Abstract
Background
The aim of our study was to evaluate all lesions in the adenoma-dysplasia-cancer sequence of the colon and to examine whether the neutrophil-to-lymphocyte ratio (NLR) can distinguish polyps indicating dysplasia and cancer.
Material/Methods
A total of 397 patients who had colonoscopic polypectomy between January 2010 and December 2014 were included in our retrospective study. The patients were divided into four groups: patients with hyperplastic polyps, patients with adenomatous polyps, patients with dysplasia, and patients with cancer. The NLR was calculated as a simple ratio indicating the relationship between counts of absolute neutrophil and absolute lymphocyte.
Results
The NLR increased in line with the adenomatous polyp-dysplasia-cancer sequence, with the highest ratio established among cancer patients (2.05 (0.27–10), 2.34 (0.83–14.70) and 3.25 (0.81–10.0), respectively). The NLR was significantly higher among cancer patients than among patients with adenomatous polyps and hyperplastic polyps (p values were 0.001 and 0.004, respectively). The lymphocyte count of cancer patients was prominently lower when compared to those in groups with adenomatous polyps and hyperplastic polyps (p values were 0.001 and 0.003, respectively). The NLR was found to be significantly higher in patients with polyps larger than 10 mm [2.71 (0.90–14.70)] when compared to those with polyps smaller than 10 mm [2.28 (0.27–11.67)] (p<0.001). With the NLR threshold set at 2.20, it was possible to predict cancerous polyps with a sensitivity of 71.4% and a specificity of 52.5% (AUC: 0.665, 95% CI: 0.559–0.772, p=0.001).
Conclusions
NLR is a cheap, universally available, simple and reliable test that can help predict cancerous polyps. It can be used as a non-invasive test for monitoring polyps.
MeSH Keywords: Colonic Polyps, Colorectal Neoplasms, Lymphocyte Count, Neutrophils
Background
Colorectal cancer (CRC) occupies an important place among cancers that affect both men and women; it ranks third among all cancers in terms of its incidence among both men and women, as well as being the third most common cause of death from cancer in the USA [1]. The estimated number of new cases and of deaths from CRC in the USA was reported to be 136,830 and 50,310, respectively [1]. Changes in lifestyle have led to a rapid increase in CRC incidence in developing countries [2].
The majority of CRCs are caused by adenomas. Many adenomas are polyps which advance from small to large (>1 cm) size, and then to dysplasia and then cancer [3]. Many colorectal polyps are either adenomatous or hyperplastic. Gross appearance cannot be used to distinguish these forms reliably, and diagnosis requires a biopsy and pathology evaluation. If adenoma advances to carcinoma, the process can take at least 10 years, on average [4].
The association between cancer and inflammation has been known for some time [5]. A number of inflammatory tests, including CRP and Glasgow prognostic score, have been found to correlate with poor prognosis of cancer patients [6]. As a reflection of systemic inflammation, the neutrophil-to-lymphocyte ratio (NLR) was initially established as a prognostic factor among patients in intensive care, then found also to be associated with poor prognosis in various types of cancer, including colon cancer [7–10]. CRC has a low morbidity and mortality rate when diagnosed at early stages, and it is possible to treat CRC through surgery in these early cancer stages. Since hematological testing is a routine procedure for most patients, NLR constitutes a simple, reliable, and easy-to-use indicator for the inflammatory response. The aim of the present study was to conduct research into the relationship between NLR and the colorectal adenoma–dysplasia-cancer sequence.
Material and Methods
The study sample included 397 patients who had colonoscopic polypectomy between January 2010 and December 2014 at the Endoscopy Unit of the Faculty of Medicine, Dicle University. This study was approved by the Ethics Board of the Faculty of Medicine of Dicle University.
Patient pre-operative demographic information and laboratory results of these patients was obtained retrospectively through the hospital’s automated record system. The study excluded patients with active infectious diseases, hematological or solid organ tumors, obstructive or ulcerated lesions identified distinctly in colonoscopy, more than 15 polyps, use of cyclooxygenase-2 (COX-2) inhibitors, and a history of surgery for colon tumors. The patients were divided into four groups: patients with hyperplastic polyps (Group A), patients with adenomatous polyps (Group B), patients with adenomatous polyps indicating dysplasia (Group C), and patients with polyps diagnosed as adenocarcinoma (Group D).
Hematological parameters were measured using an automated hematology analyzing system (Abbott Cell-Dyn 3700; Abbott Laboratory, Illinois, USA). The NLR was calculated by the division of the neutrophil count by the lymphocyte count.
