER), resulted in slow weight gain with a return to birth weight at 21 days. Failure-to-thrive led to multiple gastrointestinal evaluations and imaging studies documenting severe GER, use of a naso-gastric feeding tube and gastrostomy tube placement at 9 months. The gastrostomy tube was used until about age 20 months, thereafter he was able to eat by mouth.
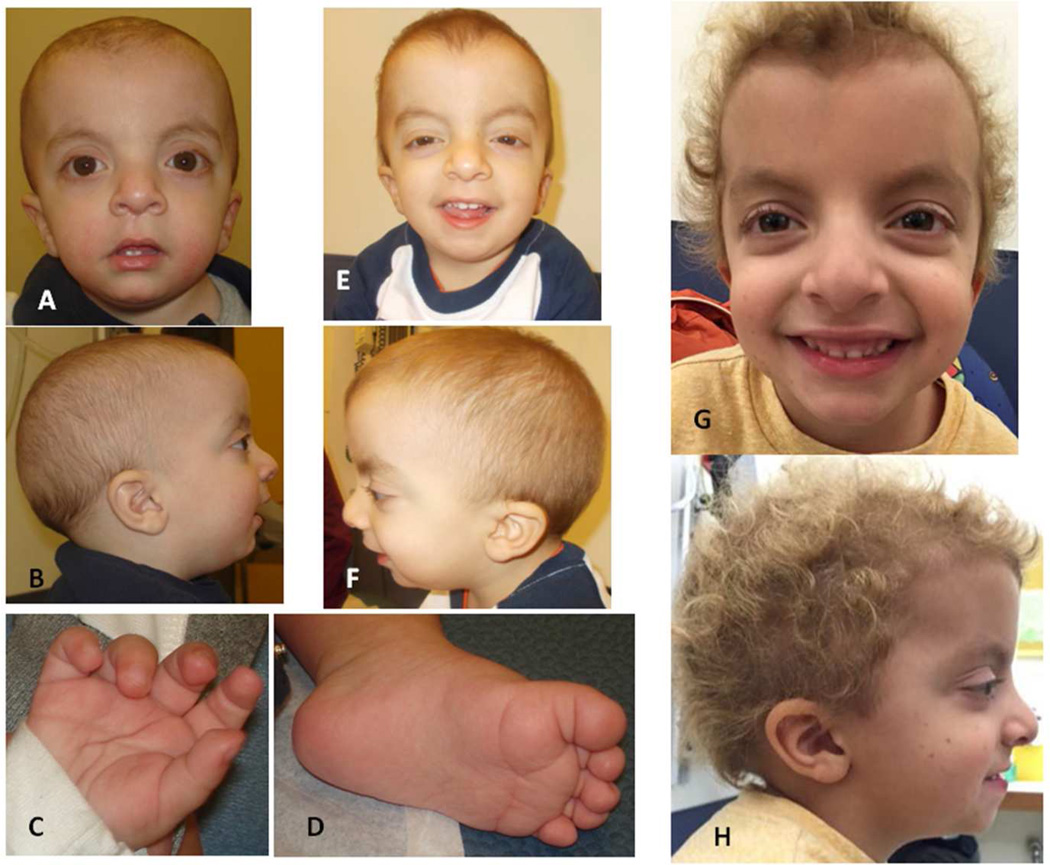
Figure 1.
Photograph of Patient 1 at age 11 months showing facial features (A, B) and prominent fetal pads on fingers (C) and slightly redundant soft tissue with deep plantar creases (D); facial features at 2 2/12 years (E, F) and at 5 2/12 years (G, H).
Table I.
Patient Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | NS-LAH [summarized Table I in Gripp et al., 2013] |
|
|---|---|---|---|---|---|
| PPP1CB mutation | De novo p.Pro49Arg |
De novo p.Ala56Pro |
De novo p.Pro49Arg | De novo p.Pro49Arg |
N/A |
| Sex | Male | Male | Female | Female | N/A |
| Age at last evaluation or contact |
5 3/12 years | 9 years | 4 7/12 years | 21 years | N/A |
| Polyhydramnios | - | - | - | - | 4/7 |
| Increased birth weight |
- | - | - | - | 3/7 |
| Short stature | + | + | + | - | 28/31 |
| Macrocephaly | Relative | Relative | Relative | + | 29/32 |
| Feeding difficulties | + | Neonatal | Neonatal | - | N/A |
| Feeding tube use | + | Neonatal | Neonatal | - | 3/5 |
| Delayed development |
+ | + | + | + | 5/5 |
| Hypotonia | - | Mild | + | - | 4/5 |
| ADHD/cognition and behavior |
Anxious | Slow processing | - | Very active, impulsive, anxious |
ADHD 14/22 |
| Down-slanted palpebral fissures |
+ | Mild | - | - | N/A |
| Hypertelorism | + | - | + | + | N/A |
| Epicanthus | - | - | + | - | N/A |
| Low-set, posteriorly angulated ears |
+ | + | + | + | N/A |
| Pulmonic valve stenosis |
- | - | + | N/A | 11/29 |
| Mitral/tricuspid valve anomaly |
Mitral valve thickening |
- | - | N/A | 9/28 |
| ASD/VSD | - | - | - | N/A | 10/25 ASD |
| Hypertrophic cardiomyopathy |
- | - | - | N/A | 6/29 |
| Pigmentation | Freckling | Café-au-lait lesions |
Irregular hypopigmentation on back |
- | 23/31 |
| Slow growing hair | + | - | + | + | 19/19 for any manifestation of LAH |
| Curly hair | + | - | - | - | |
| Unruly hair texture | + | + | - | + | |
| Hemangioma | - | - | Large, on chin | - | 2/5 |
| Eye anomalies | Proptosis, tear duct stenosis |
Strabismus repair |
- | Optic nerve hypoplasia, nystagmus, impaired vision |
N/A |
| Hearing loss | - | Possible | Mild | - | N/A |
| Webbed/short neck |
- | Short | - | - | N/A |
| Pectus anomaly | - | + | - | - | N/A |
| GU anomaly | cryptorchidism | - | N/A | N/A | N/A |
| Malignancy | - | - | - | - | Myelofibrosis in 1 |
| Other | Redundant arytenoid tissue; right bundle branch block |
N/A | prenatal edema of face and neck; hirsutism on back; coxa valga bilaterally |
Dandy-Walker malformation; preauricular pits (familial) |
N/A |
| Genetic testing with normal results |
46, XY Array CGH |
Array CGH Mitochondrial DNA |
SNP array, TSH and CK, urine oligosaccharide and free glycan, lysosomal enzymes |
N/A | N/A |
| Normal sequence results |
BRAF, HRAS KRAS, MEK1 MEK2, SHOC2 NRAS,PTPN11 SOS1, RAF1 CBL, RIT1 |
BRAF, HRAS KRAS, MEK1 MEK2, SHOC2 NRAS,PTPN11 SOS1, RAF1, CBL RIT1 |
BRAF, HRAS, KRAS MEK1, MEK2, SHOC2 NRAS, PTPN11, SOS1 RAF1, CBL (maternally inherited VUS in BRAF) NM_004333:c.967T>C(p. Ser323Pro) |
N/A | N/A |
ASD: Atrio-septal defect. N/A: Not available or not assessed. NS-LAH: Noonan syndrome with loose anagen hair. LAH: Loose anagen hair.VSD: Ventriculo-septal defect.