Statistical analysis
Statistical analyses were performed using the SPSS 18.0 for Windows software package (SPSS Inc., Chicago, IL, USA). The normality of data was evaluated using the Kolmogorov-Smirnov test. Descriptive statistics pertaining to continuous variables were indicated as average and standard deviation (SD) values. Variables that lacked a normal distribution were expressed as mean (min–max range) values. Variance analysis (ANOVA) was used for variables with normal distributions during the comparison of three groups. The Tukey test was used for subgroup analysis. The Kruskall-Wallis test was used for variables that lacked normal distributions during the comparison of three groups, and the Mann-Whitney U test was used for subgroup comparisons. The threshold value of NLR in the prediction of polyps indicating dysplasia-cancer was identified by receiver operating characteristic (ROC) curve analysis. Hypotheses were bidirectional and p<0.05 was considered statistically significant.
Results
The age range of the patients was 17–90 years and the mean age was 57.7±15.7 years. Of the 379 patients, 240 (60.4%) were male. In all, 31.5% of the patients had hyperplastic polyps (Group A, n=125); 54.7% had adenomatous polyps (Group B, n=217: 185 tubular, 37 tubulovillous and 5 villous); 5.0% of patients had polyps indicating dysplasia (Group C, n=20), and 8.8% had polyps indicating cancer (Group D, n=35).
The ratio of smoking was 26.7% among men and 8.6% among women (p<0.001). The rates of smoking for patients in Groups A, B, C, and D were 41%, 34%, 25%, and 33%, respectively (p=0.49). No correlation was identified between dysplasia, cancer, and smoking. Only 7.3% of patients had used aspirin before their colonoscopy. There was no statistically significant difference between groups (p=0.89).
Table 1 presents the median (min–max range) for white blood cells, neutrophils, lymphocytes, and NLR values of the groups. The comparison of the groups showed significant differences only in the lymphocyte count and NLR (p values were 0.002 and 0.007, respectively). NLR values increased in accordance with the adenomatous polyp–dysplasia-cancer sequence (2.05 (0.27–10), 2.34 (0.83–14.70) and 3.25 (0.81–10.0), respectively). When the groups were compared, the lymphocyte count was significantly lower among cancer patients than among the groups with hyperplastic and adenomatous polyps (p values were 0.011 and 0.003, respectively). The lymphocyte count was significantly lower among patients with dysplasia only when compared to the adenomatous group (p=0.009).
Table 1.
Statistical analysis of the groups.
| WBC count* (×103/μL) | Neutrophil count* (×103/μL) | Lymphocyte count* (×103/μL) | NLR* | |
|---|---|---|---|---|
| Group A (n=125) | 7.600 (1.300–18.000) | 4.600 (600–13.000) | 2.000 (500–5.600) | 2.24 (0.81–11.67) |
| Group B (n=217) | 7.300 (2.600–20.000) | 4.400 (570–18.000) | 2.100 (520–10.000) | 2.05 (0.27–10.00) |
| Group C (n=20) | 6.400 (2.800–16.500) | 4.050 (1.700–14.700) | 1.700 (580–3.100) | 2.34 (0.83–14.70) |
| Group D (n=35) | 8.000 (3.500–16.000) | 5.700 (1.800–13.000) | 1.700 (860–3.700) | 3.25 (0.81–10.00) |
| p** | 0.263 | 0.158 | 0.002 | 0.007 |
| Meaningful comparisons# | None | 2 & 4 | 1 & 4, 2 & 3, 2 & 4 | 1 & 4, 2 & 4 |
WBC – white blood cell; NLR – neutrophil lymphocyte ratio, Group A – hyperplastic polips; Group B – adenomatous polyps; Group C – adenomatous Polyps with dysplasi; Group D – polyps with adenocancer.
All values are given as median (min-max range);
Comparasions was done by Kruskal Wallis test;
Comparasions was done by Man Whitney U test.
NLR comparison included age, sex, polyp size, and polyp location (Table 2). The only significant difference was in polyp size. NLR median value was found to be significantly higher among those with polyps larger than 10 mm [2.71 (0.90–14.70)] compared to those with polyps smaller than 10 mm [2.28 (0.27–11.67)] (p<0.001).
Table 2.