A laryngoscopy for persistent stridor showed redundant arytenoid tissue and resulted in supraglottoplasty at 3 months. Chronic otitis media resulted in tympanostomy tube placement. Cryptorchidism and inguinal hernia were surgically repaired at 9 months.
Echocardiography performed at age 3 months was limited due to poor patient cooperation but did not show structural anomalies. At 2 years, mild mitral valve thickening without prolapse or regurgitation was seen on echocardiography. At 5 years, a cardiology evaluation with echography and ECG showed no significant heart disease or cardiomyopathy, but a mildly thickened myxomatous mitral valve without regurgitation, a probable small coronary fistula to the main pulmonary artery, and right bundle branch block.
Short stature (supplementary Figure 1A) resulted in bone age studies, which had normal results at age 23 months. Based on a clinical diagnosis of Noonan syndrome and short stature, growth hormone (GH) treatment was begun at 5 5/12 years (with no follow up data available at the time of this report).
Relative macrocephaly and developmental delay at 2.5 years led to a brain MRI, which showed ventricular dilatation and cerebellar tonsillar herniation (Table II). This herniation progressed to a Chiari 1 malformation by 2.5 years and remained stable at 5 3/12 years (supplementary Figure 2).
Table II.
Central Nervous System Imaging Findings
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | NS-LAH [summarized from Table II in Gripp et al., 2013] |
|
|---|---|---|---|---|---|
| MRI obtained at age (years) |
1 5/12 2 6/12 5 3/12 |
5 5/12 6 8 6/12 |
1 6/12 | 6 7/12 | N/A |
| Macrocephaly | Relative | Relative | Relative | + | + |
| Prominent forehead/ dolichocephaly |
+ | + | + | + | + |
| Ventriculomegaly | Mild third and lateral ventricles |
Borderline lateral ventricles |
+, third and lateral ventricles |
Mild third and lateral ventricles; cystic fourth ventricle |
+, enlarged cisterna magna in 1 |
| Arachnoid space, extra-axial fluid |
Normal | Arachnoid cyst |
Mildly prominent frontally |
Normal | Enlarged; arachnoid cysts in 2 |
| Cerebrum, heterotopia |
Normal gyral pattern |
Normal gyral pattern |
Normal gyral pattern |
Distorted gyral pattern but no dysplasia |
Periventricular nodular heterotopias in 1 |
| Corpus callosum | Mildly stretched |
Normal | Mildly stretched | Normal | Dysplastic or hypoplastic in 1 each |
| White matter | Normal | Normal | Mildly decreased white matter volume |
Normal | “Diffuse reduction in white matter” in several |
| Cerebellum | Cerebellar tonsillar ectopia progressing to Chiari 1 |
Normal | Mild cerebellar tonsillar ectopia |
Dandy-Walker malformation |
Small vermis and hemispheres in 1 |
| Chiari 1 | + | - | - | - | 3 |
| Small optic nerves | - | - | - | + | 1 |
N/A: Not available or not assessed. NS-LAH: Noonan syndrome with loose anagen hair.
Patient 1 had developmental delay with walking independently at 22 months. He received therapy services and had a special education teacher’s service twice weekly at age 5 years while attending preschool.
At age 5 3/12 years his height was 97 cm (<2nd centile, 50th centile for 3.5 years), weight was 14.9 kg (<2nd centile, 50th centile for 3.25 years) and OFC 52.9 (75–90th centile). Facial features included apparent macro- and dolichocephaly (Figure 1) with mild frontal bossing; proptotic and hyperteloric appearing eyes with down-slanted palpebral fissures; posteriorly angulated and low-set ears; and coarse, curly hair that had never been cut. Scattered small hyperpigmented lesions were noted on the cheeks and legs, and a single larger hyperpigmented lesion on his back. His neck was not wide and he had no pectus. Hands showed a bridged crease bilaterally and mild redundancy of the soft tissue, nails were normal. Muscle tone and reflexes were grossly normal. He spoke in full sentences, but his social interactions appeared immature.
Patient 2 (LR16–082)
This boy (Figure 2 A-C) was born to a 28-year-old Caucasian mother and 29-year-old Caucasian father, he subsequently had one younger brother and his parents lost one pregnancy in the first trimester. Pregnancy was complicated by placenta previa and preterm labor treated with magnesium sulfate. Delivery occurred at 34 weeks of gestation by emergent cesarean for a placental abruption. His birth length was 47.6 cm (75th centile for gestational age); weight 2.61 kg (75th-90th centile for gestational age) and OFC was not recorded. Apgar values were 7 and 9, at one and five minutes. He was treated in the neonatal intensive care unit for 12 days due to feeding difficulties. Hearing evaluations had inconsistent results, with possible bilateral mild conductive or sensorineural hearing loss without chronic otitis. Intermittent alternating exotropia resulted in bilateral strabismus repair at age 1 5/12 years.
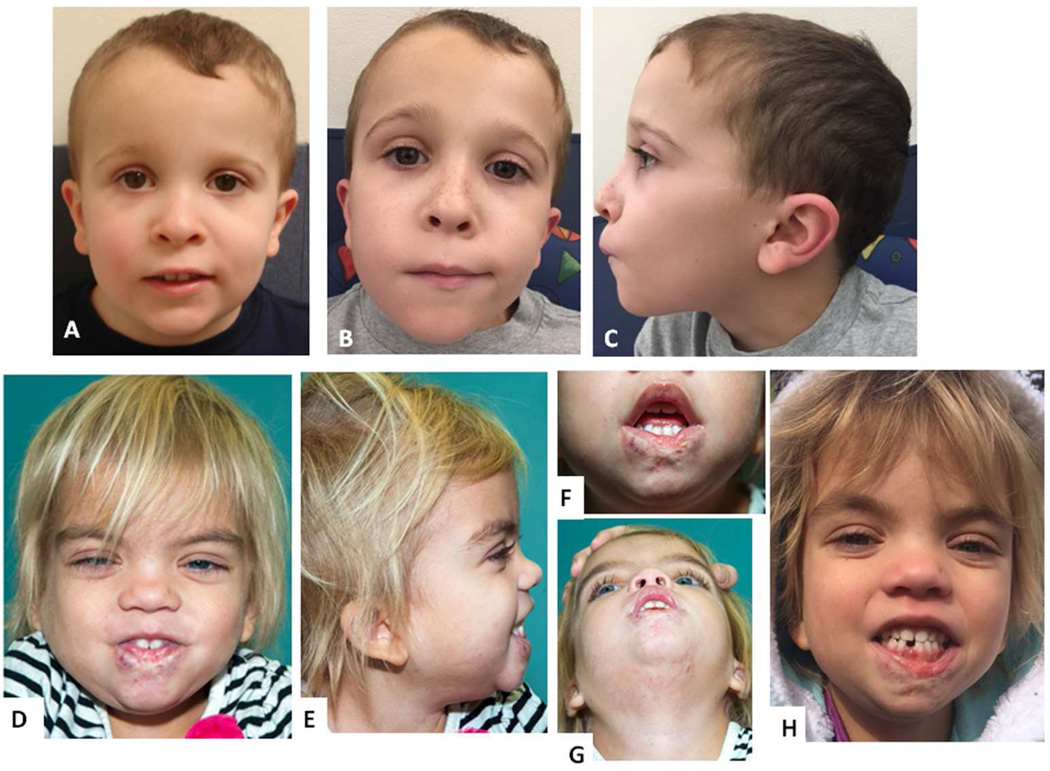
Figure 2.
Facial photographs of Patient 2 at ages 5 5/12 years (A) and 9 years (B, C), note low-set, posteriorly angulated ears; facial photographs of Patient 3 at age 4 years, note hypertelorism and epicanthus, fine blond hair, low-set ears (D, E), scarring of her lower lip after hemangioma treatment (F, G), and at age 5 years (H).
Development was delayed with independent walking at 19 months. He received speech, physical and occupational therapies and special education services. Cognitive testing using the Wechsler Intelligence Scale for Children (WISC-V) performed at 8 5/12 years showed a full scale IQ of 87 (low average), with significantly decreased processing speed which was considered uninterpretable due to the variability in his performance on the subtests. At 9 years he attended 3rd grade in a regular classroom setting with an individualized education plan and occupational, physical and speech therapy. He had preferential seating due to the suspected hearing loss.
Developmental delay and intention tremor resulted in a brain MRI at 5 5/12 years, which was reported as “subtle inferior vermis hypoplasia” and “small arachnoid cyst in the extra-axial space posterior and medial to the right thalamus”. A re-evaluation at age 8 years did not show vermis hypoplasia. A cardiology evaluation at age 5 years included an echocardiography and ECG and had normal results. Bone age studies showed delayed bone age appropriate for age 6 years at chronological age 8 8/12 years.
At 9 years, his height was 114 cm (<2nd centile; 50th centile for age 5.5 years; Z= −3.4) (supplemental Figure 1B), weight was 20.2 kg (Z= −2.69) and OFC 52.4 cm (50th centile). His facial features (Figure 2 A-C) included a tall forehead with frontal upsweep of his coarse hair, mild apparent hypertelorism, a long and smooth philtrum and borderline low-set ears with full lobes bilaterally. His neck appeared short, but not wide. Mild pectus excavatum was present. He had mildly low muscle tone with normal bulk and symmetric reflexes. Several faint café-au-lait spots were present.
Patient 3 (LR16–100)
This girl (Figure 2 D-H) was born to a G1 21-year-old mother and 24-year-old father. Her mother had a history of two seizures and had an infantile hemangioma that resolved at about 9 months of age. Her ancestry was Caucasian and Filipino. Patient 3’s father had pyloric stenosis and was of Caucasian ancestry. There was no known consanguinity, and neither parent had features suggestive of Noonan syndrome.