The relation between NLR and the clinical and demographic features of patients.
| NLR | p* | |
|---|---|---|
| Age (years) | ||
|
| ||
| <50 (n=124) | 2.21 (0.40–11.67) | 0.108 |
| >50 (n=273) | 2.15 (0.27–14.70) | |
|
| ||
| Gender | ||
|
| ||
| Female (157) | 2.14 (0.27–10.00) | 0.486 |
| Male (240) | 2.24 (0.40–14.70) | |
|
| ||
| Polyp size (mm) | ||
|
| ||
| <10 mm (n=305) | 2.28 (0.27–11.67) | <0.001 |
| >10 mm (n=92) | 2.71 (0.90–14.70) | |
|
| ||
| Localization | ||
|
| ||
| Right colon (n=42) | 2.13 (1.03–7.56) | 0.676 |
| Left colon (n=355) | 2.20 (0.27–14.70) | |
NLR – neutrophil lymphocyte ratio.
Comparasions was done by Man Whitney U test.
The relationship between NLR and cancerous polyps was evaluated by ROC curve analysis. With the NLR threshold value set at 2.20, it was possible to predict the diagnosis of cancerous polyps with a sensitivity of 71.4% and a specificity of 52.5% (AUC: 0.665, 95% CI: 0.559–0.772, p=0.001), (Figure 1).
Figure 1.
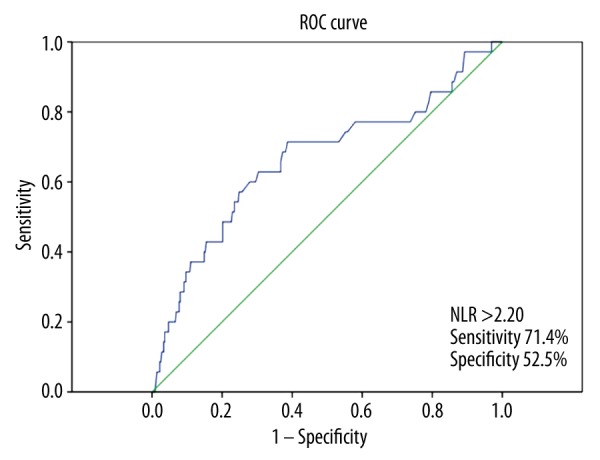
Receiver operating characteristic (ROC) curve analysis of NLR for predicting the presence of cancerous polyps.
Discussion
Previous studies have established the prognostic relationship between CRC and NLR. A limited number of studies have evaluated the relationship between polyps and NLR, and these studies addressed patients with neoplastic polyps and CRC patients separately. To the best of our knowledge, the present study is the first study that approaches non-neoplastic polyps and all lesions in the adenoma-dysplasia-cancer sequence of the colon together. The present study found a gradual increase in NLR as the patient approaches cancer diagnosis in line with the adenoma-dysplasia-cancer sequence. NLR was found to be significantly higher among cancer patients than among patients with neoplastic or non-neoplastic polyps. Again, with the NLR threshold set at 2.20, we found that cancerous polyps could be predicted with a sensitivity of 71.4% and a specificity of 52.5%. Furthermore, a significant relationship was established between polyp size, which represents an important factor in the progression from neoplastic polyps to cancer, and NLR.
The relationship between cancer and inflammation was reported by Virchow in the nineteenth century, and ample evidence has been gathered since then to support this relationship [5]. Levels of white blood cells, neutrophils, lymphocytes, and platelets in serum, and acute-phase proteins, including C-reactive protein (CRP) and albumin, are known indicators of a response to inflammation. These simple and easy-to-measure parameters are widely used as standard assays [11]. NLR can be used as a tool to indicate the balance between the activation of the pro-tumor inflammatory pathway and the anti-tumor function of the immune system. Current theories focus on the relative neutrophilia and lymphocytopenia that emerges as part of the cancer-induced systemic response to inflammation [12]. In malignant tumors, systematic inflammation is considered to be created by tumor hypoxia or necrosis and the relevant anti-apoptosis signaling pathway motivation [13]. Mechanisms proposed to explain neutrophilia include the release of G-CSF from tumor cells and inflammation in cancer through the release of IL-1 and TNF-alpha [14,15]. Relative neutrophilia leads to an increase in the number of inflammatory markers that incorporate pro-angiogenic factors (VEGF), growth factors (CXCL8), proteases (metalloproteinase tissue inhibitors), and anti-apoptotic markers (NF-κB) that extend support to tumor growth and progression [16–18]. Lymphocyte response is a prominent component of efforts to control cancer progression. A number of studies have established that a decrease in the T cell activity in tumors is correlated with the progression of the primary tumor [19]. It has been argued for some time that anti-tumor activity is primarily realized through the mediation provided by cellular immune reactions dependent on lymphocytes. Lymphopenia has been shown to be associated with disease severity and immunological escape of tumor cells from infiltrating lymphocytes [20–23]. The present study established a significant difference among the patient groups in terms of lymphocyte count, despite the absence of any significant difference identified among the groups in terms of the neutrophil count. According to inter-group comparisons, lymphocyte count was determined to be significantly lower among patients with cancer and dysplasia when compared to patients with non-dysplastic adenomatous polyps. This, in turn, signifies that the increase in NLR correlates with the decrease in lymphocyte count, as has been emphasized in the literature. An increased count of lymphocytes infiltrating tumors has been acknowledged as a factor predicting good prognosis [24]. It is possible to utilize NLR as a means to indicate the balance between the activation of the pro-tumor inflammatory pathway and the immunological anti-tumor function.