The pregnancy was complicated at 11 weeks by increased nuchal translucency. This was re-evaluated by serial ultrasounds and resolved by 27 weeks gestation. Edema of the face and anterior neck was identified prenatally, and postnatally found to be caused by a hemangioma involving the lip, chin and chest. Patient 3 was born vaginally at 35 weeks gestation, with a birth weight of 2.83 kg (75th-90th centile for gestational age) and birth length of 46 cm (50th centile for gestational age). OFC was not recorded. She had difficulty coordinating suck and swallow, partially attributed to the hemangioma, and required a feeding tube for two weeks. Propanolol treatment for her hemangioma was begun at age 2 weeks. An echocardiogram identified pulmonic valve stenosis, and repeat echocardiogram in the first year showed mild pulmonic stenosis.
Hypotonia, recurrent cough with pneumonia and dysmorphic features were recognized in infancy. Developmental delay manifested with sitting independently at 11 months and independent walking at 20 months. She had speech delays, with a two-word vocabulary at 22 months. The hemangioma may have contributed to the speech delay. A brain MRI performed at 1 7/12 years due to relative macrocephaly and developmental delay showed mildly enlarged third and lateral ventricles and a mildly thin corpus callosum, but no cortical malformation.
At age 2 years coarse facial features led to consideration of a lyosomal storage disorder. An eye examination had normal results and a skeletal survey identified coxa valga bilaterally. Urine oligosaccharides, free glycan, and leukocyte-based lysosomal enzyme screen were normal (Table I). A SNP array (Affymetrix Cytoscan HD, 2.67 million probes) had a normal female result, and a Noonan gene panel identified a variant of uncertain significance (VUS) in BRAF: NM_004333:c.967T>C, resulting in p.Ser323Pro (Table I). Parental testing showed this BRAF variant to be maternally inherited and therefore unlikely to be pathogenic.
The hemangioma was debulked at age 3 years. Tonsilladenoidectomy with placement of typanostomy tubes was performed at 4 years and her chronic cough and pneumonias improved thereafter. Audiology evaluation showed mild hearing loss at lower (500 Hz) frequencies. A renal ultrasound was normal.
Evaluation at 4 7/12 years showed further development without regression. Her weight was at the 25th centile, with height maintaining its trajectory along the 3rd centile (supplemental Figure 1C) and head circumference at the 98th centile. Her dysmorphic features included hypertelorism (interpupillary distance at +2.1 SD), a flat and retracted midface with a broad nasal root, epicanthus and low-set and posteriorly angulated ears. Ectodermal findings included areas of irregular hypopigmentation on her back and lower legs, and very fine and blonde hair on her scalp. Her scalp hair appeared quite different from her eyebrows, which were dark and bushy (Figure 2). Her hair grew slowly and fell out easily, and she had never needed a haircut.
Patient 4 (LR06–085)
This young woman (Figure 3) was born to her 31-year-old mother and 37-year-old father after a pregnancy complicated by a brief febrile illness in the late first trimester. Her Caucasian parents were not consanguineous and an older brother was in good health. Her father had bilateral pre-auricular ear pits and had unilateral surgical intervention for conductive hearing loss at age 10 years.
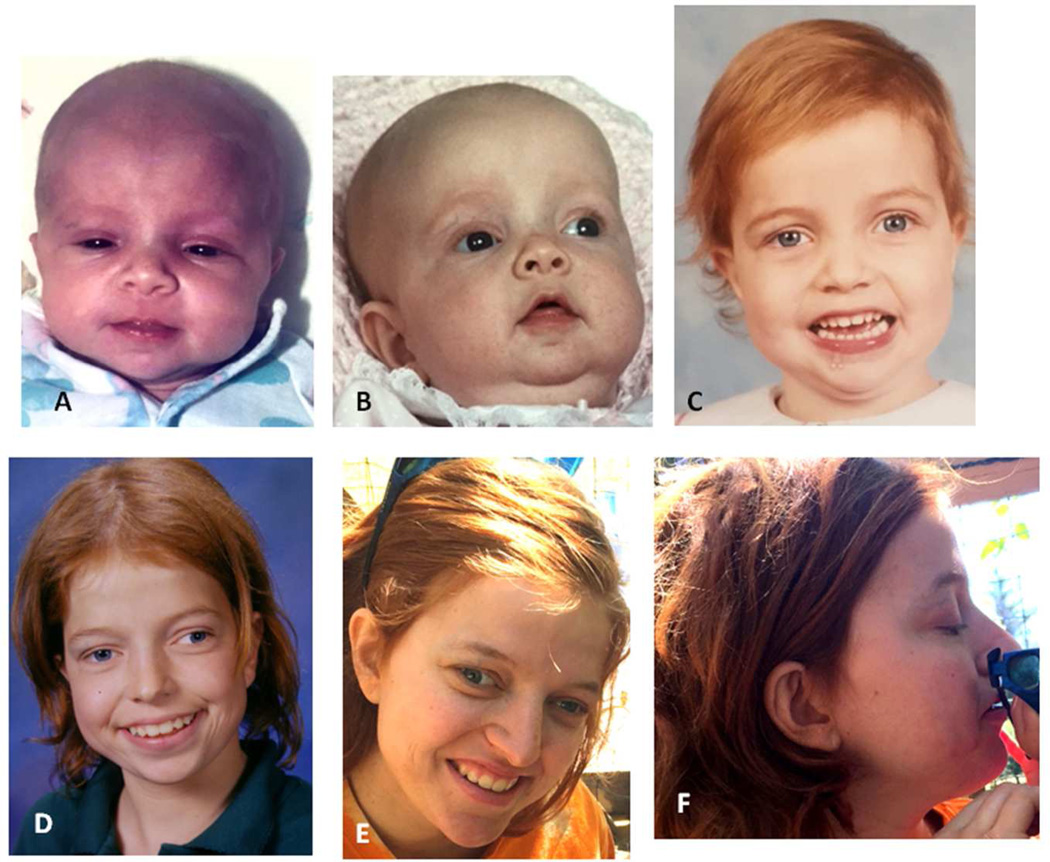
Figure 3.