Neutrophils in tumor tissue have been shown in histopathology studies to be a factor for poor prognosis in patients with CRC [25]. In the present study, the high level of intratumoral CD66b neutrophils was positively correlated with pathologic and clinical stages. Neutrophils in the blood have also been found to be an independent prognostic marker for poor survival in CRC exhibiting metastasis [10]. One study found that for patients with advanced CRC who receive oxaliplatin-based chemotherapy, increased NLR predicted poor prognosis as an independent factor [26]. An increase in NLR (>5) also provided an independent prediction of poor prognosis for colorectal metastasis in the liver following percutaneous radiofrequency ablation [27]. A recent study identified the association of systemic inflammation before the operation (Glasgow prognostic score criteria), peritumoral inflammatory infiltrate, and cancer-specific survival among patients who had undergone resection with a remedial potential for colorectal cancer. In addition, the aforementioned study demonstrated that a low-grade peritumoral infiltrate was associated with an increase in the Dukes’ stage and in the total white cell count and neutrophil count [6]. Another recent study established a positive correlation between CRP and metastatic lymph node ratio, an independent indicator of prognosis among patients with stage III colon cancer [28]. The seemingly inverse proportion between markers of the response to systemic inflammation and the response to local inflammation most probably reflects the imbalances in the innate and adaptive immune systems that disrupt effective host-tumor immune responses.
In one study, the presence of increased inflammation was identified by histopathology in adenomatous and hyperplastic polyps [29]; however, both acute and chronic inflammation was found to be prominent only when compared to both normal tissue and hyperplastic polyps among adenomatous polyps with neoplastic potential [29]. Most colon cancers are known to be induced by adenomatous polyps with neoplastic potential. COX-2 proteins, which are negative in normal colon epithelia, increase in both adenomatous polyps and CRC cells [30]. Clinical and experimental studies have established that selective and nonselective nonsteroidal anti-inflammatory (NSAIDs) prevent the development of cancer from neoplastic polyps. Studies that showed COX-2 to be a significant factor that regulates apoptosis, angiogenesis (VEGF), and invasiveness in tumor cells also provided mechanistic insights into the position of COX-2 in intestinal tumorigenesis [30]. In addition, studies have indicated that NSAIDs inhibit cell proliferation and induce apoptosis in colon and other tumor cell lines in culture [31].
Karaman et al. reported that NLR was able to distinguish neoplastic polyps from non-neoplastic ones [32]. In the present study, NLR values >1.9 could predict neoplastic polyps with a sensitivity of 71% and a specificity of 50%. However, we could not establish a significant difference in the NLR between these two groups. This situation may be associated with the low villous adenoma ratio (7.4% and 2.3%) despite the relatively high total number of patients included in the study. Another study looking at the polyp-NLR relationship found that NLR was able to distinguish CRC from neoplastic polyps [33]. In the present study, NLR was found to be significantly higher in the CRC group than in both the group with neoplastic polyps and the healthy group, whereas no difference was identified between the NLR values of patients with neoplastic polyps compared to healthy controls. The earlier study established NLR values >2.28 as able to predict CRC with a sensitivity of 68% and a specificity of 42%. These values correspond to the results of our study.
In histopathologic terms, a correlation was determined between polyp size and both acute and chronic inflammation [29]. The present study identified significantly higher NLRs in polyps larger than 10 mm and demonstrated the same finding in systemic terms.
The first, most important limitation of the present study is its concentric and retrospective nature. Second, the study could not assess the effects on the immune system fully due to its retrospective design. Another weakness of the study was the low number of patients with cancerous and dysplastic polyps compared to the number of patients with hyperplastic and adenomatous polyps. Furthermore, NLR remained insufficient in predicting dysplastic polyps and could only predict cancer in the adenoma-dysplasia-cancer sequence. This may have been the result of the small number of patients in the dysplasia group, as well as the low number of high-grade dysplastic patients in this group (n=3). These results provide preliminary information for multi-centered, prospective and long-term studies to be undertaken in the future.