Facial photographs of Patient 4 as neonate (A), at age 3 months (B), 4 years (C), teenager (E) and age 21 years (F, G), note unruly hair texture, apparent hypertelorism and low-set, posteriorly angulated ears.
Uncomplicated vaginal delivery occurred at term. Birth weight was 3.91 kg (75th centile), length 53.4 cm (75th-90th centile) and OFC 38.1 cm (>97th centile; 50th centile for age 2 months). She had no neonatal problems and breastfed without difficulties. From early infancy she cried unless held or carried.
Bilateral preauricular ear pits required excision at age 5 years after recurrent infections. She did not have hearing loss. Her skin was dry with eczema, and her hair was very sparse with an unusual texture described as straw-like. The hair grew very slowly, broke easily and at times appeared to be pulled out painlessly upon brushing. The patient had two typical haircuts by age 21 years, additionally she had occasional trimming of her hair ends.
At age 4.5 years an eye examination showed bilateral optic nerve hypoplasia, nystagmus and poor peripheral vision in her left eye. Cortical visual impairment was diagnosed. A brain MRI study was performed at age 6 7/12 years demonstrated a mildly prominent forehead, mildly enlarged 3rd and lateral ventricles, and classic Dandy-Walker malformation (DWM) with small, bi-lobed and upwardly rotated vermis, and markedly enlarged posterior fossa. The cerebellar abnormalities (Supplemental Figure 2 I, J) were considered causally related to her poor and inconsistent motor coordination. Her development was delayed with walking independently at age 18 months. She was able to use 2-word sentences at age 2 years. Schooling was complicated by poor motor skills, impaired vision, emotional problems and delayed social skills. Her activity level was very high with a short attention span. She attended a combined classroom with the help of a one-on-one aide. Subsequently she was in a mainstream class with additional special education service. She required special education for reading until 8th grade and had poor retention of visually presented information. During high school she was exclusively in a mainstream classroom but received extensive help outside of the school system. At age 21 years she attended college independently, but required significant family support. She had a discrepancy between her intellectual abilities and her performance, with difficulties managing emotions and requiring medication for anxiety and frequent headaches. At age 21 years her self-reported height was 167 cm (50–75th centile), weight 63.5 kg (50–75th centile) and OFC 58.4 cm (>3 SD above mean for adult females). Facial features included macrocephaly, hypertelorism and low-set posteriorly angulated ears (Figure 3 A-F).
RESULTS
Exome sequencing identified de novo heterozygous missense mutations in PPP1CB in all four patients. Three (Patients 1, 3 and 4) had the same, recurrent mutation: c.146G>C, predicted to result in a p.Pro49Arg substitution (NM_206876.1:c.146C>G: p.Pro49Arg). Patient 2 had a c.166G>C mutation predicted to result in a p.Ala56Pro substitution (NM_206876: c.166G>C: p.Ala56Pro) in exon 3. The variants were confirmed in each proband by Sanger sequencing. Both substitutions affect conserved amino acids and were predicted to be likely damaging by in silico analyses. Specifically, the recurrent PPP1CB c.146G>C, p.Pro49Arg variant was considered damaging by PolyPhen2 (0.953), Mutation Taster (1.0) and SIFT (1). The PPP1CB c.166G>C, p.Ala56Pro variant was considered damaging by PolyPhen2 (0.941), Mutation Taster (1.0) and SIFT (0.99).
Neither variant was present in the 1000 Genomes, NHLBI Exome Variant Server, Exome Aggregation Consortium or dbSNP version 137 databases. Using the standards for the interpretation of sequence variants outlined by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [Richards et al., 2015] and optimized for rasopathies by Vincent et al. [2016], these variants collectively meet the standard pathogenicity due to fulfilling two strong criteria for pathogenicity (PS), namely occurring de novo with confirmed paternity and maternity in an individual with a consistent phenotype and without a family history (PS2), and by the prevalence of the variant in affected individuals being significantly increased compared to the prevalence in controls (PS4).
The three individuals in whom exome analyses were performed clinically (Patients 1–3) shared a clinical diagnosis of Noonan syndrome or related rasopathy, and each had previous testing for genes related to rasopathies (Table I). Delayed development, relative or absolute macrocephaly, hair abnormalities and low-set, posteriorly angulated ears were seen in all four individuals. Short stature, feeding difficulties and apparent or documented hypertelorism were present in three. Very slow growing hair with a fine or unruly hair texture was seen in three individuals, while one had an unruly hair texture with normal growth.
Brain MRI studies (Table II) (supplemental Figure 2) showed only mild abnormalities except for a classic Dandy-Walker malformation with small bi-lobed and upwardly rotated vermis, and markedly enlarged posterior fossa in patient 4. Other findings included prominent forehead in all four, mild ventriculomegaly in three, and subtle downward displacement of the cerebellar tonsils to fill the low posterior fossa in two individuals, progressing to a Chiari 1 malformation in one (supplemental Figure 2).
DISCUSSION
We identified de novo missense mutations in PPP1CB in four individuals, with three sharing a recurrent mutation. Three patients were ascertained due to clinical overlap with Noonan syndrome. Noonan syndrome was considered in these patients due to the combination of short stature, macrocephaly, low-set and posteriorly angulated ears and developmental delay, but sequencing did not identify a pathogenic mutations in the 12 known Noonan syndrome/rasopathy causing genes. A fourth individual (Patient 4) was ascertained into a research study due to her Dandy-Walker malformation (DWM), although retrospectively this individual had features of Noonan syndrome, including macrocephaly, developmental delay, low-set, posteriorly angulated ears, and hypertelorism. It is not known if Patient 4’s DWM is causally related to the PPP1CB mutation. Of the three patients who underwent echocardiography, one had no structural heart defect (Patient 2) and two had mild defects (mitral valve thickening in Patient 1 and mild pulmonic valve stenosis in Patient 3). Although cardiac anomalies are common in Noonan syndrome, they are not seen in all patients. Structural anomalies are more frequent in individuals with PTPN11 mutations, and hypertrophic cardiomyopathy is particularly common in individuals with RAF1 [Roberts et al., 2013] or RIT1 mutations [Yaoita et al., 2016]. Cryptorchidism, common in Noonan syndrome, occurred in one of two male individuals reported here. Interestingly, all four patients had some hair abnormality (slow growing, very fine or unruly hair texture), which may be a major clinical clue suggesting a PPP1CB-related disorder.
Similarities to Noonan syndrome with loose anagen hair
Three of the individuals reported here have very slow growing hair with an unruly texture. Patient 3’s parents reported that her hair “falls out easily”. Patient 3’s sparse, light blond hair with an unruly texture (Fig. 2 D-H) not requiring a hair cut to her current age 5 years is suggestive of loose anagen hair. Patient 4 reported that her hair is pulled out easily without inflicting pain. These characteristics are suggestive of loose anagen hair, an abnormality resulting from abnormal root sheaths. Loose anagen hair is seen in most individuals with a recurrent point mutation in SHOC2 resulting in Noonan syndrome with loose anagen hair (NS-LAH) [Cordeddu et al., 2009; Gripp et al., 2013; Baldassare et al., 2014], in Costello syndrome due to the HRAS p.G13C mutation [Gripp et al., 2011] and rarely in cardio-facio-cutaneous syndrome [Wong Ramsey et al., 2014]. Short stature, often due to growth hormone deficiency, is common in NS-LAH. Three patients reported here had short stature, none had growth hormone (GH) deficiency. One started GH treatment (Patient 1), but no growth data after treatment initiation were available at the time of this report. Ocular abnormalities occurred in three individuals reported here, these are common in patients with NS-LAH [Baldassare et al., 2014]. Relative or absolute macrocephaly with mildly enlarged ventricles and arachnoid spaces are seen in NS-LAH [Gripp et al., 2013] and were present in all patients reported here. Patient 1 had cerebellar tonsillar ectopia progressing to Chiari 1 malformation, Chiari 1 malformation has been reported in several individuals with NS-LAH [Gripp et al., 2013]. While the individuals reported here had faint café-au-lait lesions or hyperpigmented lesions and freckles developing over time (Table I), they did not show the generalized hyperpigmentation often noted in individuals with NS-LAH. While hyperkeratosis was reported in some patients with NS-LAH [Cordeddu et al., 2009], no individual reported here had hyperkeratosis. Attention deficit hyperactivity disorder (ADHD) is often seen in individuals with NS-LAH. No individual reported here was formally diagnosed with ADHD, however, each had developmental, learning or behavior difficulties. Patient 1 reported here had a mitral valve abnormality. While mitral valve abnormalities are uncommon in Noonan syndrome in general, 8 of 24 individuals with NS-LAH had mitral or tricuspid anomalies [Cordeddu et al., 2009] and 1 of 5 reported in Gripp et al. [2013] had a mitral valve anomaly.
PPP1CB mutations in the literature
PPP1CB is highly expressed in the human brain throughout development [Johnson et al., 2009]. In a cohort of 41 individuals with moderate to severe intellectual disability, exome sequencing in patients and parents identified a de novo PPPC1B mutation in one patient with 1 bp insertion (c.909dupA) predicted to cause a frameshift and premature truncation of the protein (p.Tyr450Ilefs*92) [Hamdan et al., 2014]. The limited information provided on this patient included severe ID, short stature and relative macrocephaly, a large mouth and malar hypoplasia. Mildly increased cerebral spinal fluid spaces were reported. As the mutation in the patient reported by Hamdan et al. [2014] is predicted to result in a premature protein termination, it is not known if the effect on the abnormal protein product is comparable to that of the missense mutations reported here.
PPP1CB and SHOC2
Protein phosphatase 1 (PP1) is a serine/threonine specific phosphatase. Protein phosphatases counterbalance kinases, controlling the level of phosphorylation and related activation of signaling proteins, such as the mitogen activated protein kinases of the RAS/MAPK pathway. Protein phosphatase 1 has three catalytic subunits, alpha, beta (encoded by PPP1CB) and gamma. PP1C and SHOC2 form a complex which, after stimulation by MRAS, dephosphorylates RAFs at an inhibitory serine, resulting in activation of the signaling cascade [Abraham et al., 2000; Rodriguez-Viciana et al., 2006; Young et al., 2013]. Rodriguez-Viciana et al. [2006] concluded that Shoc2 is a regulatory subunit of PP1C, controlling the activity of the PP1 catalytic site to specifically target the Ser259 phosphorylation site of CRAF/RAF1. The subsequent dephosphorylation of Raf results in ERK activation. This data demonstrates a clear role for PPP1CB in the regulation of the RAS/MAPK pathway activation. Since the p.Ser2Gly substitution in SHOC2 causing NS-LAH is predicted to result in sustained RAF1-stimulated MAPK activation [Cordeddu et al., 2009], we speculate that the PPP1CB mutations described here are likely to result in MAPK activation by activating RAF though increased dephosphorylation of the inhibitory serine residue [Young et al., 2006], but further studies are necessary to prove this. The PPP1CB amino acid substitutions may, for example, result in enhanced substrate binding by the PP1/SHOC2 complex or in its prolonged activation after stimulation. Functional studies are beyond the scope of this article. At the least, the direct interaction between SHOC2 and PPP1CB provides a molecular correlate to the notable phenotypic similarities between the patients with NS-LAH and the phenotype reported here.