Conclusions
The present study established that NLR increased gradually in the pathway extending from adenoma to cancer. We detected that NLR was significantly higher in cancerous polyps when compared to non-neoplastic polyps and adenomatous polyps. Furthermore, with the NLR cutoff value set at 2.2, we were able to predict patients with cancerous polyps from amongst all patients exhibiting polyps with a medium level of sensitivity and specificity. NLR is a cheap, universally available, simple, and reliable test that can predict cancerous polyps. Large-scale prospective studies to be conducted in the future may enable NLR to be utilized as a useful biomarker in the follow-up of patients with polyps and the selection of patients for such modes of treatment as COX-2 inhibitors that inhibit the progression of CRC.
Footnotes
Conflict of interest
None.
Source of support: This study was financed by the authors themselves
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: Clinical guidelines and rationale. Gastroenterology. 1997;112(2):594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Roxburgh CS, Salmond JM, Horgan PG, et al. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249(5):788–93. doi: 10.1097/SLA.0b013e3181a3e738. [DOI] [PubMed] [Google Scholar]
- 7.Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 8.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–5. doi: 10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 9.Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–76. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 10.Cetin B, Kaplan MA, Berk V, et al. Prognostic factors for overall survival in patients with metastatic colorectal carcinoma treated with vascular endothelial growth factor-targeting agents. Asian Pac J Cancer Prev. 2012;13(3):1059–63. doi: 10.7314/apjcp.2012.13.3.1059. [DOI] [PubMed] [Google Scholar]
- 11.Maeda K, Shibutani M, Otani H, et al. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. 2015;7(8):111–17. doi: 10.4251/wjgo.v7.i8.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23(1):31–39. doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 14.Hirashima M, Higuchi S, Sakamoto K, et al. The ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancer. J Cancer Res Clin Oncol. 1998;124:329–34. doi: 10.1007/s004320050178. [DOI] [PubMed] [Google Scholar]
- 15.Lord BI, Bronchud MH, Owens S, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA. 1989;86:9499–503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azab B, Bhatt V, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 17.Halazun KJ, Hardy MA, Rana AA, et al. Negative Impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–51. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 18.Kusumanto Y, Dam W, Hospers GP, et al. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–87. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 19.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–18. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 20.Nind AP, Nairn RC, Rolland JM, et al. Lymphocyte anergy in patients with carcinoma. Br J Cancer. 1973;28:108–17. doi: 10.1038/bjc.1973.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calman KC. Tumour immunology and the gut. Gut. 1975;16:490–99. doi: 10.1136/gut.16.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite |instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–20. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: The role of tumor invading T cells. World J Gastroenterol. 2006;12:7233–38. doi: 10.3748/wjg.v12.i45.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Rao HL, Chen JW, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One. 2012;7(1):e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko M, Nozawa H, Sasaki K, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin-based chemotherapy. Oncology. 2012;82(5):261–68. doi: 10.1159/000337228. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Peng Z, Chen M, et al. Elevated neutrophil to lymphocyte ratio might predict poor prognosis for colorectal liver metastasis after percutaneous radiofrequency ablation. Int J Hyperthermia. 2012;28(2):132–40. doi: 10.3109/02656736.2011.654374. [DOI] [PubMed] [Google Scholar]
- 28.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilinski C, Burleson J, Forouhar F. Inflammation associated with neoplastic colonic polyps. Ann Clin Lab Sci. 2012;42(3):266–70. [PubMed] [Google Scholar]
- 30.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metast Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 31.Rigas B, Shiff SJ. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenases, and the cell cycle. Their interactions in colon cancer. Adv Exp Med Biol. 1999;470:119–26. doi: 10.1007/978-1-4615-4149-3_13. [DOI] [PubMed] [Google Scholar]
- 32.Karaman H, Karaman A, Erden A, Poyrazoglu OK, et al. Relationship between colonic polyp type and the neutrophil/lymphocyte ratio as a biomarker. Asian Pac J Cancer Prev. 2013;14(5):3159–61. doi: 10.7314/apjcp.2013.14.5.3159. [DOI] [PubMed] [Google Scholar]
- 33.Emir S, Aydin M, Can G, et al. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur Rev Med Pharmacol Sci. 2015;19(19):3613–18. [PubMed] [Google Scholar]