Brain abnormalities and optic nerve hypoplasia in rasopathies
Structural brain abnormalities, in particular Chiari type I malformations, have been reported in several individuals with Noonan syndrome [Holder-Espinasse and Winter, 2003; Beier et al., 2009; Gripp et al., 2013; Keh et al., 2013; Zarate et al., 2014; Mitsuhara et al., 2014, Ejarque et al., 2015], but the true incidence of Chiari I in Noonan syndrome is unknown. Two patients reported here had mild cerebellar tonsillar ectopia, with the cerebellum, particularly the cerebellar tonsils, filling the posterior fossa. This progressed to a mild Chiari 1 malformation in one. Patient 4 had a Dandy-Walker malformation, bilateral preauricular pits, and optic nerve hypoplasia. Her preauricular pits are unlikely to be due to her PPP1CB mutation, because her father also had preauricular pits but did not have a PPP1CB mutation. It is unknown if Patient 4’s DWM is functionally related to her PPP1CB mutation. Dandy-Walker malformation is a developmental abnormality of the cerebellum not typical for Noonan syndrome or NS-LAH. However, DWM occurred in an individual with a very rare HRAS mutation (p.S89C) [Gripp et al., 2012]. This contrasts with the cerebellar tonsillar herniation or Chiari 1 malformation typically seen in individuals with the most common Costello syndrome causing HRAS mutations [Gripp et al., 2010]. In light of the DWM in a patient with a rasopathy [Gripp et al., 2012] and the possibility that DWM and Chiari I are caused by dysregulation of posterior fossa mesenchyme [Aldinger et al., 2009], it is tempting to speculate that Chiari I and DWM are rare manifestation of rasopathies. Sequencing of PPP1CB in larger DWM and Chiari 1 cohorts will be required to address this.
Optic nerve hypoplasia, as seen in Patient 4, was noted in one individual with NS-LAH as “small optic nerves on MRI” [Patient I in Gripp et al., 2013], is relatively common in patients with cardio-facio-cutaneous syndrome [Pierpont et al., 2014]. Thus, this is probably related to the PPP1CB mutation.
Recurrent and unique PPP1CB mutations
Three individuals share a recurrent mutation affecting the proline residue in position 49, while the fourth (Patient 2) has a mutation affecting the alanine residue in position 56 of this 327 amino acid protein product. While Patient 2 is the only individual without slow growing hair and has subjectively the least distinctive facial features, this does not necessarily suggest mutation specific findings. Patient 2 shows the short stature and relative macrocephaly similar to Patients 1 and 3, has learning difficulties and mild pectus excavatum. Larger cohort studies are required to elucidate phenotypic difference between patients with the recurrent mutation and other missense mutations in PPP1CB.
Conclusions
Missense mutations in PPP1CB are associated with a phenotype resembling NS-LAH. Based on this phenotype and PPP1CB’s known role within the RAS/MAPK pathway, we think that this condition is a novel rasopathy, which may be provisionally termed “PPP1CB-related Noonan syndrome with loose anagen hair” (P-NS-LAH). While the loose anagen hair is a distinctive finding shared between SHOC2 and PPP1CB related conditions, the growth hormone deficiency often seen in individuals with a SHOC2 mutation was absent in the patients reported here. Analysis of phenotypic findings in additional individuals with PPP1CB mutations will determine if PPP1CB should be considered a second locus for NS-LAH, or if P-NS-LAH is a distinctive condition. Similarly, larger cohort studies are required to elucidate the relative frequency of the recurrent mutation p.Pro49Arg and mutation specific phenotypic variations. This PPP1CB-related condition should be considered in the differential diagnosis of NS-LAH and other rasopathies.
Supplementary Material
Acknowledgments
We thank the patients and their families for sharing this information. Genematcher (genematcher.org) was instrumental in establishing the connection between the investigators. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grants number P30GM114736 (COBRE) and P20GM103446 (INBRE), the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number R01NS050375 (to W.B.D.), an internal award from Seattle Children’s Research Institute and a Burroughs Wellcome Fund Career Award for Medical Scientists (to J.T.B.), and by the Dandy-Walker Alliance. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W, Baccarini M. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem. 2000;275:22300–22304. doi: 10.1074/jbc.M003259200. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Elsen GE, Prince VE, Millen KJ. Model organisms inform the search for the genes and developmental pathology underlying malformations of the human hindbrain. Semin Pediatr Neurol. 2009;16:155–163. doi: 10.1016/j.spen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre G, Mussa A, Banaudi E, Rossi C, Tartaglia M, Silengo M, Ferrero GB. Phenotypic variability associated with the invariant SHOC2 c.4A>G (p.Ser2Gly) missense mutation. Am J Med Genet A. 2014;164:3120–3125. doi: 10.1002/ajmg.a.36697. [DOI] [PubMed] [Google Scholar]
- Beier AD, Barrett RJ, Burke K, Kole B, Soo TM. Leopard syndrome and Chiari type I malformation: A case report and review of the literature. Neurologist. 2009;15:37–39. doi: 10.1097/NRL.0b013e31817833c4. [DOI] [PubMed] [Google Scholar]
- Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, Fodale V, Cecchetti S, Cardinale A, Martin J, Schackwitz W, Lipzen A, Zampino G, Mazzanti L, Diglio MC, Martinelli S, Flex E, Lepri F, Bartholdi D, Kutsche K, Ferrero GB, Anichini C, Selicorni A, Rossi C, Tenconi R, Zenker M, Merlo D, Dallapiccola B, Iyengar R, Bazzicalupo P, Gelb BD, Tartaglia M. Mutation of SHOC2 promotes aberrant protein N-myristolation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejarque I, Millan-Salvador JM, Oltra S, Pesudo-Martinez JV, Beneyto M, Perez-Aytes A. Arnold-Chiari malformation in Noonan syndrome and other syndromes of the RAS/MAPK pathway. Rev Neurol. 2015;60:408–412. [PubMed] [Google Scholar]
- Gripp KW, Bifeld E, Stabley DL, Hopkins E, Meien S, Vinette K, Sol-Church K, Rosenberger G. A novel HRAS substitution (c.266C>G; p.S89C) resulting in decreased downstream signaling suggests a new dimension of RASpathway dysregulation in human development. Am J Med Genet A. 2012;158A:2106–2118. doi: 10.1002/ajmg.a.35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Doyle D, Dobyns WB. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: Brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A. 2010;152A:1161–1168. doi: 10.1002/ajmg.a.33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, Dobyns WB, Hudson C, Johnson J, Tenconi R, Graham GE, Sousa AB, Heller R, Piccione M, Corsello G, Herman GE, Tartaglia M, Lin AE. Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. Am J Med Genet A. 2011;155A:706–716. doi: 10.1002/ajmg.a.33884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Zand DJ, Demmer L, Anderson CE, Dobyns WB, Zackai EH, Denenberg E, Jenny K, Stabley DL, Sol-Church K. Expanding the SHOC2 mutation associated phenotype of Noonan syndrome with loose anagen hair: Structural brain anomalies and myelofibrosis. Am J Med Genet A. 2013;161:2420–2430. doi: 10.1002/ajmg.a.36098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Srour M, Capo-Chichi JM, Daoud H, Nassif C, Patry L, Massicotte C, Ambalavanan A, Spiegelman D, Diallo O, Henrion E, Dionne-Laporte A, Fougerat A, Pshezhetsky AV, Venkateswaran S, Rouleau GA, Michaud JL. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10(10):e1004772. doi: 10.1371/journal.pgen.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder-Espinasse M, Winter RM. Type 1 Arnold-Chiari malformation and Noonan syndrome: A new diagnostic feature? Clin Dysmorphol. 2003;12:275. doi: 10.1097/00019605-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keh YS, Abernethy L, Pettorini B. Association between Noonan syndrome and Chiari 1 malformation: a case based update. Childs Nerv Syst. 2013;29:749–752. doi: 10.1007/s00381-012-2000-9. [DOI] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimationg the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti L, Cacciari E, Cicognani A, Bergamaschi R, Scarano E, Forabosco A. Noonan-like Syndrome with Loose anagen Hair: A New Syndrome? Am J Med Genet. 2003;118A:279–286. doi: 10.1002/ajmg.a.10923. [DOI] [PubMed] [Google Scholar]
- Mitsuhara T, Yamaguchi S, Takeda M, Kurisu K. Gowers’ intrasyringeal hemorrhage associated with Chiari type I malformation in Noonan syndrome. Surg Neurol Int. 2014 doi: 10.4103/2152-7806.125546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME, Magoulas PL, Adi S, Kavamura MI, Neri G, Noonan J, Pierpont EI, Reinker K, Roberts AE, Shankar S, Sullivan J, Wolford M, Conger B, Santa Cruz M, Rauen KA. Cardio-facio-cutaneous syndrome: Clinical features, diagnosis, and management guidelines. Pediatrics. 2014;134:e1149–e1162. doi: 10.1542/peds.2013-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, Vertino-Bell A, Smaoui N, Neidich J, Monaghan KG, McKnight D, Bai R, Suchy S, Friedman B, Tahiliani J, Pineda-Alvarez D, Richard G, Brandt T, Haverfield E, Chung WK, Bale S. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2015 doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381:333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Oses-Prieto J, Burlinghame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Molecular Cell. 2006;22:217–230. doi: 10.1016/j.molcel.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Vincent LM, Mason-Suares H, Mao R, Dillon MW, Williams B, Smpokou P, Gripp KW, Rauen KA, Roberts AE, Gelb BD, Bale S. Optimization and Utilization of ACMG variant classification criteria for the rasopathies: A ClinGen Initiative. American College of Medical Genetics and Genomics; Annual meeting 2016; March 8-12th; Tampa, FL. [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acid Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Ramsey KN, Loichinger MH, Slavin TP, Kuo S, Seaver LH. The perinatal presentation of cardiofaciocutaneous syndrome. Am J Med Genet Part A. 2014;164A:2036–2042. doi: 10.1002/ajmg.a.36558. [DOI] [PubMed] [Google Scholar]
- Yaoita M, Niihori T, Mizuno S, Okamoto N, Hayashi S, Watanabe A, Yokozawa M, Suzumura H, Nakahara A, Nakano Y, Hokosaki T, Ohmori A, Sawada H, Migita O, Mima A, Lapunzina P, Santos-Simarro F, García-Miñaúr S, Ogata T, Kawame H, Kurosawa K, Ohashi H, Inoue S, Matsubara Y, Kure S, Aoki Y. Spectrum of mutations and genotype-phenotype analysis in Noonan syndrome patients with RIT1 mutations. Hum Genet. 2016;135:209–222. doi: 10.1007/s00439-015-1627-5. [DOI] [PubMed] [Google Scholar]
- Young LC, Hartig N, Muñoz-Alegre M, Oses-Prieto JA, Durdu S, Bender S, Vijayakumar V, Vietri Rudan M, Gewinner C, Henderson S, Jathoul AP, Ghatrora R, Lythgoe MF, Burlingame AL, Rodriguez-Viciana P. An MRAS, SHOC2, and SCRIB complex coordinates ERK pathway activation with polarity and tumorigenic growth. Mol Cell. 2013;52:679–692. doi: 10.1016/j.molcel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Zarate YA, Lichty AW, Champion KJ, Clarkson LK, Holden KR, Matheus MG. Unique cerebrovascular anomalies in Noonan syndrome with RAF1 mutation. J Child Neurol. 2014;29:13–17. doi: 10.1177/0883073813492384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.